Poria cocos Lanostane Triterpenoids Extract Promotes Collagen and Hyaluronic Acid Production in D-Galactose-Induced Aging Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Poria cocos Extract Preparation (PCE) (Lipucan®)
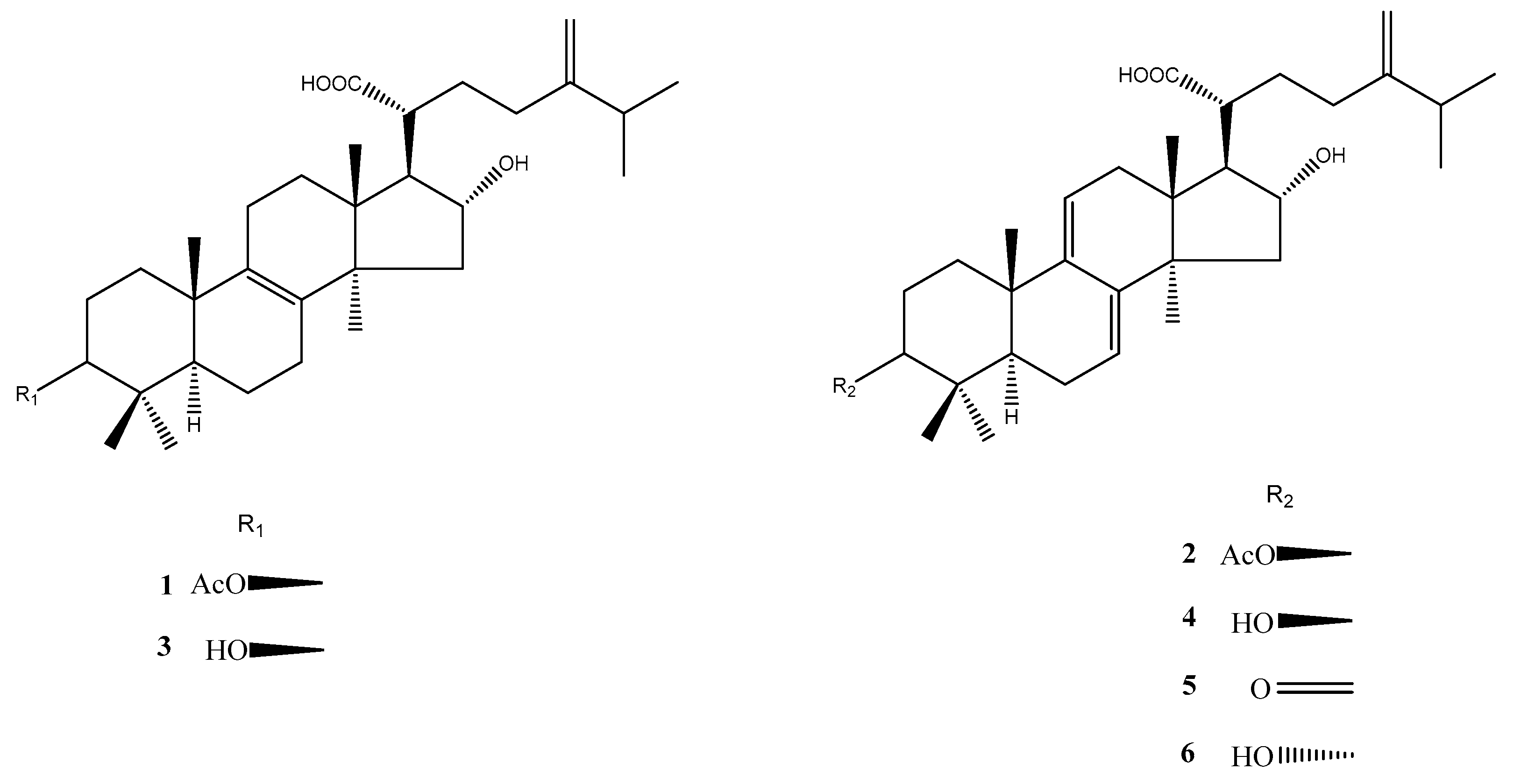
2.2. Isolation and Purification of Lanostane Triterpenoid Compounds (1–6)
2.3. Animals
2.4. D-Galactose-Induced Rat Skin Aging Model
2.5. Immunohistochemical (IHC) Staining Procedure
2.6. Western Blot Analysis of Type I Collagen In Vivo
2.7. Hyaluronic Acid Measurement In Vivo
2.8. Lanostane Triterpenoid Compounds (1–6) for Collagen and Hyaluronic Acid Analysis in Primary Human Dermal Fibroblasts
2.9. Statistical Analysis
3. Results
3.1. The Anti-Aging Activity of PCE in D-Galatose-Induced Skin Aging Rats
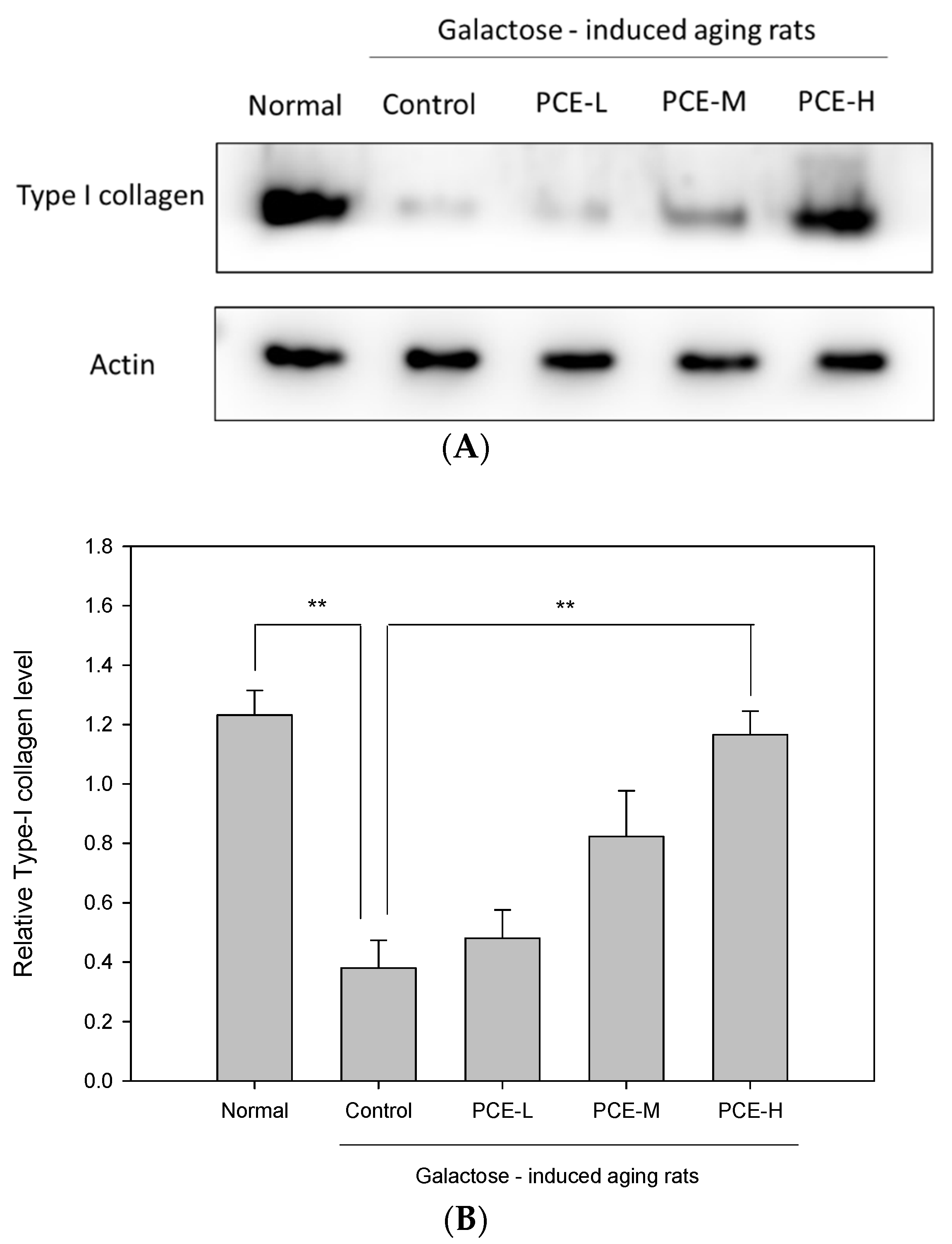
3.2. PCE Promoted Collagen Type I Production in D-Galactose-Induced Skin Aging Rats
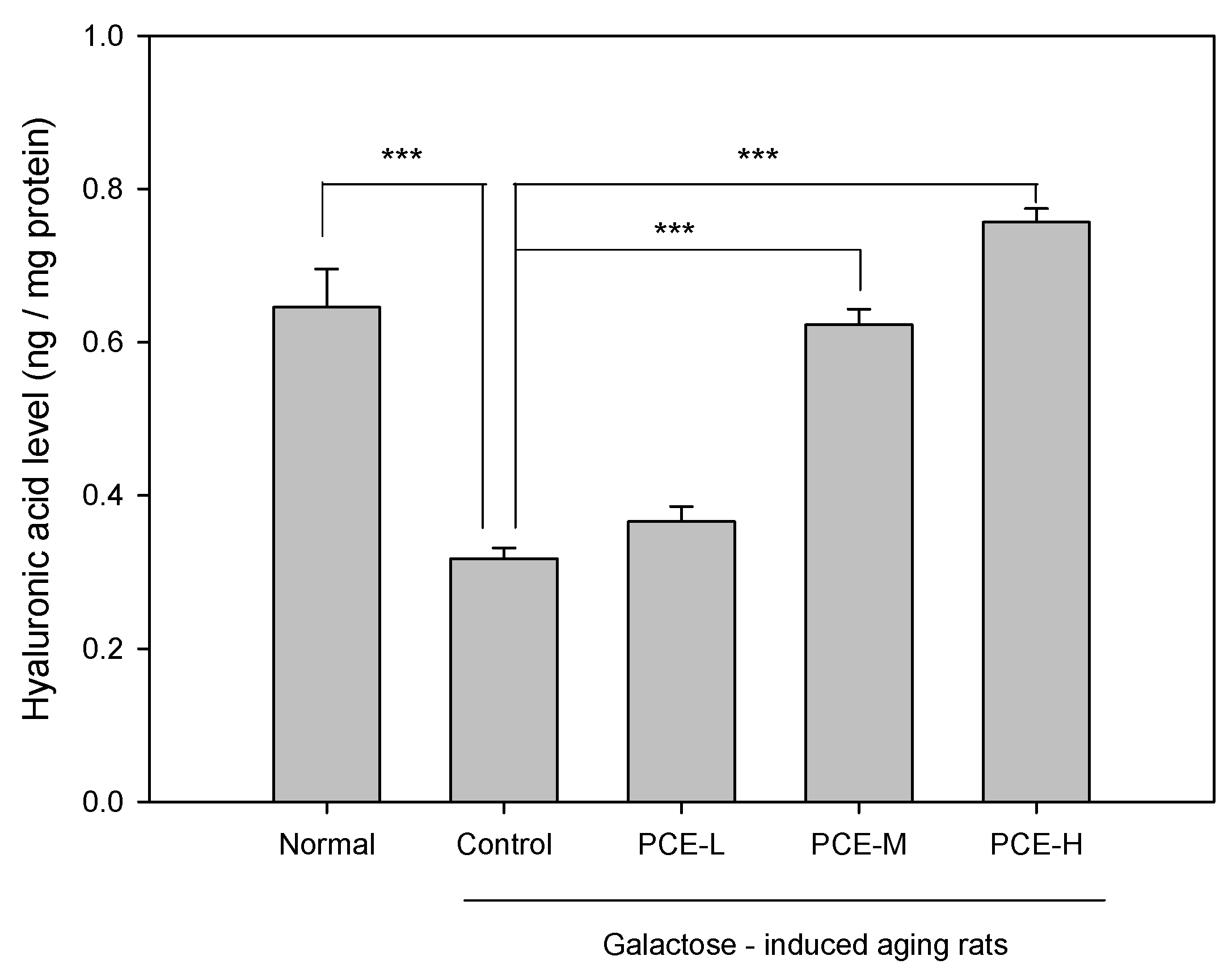
3.3. PCE Promoted Hyaluronic Acid Production in D-Galactose-Induced Skin Aging Rats
3.4. Isolation and Identification of Six Lanostane Triterpenoid Compounds 1–6 of P. cocos
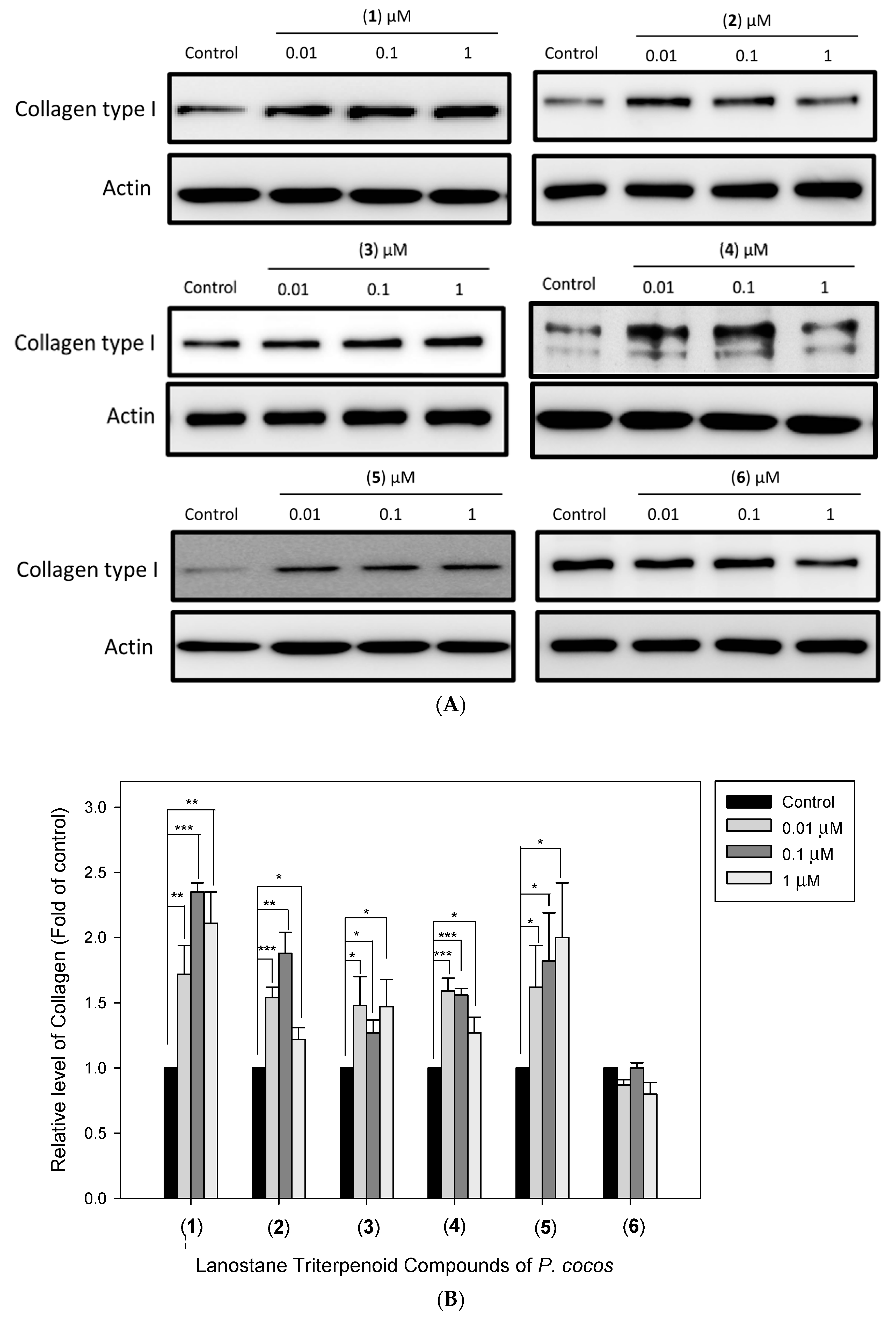
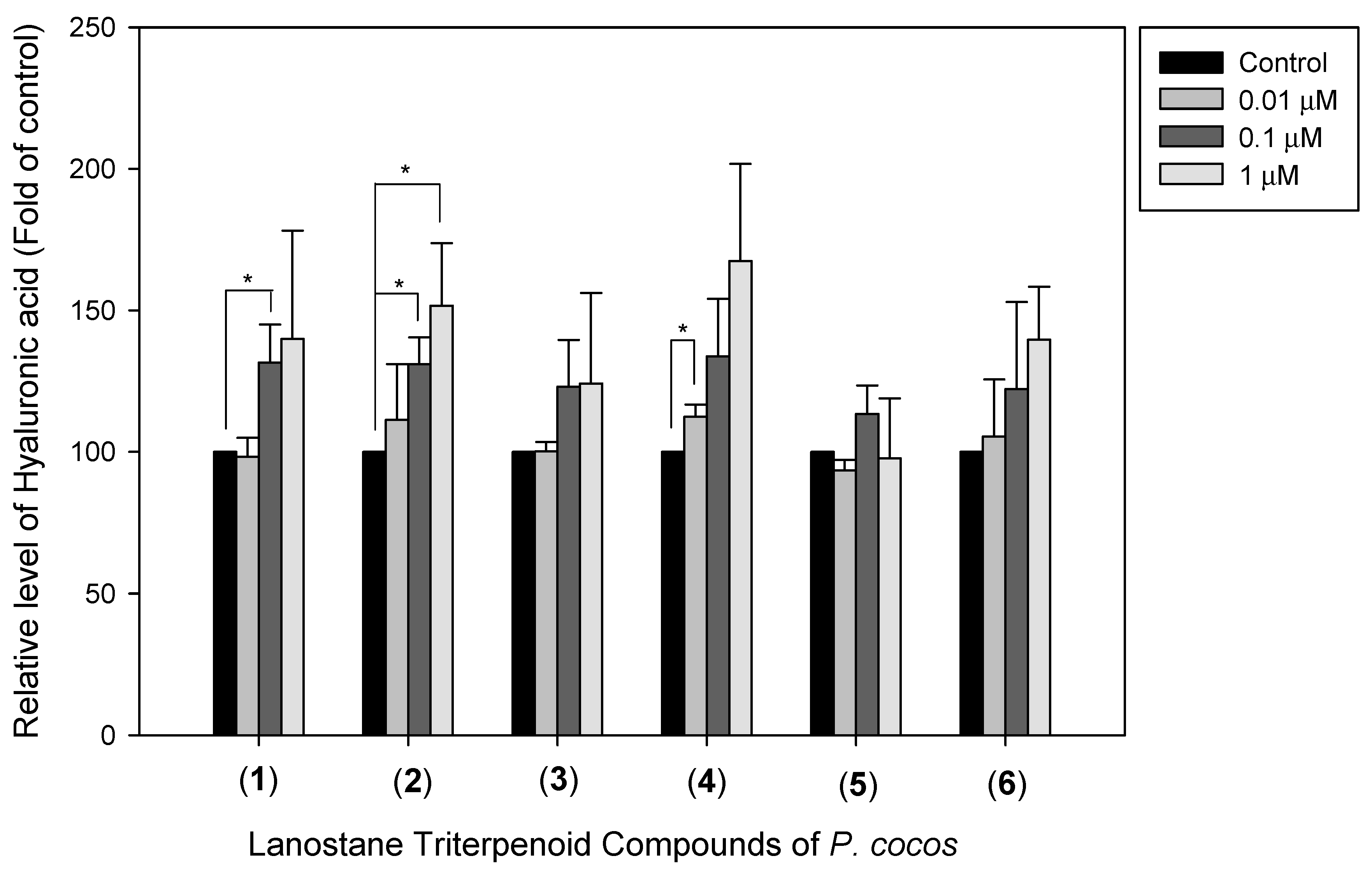
