The Use of Registries to Improve Cancer Treatment: A National Database for Patients Treated with Interleukin-2 (IL-2)
Abstract
:1. Introduction
2. IL-2 Therapy over the Past Two Decades
3. Predictive Biomarkers of IL-2 Clinical Response
4. Managing IL-2 Toxicity
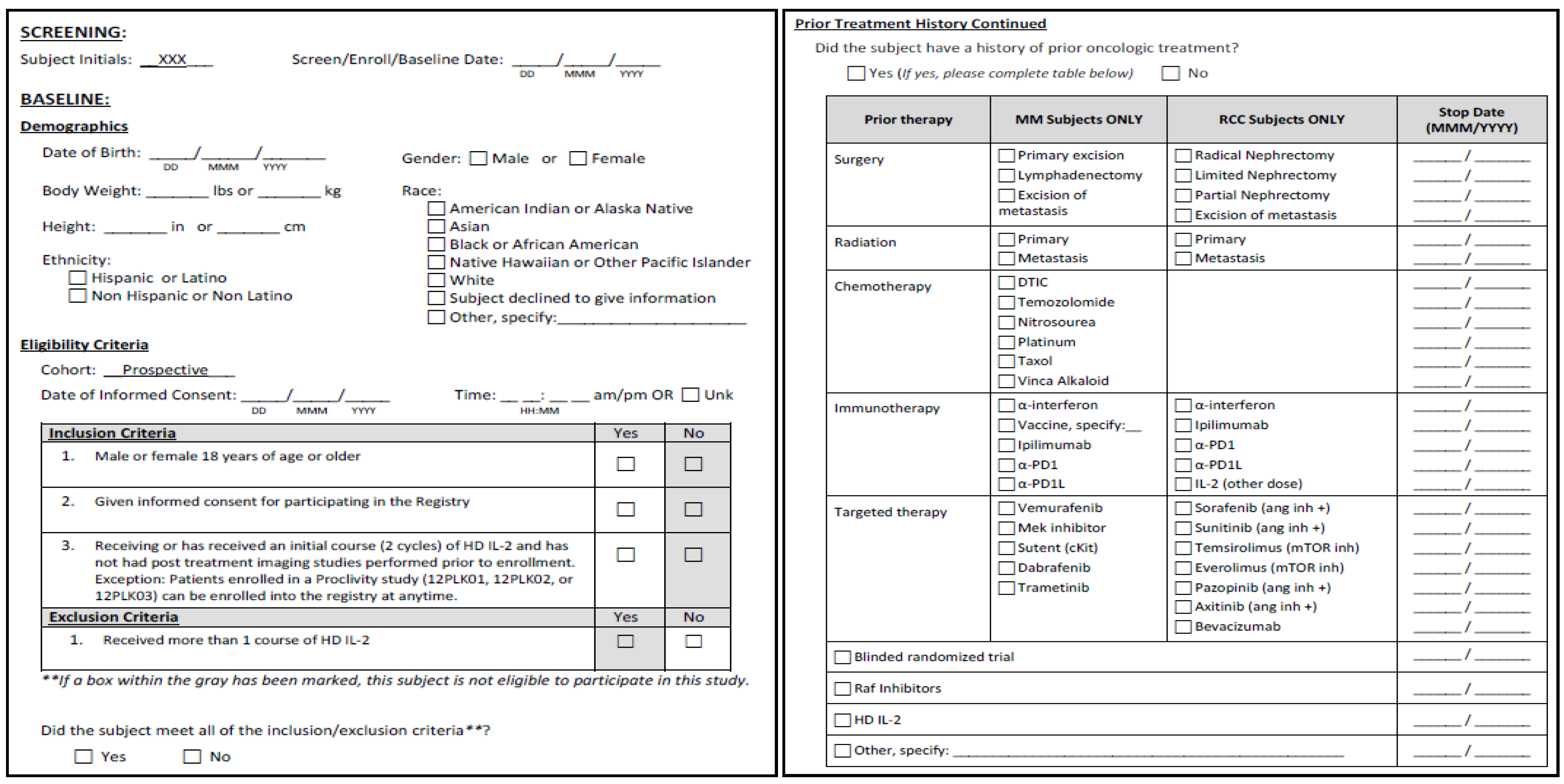
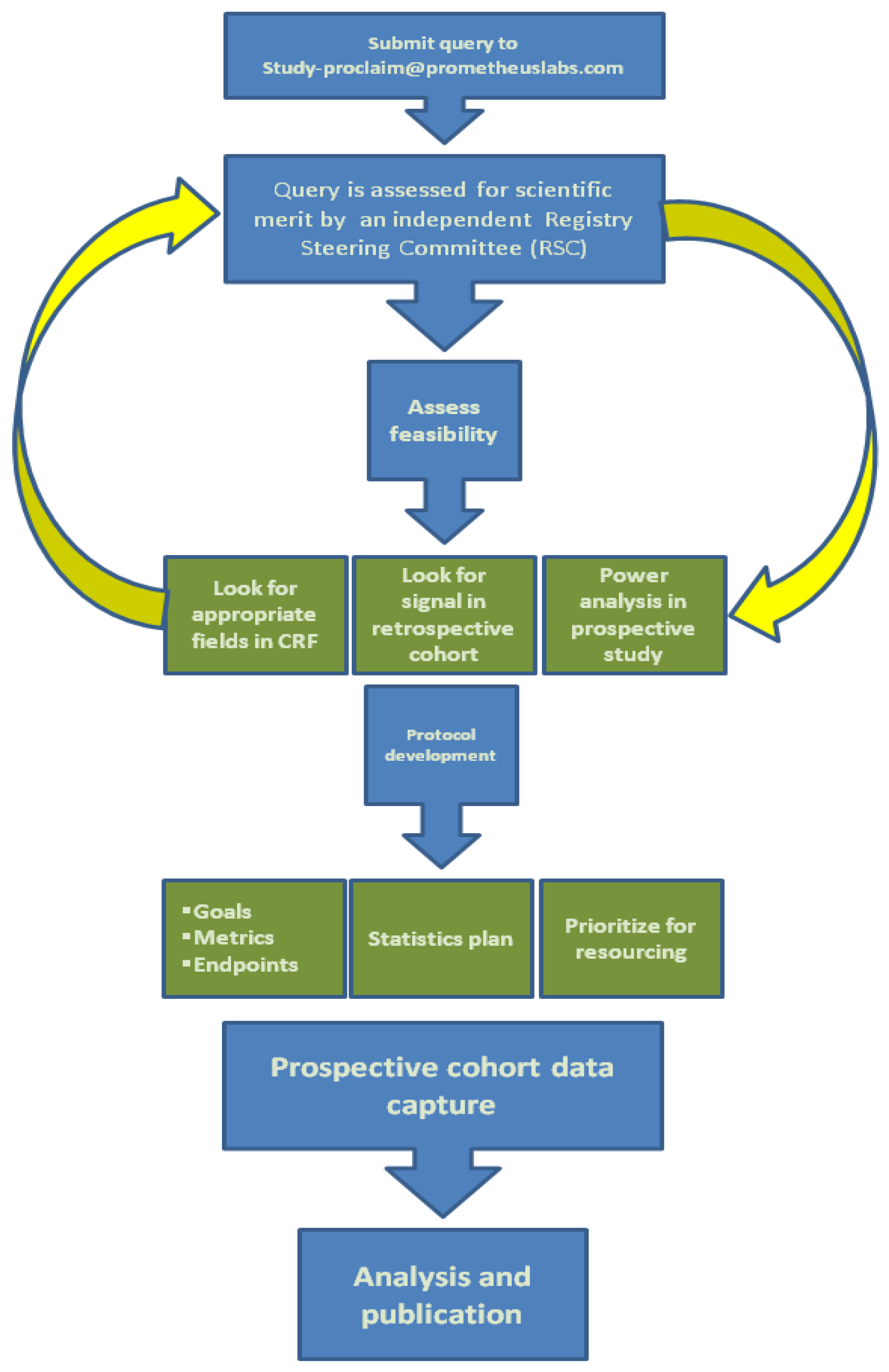
5. Interleukin-2 Registry
|
|
|
|
|
| # | State | Registry Site | Principle Investigator |
|---|---|---|---|
| 1 | AZ | The University of Arizona Cancer Center | Joanne Jeter MD |
| 2 | CA | University of California San Diego | Gregory Daniels MD PhD |
| 3 | CA | USC Norris Cancer Center | Michael Wong MD PhD |
| 4 | CO | University of Colorado Cancer Center | Rene Gonzalez MD |
| 5 | FL | H. Lee Moffitt Cancer Center and Research Institute | Mayer Fishman MD PhD |
| 6 | FL | Mount Sinai Medical Center Comprehensive Cancer Center | Jose Lutzky MD |
| 7 | GA | Emory University Winship Cancer Institute | David Lawson MD |
| 8 | IA | University of Iowa Hospitals and Clinics | Mohammed Milhem MD |
| 9 | IL | Loyola University Medical Center | Joseph Clark MD |
| 10 | IL | Oncology Specialists, SC | Sigrun Hallmeyer MD |
| 11 | IL | Rush University Medical Center | Howard Kaufman MD |
| 12 | IN | Indiana University Simon Cancer Center | Theodore Logan MD |
| 13 | KS | University of Kansas Hospital | Peter Van Veldhuizen MD |
| 14 | LA | The Baton Rouge Clinic, AMC | Gerald Miletello MD |
| 15 | MA | Beth Israel Deaconess Medical Center | David McDermott MD |
| 16 | MI | Barbara Ann Karmanos Cancer Institute | Ulka Vaishampayan MD |
| 17 | MN | University of Minnesota Masonic Cancer Center | Venkatesh Rudrapatna MD |
| 18 | MO | Saint Louis University | John Richart MD |
| 19 | NC | Blumenthal Cancer Center | Asim Amin MD PhD |
| 20 | NC | Duke University Medical Center | Michael Morse MD |
| 21 | NC | Wake Forest University Baptist Medical Center | John Stewart IV MD |
| 22 | NE | Midwest Cancer Center—Legacy | Ralph Hauke MD |
| 23 | NH | Dartmouth Hitchcock Medical Center | Marc Emstoff MD |
| 24 | NJ | Hackensack University Medical Center | Robert Alter MD |
| 25 | NY | Saint Luke’s-Roosevelt Hospital Center | Seth Cohen MD |
