Stimulation of Hemolysis and Eryptosis by β-Caryophyllene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Blood Collection
2.3. Hemolysis
2.4. Potassium Leakage
2.5. Lactate Dehydrogenase (LDH) Activity
2.6. Aspartate Aminotransferase (AST) Activity
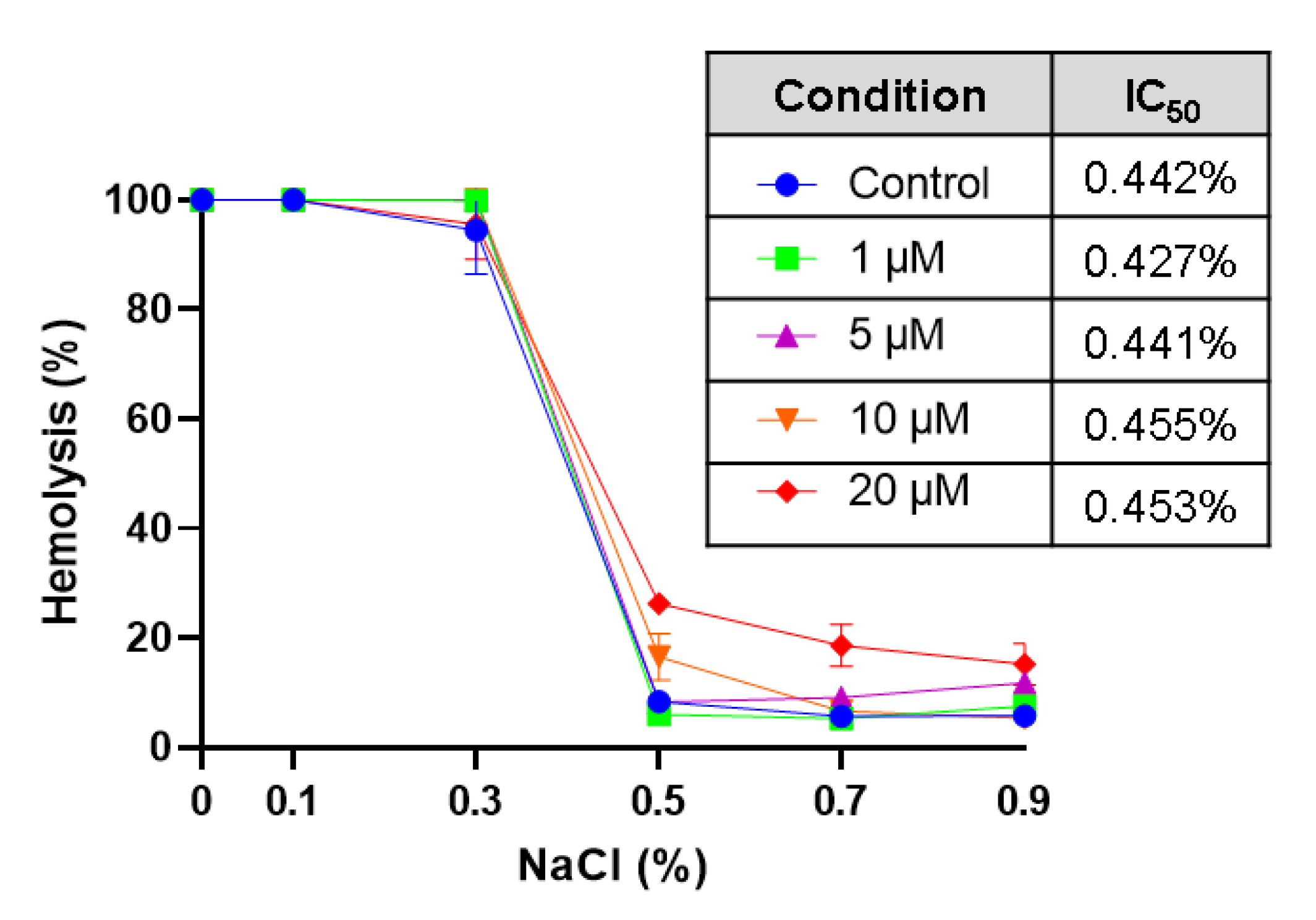
2.7. Osmotic Fragility
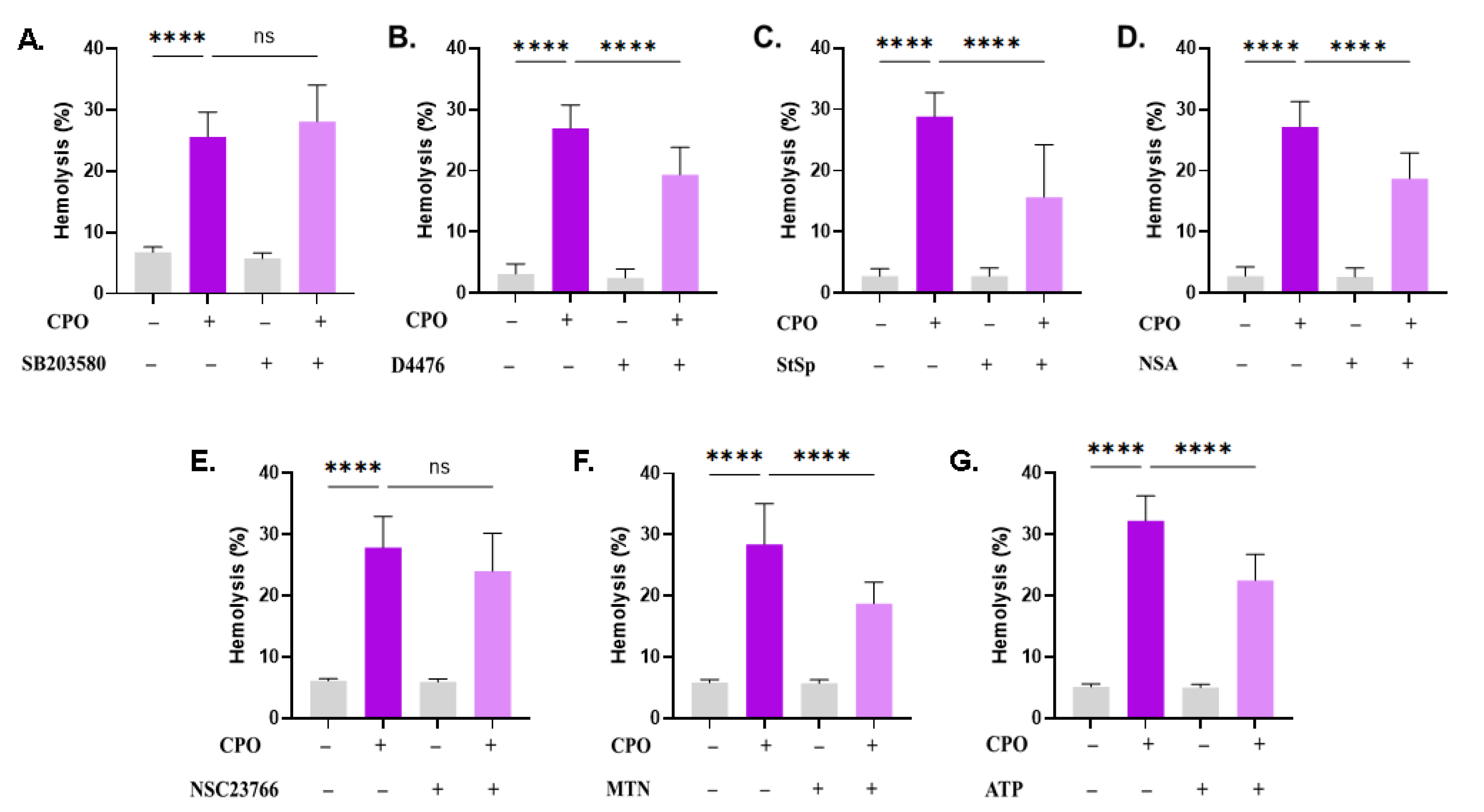