3.5. The Effects of Lanostane Triterpenoid Compounds 1–6 of P. cocos on Type I Collagen and Hyaluronic Acid Production in HDF Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier function of the skin: “la raison d’être” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Levin, J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J. Clin. Aesthet. Dermatol. 2011, 4, 22–42. [Google Scholar]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Tzaphlidou, M. The role of collagen and elastin in aged skin: An image processing approach. Micron 2004, 35, 173–177. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Juhlin, L. Hyaluronan in skin. J. Intern. Med. 1997, 242, 61–66. [Google Scholar] [CrossRef]
- Reed, R.K.; Lilja, K.; Laurent, T.C. Hyaluronan in the rat with special reference to the skin. Acta Physiol. Scand. 1988, 134, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.; Hay, R.J.; Griffiths, C.E. Age-associated skin conditions and diseases: Current perspectives and future options. Gerontologist 2016, 56, S230–S242. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Amer. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Fuster, V. Changing demographics: A new approach to global health care due to the aging population. J. Am. Coll. Cardiol. 2017, 69, 3002–3005. [Google Scholar] [CrossRef] [PubMed]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based. Complement. Alternat. Med. 2013, 2013, 827248. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Nusgens, B.V.; Colige, A.C.; Lambert, C.A.; Lapière, C.M.; Humbert, P.; Rougier, A.; Haftek, M.; Richard, A.; Creidi, P. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J. Investig. Dermatol. 2001, 116, 853–859. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huh, S.; Kim, S.; Koh, J.; Park, D. Effect of camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Lee, J.; Huh, S.; Kim, J.; Park, M.; So, J.; Ham, Y.; Jung, K.; Hyun, C.G.; et al. Panax ginseng induces human type I collagen synthesis through activation of Smad signaling. J. Ethnopharmacol. 2007, 109, 29–34. [Google Scholar] [CrossRef]
- Takasao, N.; Tsuji-Naito, K.; Ishikura, S.; Tamura, A.; Akagawa, M. Cinnamon extract promotes type I collagen biosynthesis via activation of IGF-I signaling in human dermal fibroblasts. J. Agric. Food Chem. 2012, 60, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Wakaizumi, M.; Ikami, T.; Saito, M. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J. Ethnopharmacol. 2008, 119, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.F.; Chiang, B.H. Stimulating effects of Bacillus subtilis natto-fermented radix astragali on hyaluronic acid production in human skin cells. J. Ethnopharmacol. 