| 26 | OH | University Hospitals Siedman Cancer Center | Henry Koon MD |
| 27 | OR | Providence Portland Medical Center | Brendan Curti MD |
| 28 | PA | Hillman Cancer Research Pavilion, Div. of Medical Oncology | John Kirkwood MD |
| 29 | PA | Saint Luke’s Hospital and Health Network | Sanjiv Agarwala MD |
| 30 | TX | MD Anderson Cancer Center | Sapna Patel MD |
| 31 | UT | University of Utah School of Medicine | Neeraj Agarwal, MD |
| 32 | OH | The Christ Hospital Cancer Center | Philip Leming, MD |
| 33 | MI | University of Michigan | Christopher Lao, MD |
| 34 | MD | John Hopkins University School of Medicine | William Sharfman, MD |
| 35 | NY | Columbia University Medical Center | Bret Taback, MD |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rising, K.; Bacchetti, P.; Bero, L. Reporting bias in drug trials submitted to the food and drug administration: Review of publication and presentation. PLoS Med. 2008, 5, e217. [Google Scholar] [CrossRef]
- Lee, K.; Bacchetti, P.; Sim, I. Publication of clinical trials supporting successful new drug applications: A literature analysis. PLoS Med. 2008, 5, e191. [Google Scholar] [CrossRef]
- Turner, E.H.; Matthews, A.M.; Linardatos, E.; Tell, R.A.; Rosenthal, R. Selective publication of antidepressant trials and its influence on apparent efficacy. N. Engl. J. Med. 2008, 358, 252–260. [Google Scholar] [CrossRef]
- Goldman, J.M.; Horowitz, M.M. The international bone marrow transplant registry. Int. J. Hematol. 2002, 76, 393–397. [Google Scholar] [CrossRef]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar]
- Fisher, R.I.; Rosenberg, S.A.; Fyfe, G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J. Sci. Am. 2000, 6, S55–S57. [Google Scholar]
- Atkins, M.B. Interleukin-2 in metastatic melanoma: What is the current role? Cancer J. Sci. Am. 2000, 6, S8–S10. [Google Scholar]
- Yang, J.C.; Sherry, R.M.; Steinberg, S.M.; Topalian, S.L.; Schwartzentruber, D.J.; Hwu, P.; Seipp, C.A.; Rogers-Freezer, L.; Morton, K.E.; White, D.E.; et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J. Clin. Oncol. 2003, 21, 3127–3132. [Google Scholar] [CrossRef]
- McDermott, D.F.; Regan, M.M.; Clark, J.I.; Flaherty, L.E.; Weiss, G.R.; Logan, T.F.; Kirkwood, J.M.; Gordon, M.S.; Sosman, J.A.; Ernstoff, M.S.; et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2005, 23, 133–141. [Google Scholar] [CrossRef]
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 2008, 113, 293–301. [Google Scholar] [CrossRef]
- McDermott, D.; Ghebremichael, M.; Signoretti, S.; Margolin, K.; Clark, J.; Sosman, J.; Dutcher, J.; Logan, T.; Figlin, R.; Atkins, M. The high-dose aldesleukin (HD IL-2) “select” trial in patients with metastatic renal cell carcinoma (mRCC). J. Clin. Oncol. 2010, 28. Abstract 4515. [Google Scholar]
- O’Day, S.J.; Boasberg, P.D.; Piro, L.; Kristedja, T.S.; Wang, H.J.; Martin, M.; Deck, R.; Ames, P.; Shinn, K.; Kim, H.; et al. Maintenance biotherapy for metastatic melanoma with interleukin-2 and granulocyte macrophage-colony stimulating factor improves survival for patients responding to induction concurrent biochemotherapy. Clin. Cancer Res. 2002, 8, 2775–2781. [Google Scholar]
- Dummer, R.; Hauschild, A.; Henseler, T.; Burg, G. Combined interferon-alpha and interleukin-2 as adjuvant treatment for melanoma. Lancet 1998, 352, 908–909. [Google Scholar]
- Edwards, B.S.; Merritt, J.A.; Fuhlbrigge, R.C.; Borden, E.C. Low doses of interferon alpha result in more effective clinical natural killer cell activation. J. Clin. Investig. 1985, 75, 1908–1913. [Google Scholar] [CrossRef]
- Sahasrabudhe, D.M.; Dusel, J.C. Effect of murine interferon alpha/beta on tumour-induced suppressor function. Cancer Immunol. Immunother. 1994, 39, 360–366. [Google Scholar] [CrossRef]
- Lee, K.H.; Wang, E.; Nielsen, M.B.; Wunderlich, J.; Migueles, S.; Connors, M.; Steinberg, S.M.; Rosenberg, S.A.; Marincola, F.M. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J. Immunol. 1999, 163, 6292–6300. [Google Scholar]
- Rosenberg, S.A.; Yang, J.C.; Schwartzentruber, D.J.; Hwu, P.; Marincola, F.M.; Topalian, S.L.; Restifo, N.P.; Dudley, M.E.; Schwarz, S.L.; Spiess, P.J.; et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998, 4, 321–327. [Google Scholar] [CrossRef]