2009, 125, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Giner-Larza, E.M.; Máñez, S.; Giner-Pons, R.M.; Carmen Recio, M.; Rĺos, J.L. On the anti-inflammatory and anti-phospholipase A2 activity of extracts from lanostane-rich species. J. Ethnopharmacol. 2000, 73, 61–69. [Google Scholar] [CrossRef]
- Shah, V.K.; Choi, J.J.; Han, J.Y.; Lee, M.K.; Hong, J.T.; Oh, K.W. Pachymic acid enhances pentobarbital-induced sleeping behaviors via GABAA-ergic systems in mice. Biomol. Ther. 2014, 22, 314–320. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chang, W.L.; Huang, S.F.; Lin, C.Y.; Lin, H.C.; Chang, T.C. Pachymic acid stimulates glucose uptake through enhanced GLUT4 expression and translocation. Eur. J. Pharmacol. 2010, 648, 39–49. [Google Scholar] [CrossRef]
- Yu, S.J.; Tseng, J. Fu-ling, a chinese herbal drug, modulates cytokine secretion by human peripheral blood monocytes. Int. J. Immunopharmacol. 1996, 18, 37–44. [Google Scholar] [CrossRef]
- Deqiang, D.; Hang, X.; Xiaofei, W.; Bingyou, Y.; Haixue, K. Immunoenhancing constituents of Poria cocos. Int. J. Pharmacol. 2015, 11, 463–469. [Google Scholar]
- Cuellar, M.J.; Giner, R.M.; Recio, M.C.; Just, M.J.; Manez, S.; Rios, J.L. Effect of the basidiomycete Poria cocos on experimental dermatitis and other inflammatory conditions. Chem. Pharm. Bull. 1997, 45, 492–494. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, H.H.; Han, M.H.; Kim, G.Y.; Hong, S.H.; Park, C.; Choi, Y.H. Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement. Altern. Med. 2014, 14, 101. [Google Scholar] [CrossRef]
- Chu, B.F.; Lin, H.C.; Huang, X.W.; Huang, H.Y.; Wu, C.P.; Kao, M.C. An ethanol extract of Poria cocos inhibits the proliferation of non-small cell lung cancer A549 cells via the mitochondria-mediated caspase activation pathway. J. Funct. Foods 2016, 23, 614–627. [Google Scholar] [CrossRef]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; de la Cruz, M.; Monteiro, M.C.; Melguizo, Á.; et al. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional chinese medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.F.; Hua, T.; Wang, D.; Zhao, Z.W.; Xi, G.L.; Chen, Z.F. Phytochemical and chemotaxonomic study of Poria cocos (Schw.). Wolf Biochem. Syst. Ecol. 2019, 83, 54–56. [Google Scholar] [CrossRef]
- Ding, G.; Wang, Z.Z.; Zhang, C.F.; Sheng, L.S. Study on HPLC fingerprint of the triterpene acids in Poria cocos. Zhongguo Zhong Yao Za Zhi 2002, 27, 756–758. [Google Scholar]
- Yang, H.; Shen, Y.; Chen, B.; Jia, X.; Cai, B. RP-HPLC-DAD determination of six triterpenes in a herbal tonic hoelen. J. Liq. Chromatogr. Relat. 2011, 34, 1772–1782. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci. Rep. 2018, 8, 1465. [Google Scholar] [CrossRef]