- Schwartzentruber, D.J.; Lawson, D.H.; Richards, J.M.; Conry, R.M.; Miller, D.M.; Treisman, J.; Gailani, F.; Riley, L.; Conlon, K.; Pockaj, B.; et al. gp100 Peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 2011, 364, 2119–2127. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Prieto, P.A.; Yang, J.C.; Sherry, R.M.; Hughes, M.S.; Kammula, U.S.; White, D.E.; Levy, C.L.; Rosenberg, S.A.; Phan, G.Q. CTLA-4 blockade with ipilimumab: Long-term follow-up of 177 patients with metastatic melanoma. Clin. Cancer Res. 2012, 18, 2039–2047. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin. Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef]
- Dudley, M.E.; Gross, C.A.; Somerville, R.P.; Hong, Y.; Schaub, N.P.; Rosati, S.F.; White, D.E.; Nathan, D.; Restifo, N.P.; Steinberg, S.M.; et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J. Clin. Oncol. 2013, 31, 2152–2159. [Google Scholar] [CrossRef]
- Sabatino, M.; Kim-Schulze, S.; Panelli, M.C.; Stroncek, D.; Wang, E.; Taback, B.; Kim, D.W.; Deraffele, G.; Pos, Z.; Marincola, F.M.; et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J. Clin. Oncol. 2009, 27, 2645–2652. [Google Scholar] [CrossRef]
- Bui, M.; Seligson, D.; Han, K.; Pantuck, A.; Dorey, F.J.; Huang, Y.; Horvath, S.; Leibovish, B.C.; Chopra, S.; Liao, S.Y.; et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin. Can. Res. 2003, 9, 802–811. [Google Scholar]
- Upton, M.P.; Parker, R.A.; Youmans, A.; McDermott, D.F.; Atkins, M.B. Histologic predictors of renal cell carcinoma response to interleukin-2 based therapy. J. Immunother. 2005, 28, 488–495. [Google Scholar] [CrossRef]
- Atkins, M.; Regan, M.; McDermott, D.; Mier, J.; Stanbridge, E.; Youmans, A.; Febbo, P.; Upton, M.; Lechpammer, M.; Signoretti, S. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin. Cancer Res. 2005, 11, 3714–3721. [Google Scholar] [CrossRef]
- Bailey, A.S.; Cheng, S.C.; Kwon, E.D.; Leibovich, B.C.; Signoretti, S.; Dutcher, J.P.; Appleman, L.J.; Sosman, J.A.; Margolin, K.A.; Clark, J.; Khushalani, N.I.; et al. Pdl-1/pdl-3 (programmed death ligand-1/3) tissue expression and response to treatment with IL2 and antiangiogenic therapies. In Proceedings of the 2013 ASCO Annual Meeting, Chicago, IL, USA, 31 May, 2013.
- Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Berger, A.; Bindea, G.; Meatchi, T.; Bruneval, P.; Trajanoski, Z.; Fridman, W.H.; Pagès, F.; et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 2011, 29, 610–618. [Google Scholar] [CrossRef]
- Pages, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef]
- Pages, F.; Galon, J.; Dieu-Nosjean, M.C.; Tartour, E.; Sautes-Fridman, C.; Fridman, W.H. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2010, 29, 1093–1102. [Google Scholar] [CrossRef]
- Nosho, K.; Baba, Y.; Tanaka, N.; Shima, K.; Hayashi, M.; Meyerhardt, J.A.; Giovannucci, E.; Dranoff, G.; Fuchs, C.S.; Ogino, S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J. Pathol. 2010, 222, 350–366. [Google Scholar] [CrossRef]
- Broussard, E.K.; Disis, M.L. TNM staging in colorectal cancer: T is for T cell and M is for memory. J. Clin. Oncol. 2011, 29, 601–603. [Google Scholar] [CrossRef]
- Erdag, G.; Schaefer, J.T.; Smolkin, M.E.; Deacon, D.H.; Shea, S.M.; Dengel, L.T.; Patterson, J.W.; Slingluff, C.L., Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012, 72, 1070–1080. [Google Scholar]
- Galon, J.; Pages, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012, 10, e205. [Google Scholar] [CrossRef] [Green Version]
- PROLEUKIN®. Available online: http://www.proleukin.com/assets/proleukin.pdf (assessed on 3 March 2014).
- Phan, G.Q.; Attia, P.; Steinberg, S.M.; White, D.E.; Rosenberg, S.A. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J. Clin. Oncol. 2001, 19, 3477–3482. [Google Scholar]
- Schwartz, R.N.; Stover, L.; Dutcher, J. Managing toxicities of high-dose interleukin-2. Oncology 2002, 16, 11–20. [Google Scholar]
- Fox, B.A.; Schendel, D.J.; Butterfield, L.H.; Aamdal, S.; Allison, J.P.; Ascierto, P.A.; Atkins, M.B.; Bartunkova, J.; Bergmann, L.; Berinstein, N.; et al. Defining the critical hurdles in cancer immunotherapy. J. Transl. Med. 2011, 9, e214. [Google Scholar] [CrossRef] [Green Version]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaufman, H.L.; Wong, M.K.; Daniels, G.A.; McDermott, D.F.; Aung, S.; Lowder, J.N.; Morse, M.A. The Use of Registries to Improve Cancer Treatment: A National Database for Patients Treated with Interleukin-2 (IL-2). J. Pers. Med. 2014, 4, 52-64. https://doi.org/10.3390/jpm4010052
Kaufman HL, Wong MK, Daniels GA, McDermott DF, Aung S, Lowder JN, Morse MA. The Use of Registries to Improve Cancer Treatment: A National Database for Patients Treated with Interleukin-2 (IL-2). Journal of Personalized Medicine. 2014; 4(1):52-64. https://doi.org/10.3390/jpm4010052
Chicago/Turabian StyleKaufman, Howard L., Michael K. Wong, Gregory A. Daniels, David F. McDermott, Sandra Aung, James N. Lowder, and Michael A. Morse. 2014. "The Use of Registries to Improve Cancer Treatment: A National Database for Patients Treated with Interleukin-2 (IL-2)" Journal of Personalized Medicine 4, no. 1: 52-64. https://doi.org/10.3390/jpm4010052