- Kim, S.; Kang, B.Y.; Cho, S.Y.; Sung, D.S.; Chang, H.K.; Yeom, M.H.; Kim, D.H.; Sim, Y.C.; Lee, Y.S. Compound K induces expression of hyaluronan synthase 2 gene in transformed human keratinocytes and increases hyaluronan in hairless mouse skin. Biochem. Biophys. Res. Commun. 2004, 316, 348–355. [Google Scholar] [CrossRef]
- Lynch, B.; Pageon, H.; Le Blay, H.; Brizion, S.; Bastien, P.; Bornschlögl, T.; Domanov, Y. A mechanistic view on the aging human skin through ex vivo layer-by-layer analysis of mechanics and microstructure of facial and mammary dermis. Sci. Rep. 2022, 12, 849. [Google Scholar] [CrossRef]
- Nations, U.; Economic, D.; Affairs, S. World Population Prospects 2019; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Lu, J.; Tian, J.; Zhou, L.; Meng, L.; Chen, S.; Ma, C.; Wang, J.; Liu, Z.; Li, C.; Kang, W. Phytochemistry and biological activities of Poria. J. Chem. 2021, 2021, 6659775. [Google Scholar] [CrossRef]
- Shen, L.H.; Fan, L.; Zhang, Y.; Shen, Y.; Su, Z.T.; Peng, G.N.; Deng, J.L.; Zhong, Z.J.; Wu, X.F.; Yu, S.M.; et al. Antioxidant Capacity and Protective Effect of Cow Placenta Extract on D-Galactose-Induced Skin Aging in Mice. Nutrients 2022, 14, 4659. [Google Scholar] [CrossRef]
- Fang, C.L.; Paul, C.R.; Day, C.H.; Chang, R.L.; Kuo, C.H.; Ho, T.J.; Hsieh, D.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Poria cocos (Fuling) targets TGFβ/Smad7 associated collagen accumulation and enhances Nrf2-antioxidant mechanism to exert anti-skin aging effects in human dermal fibroblasts. Environ. Toxicol. 2021, 36, 729–736. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-L.; Kuo, H.-P.; Huang, H.-W.; Cheng, M.-Y.; Chao, H.-F.; Lu, S.-M.; Lin, H.-C.; Wang, C.-J.; Chang, T.-C.; Wu, C.-R. Poria cocos Lanostane Triterpenoids Extract Promotes Collagen and Hyaluronic Acid Production in D-Galactose-Induced Aging Rats. Life 2023, 13, 2130. https://doi.org/10.3390/life13112130
Chao C-L, Kuo H-P, Huang H-W, Cheng M-Y, Chao H-F, Lu S-M, Lin H-C, Wang C-J, Chang T-C, Wu C-R. Poria cocos Lanostane Triterpenoids Extract Promotes Collagen and Hyaluronic Acid Production in D-Galactose-Induced Aging Rats. Life. 2023; 13(11):2130. https://doi.org/10.3390/life13112130
Chicago/Turabian StyleChao, Chien-Liang, Han-Peng Kuo, Hsin-Wen Huang, Maw-Yeun Cheng, Hsin-Fan Chao, Shih-Min Lu, Hang-Ching Lin, Chao-Jih Wang, Tsu-Chung Chang, and Chi-Rei Wu. 2023. "Poria cocos Lanostane Triterpenoids Extract Promotes Collagen and Hyaluronic Acid Production in D-Galactose-Induced Aging Rats" Life 13, no. 11: 2130. https://doi.org/10.3390/life13112130