2.8. Cell Signaling Analysis
2.9. Membrane Scrambling
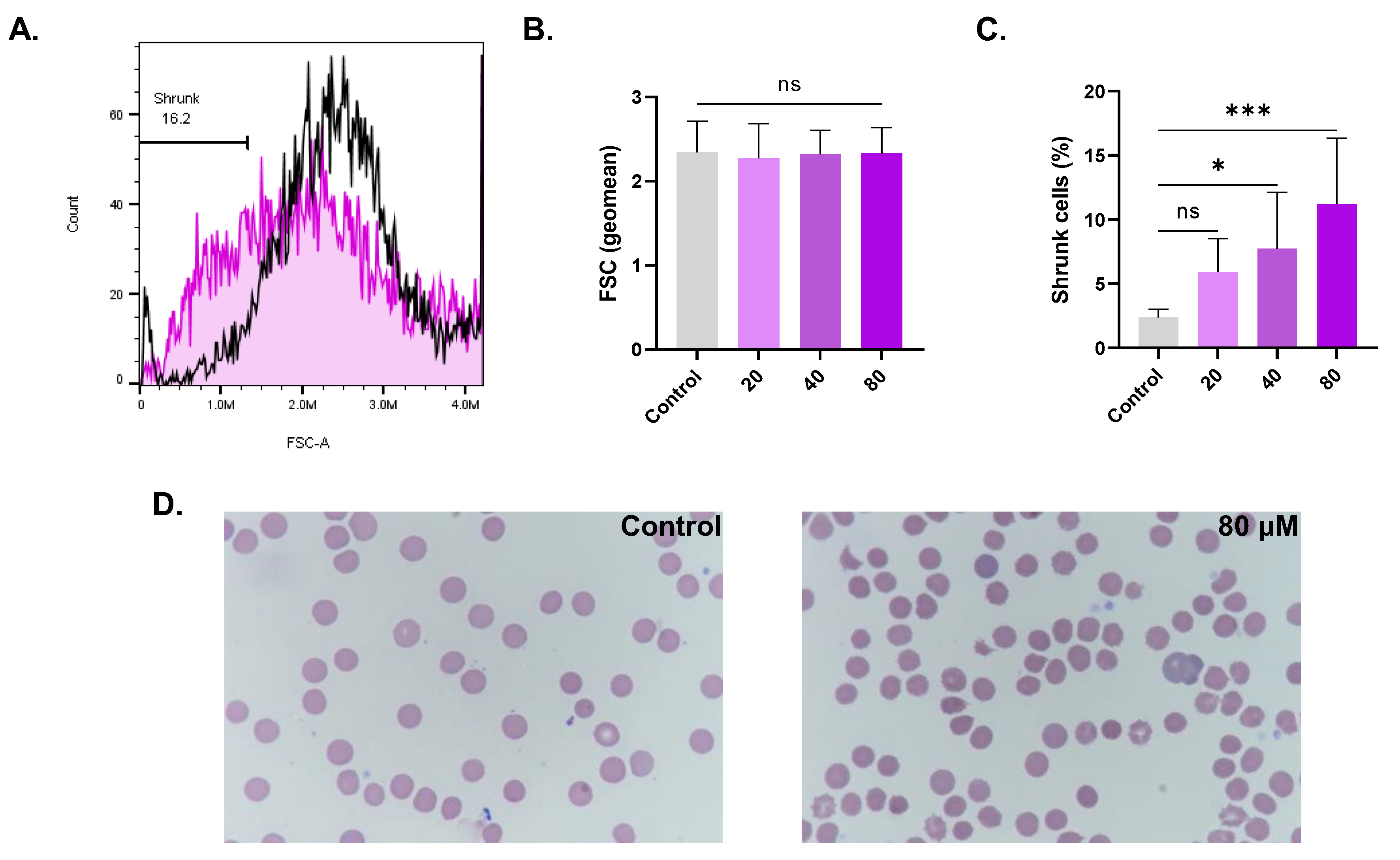
2.10. Cellular Morphology
2.11. Erythrocyte Sedimentation Rate (ESR)
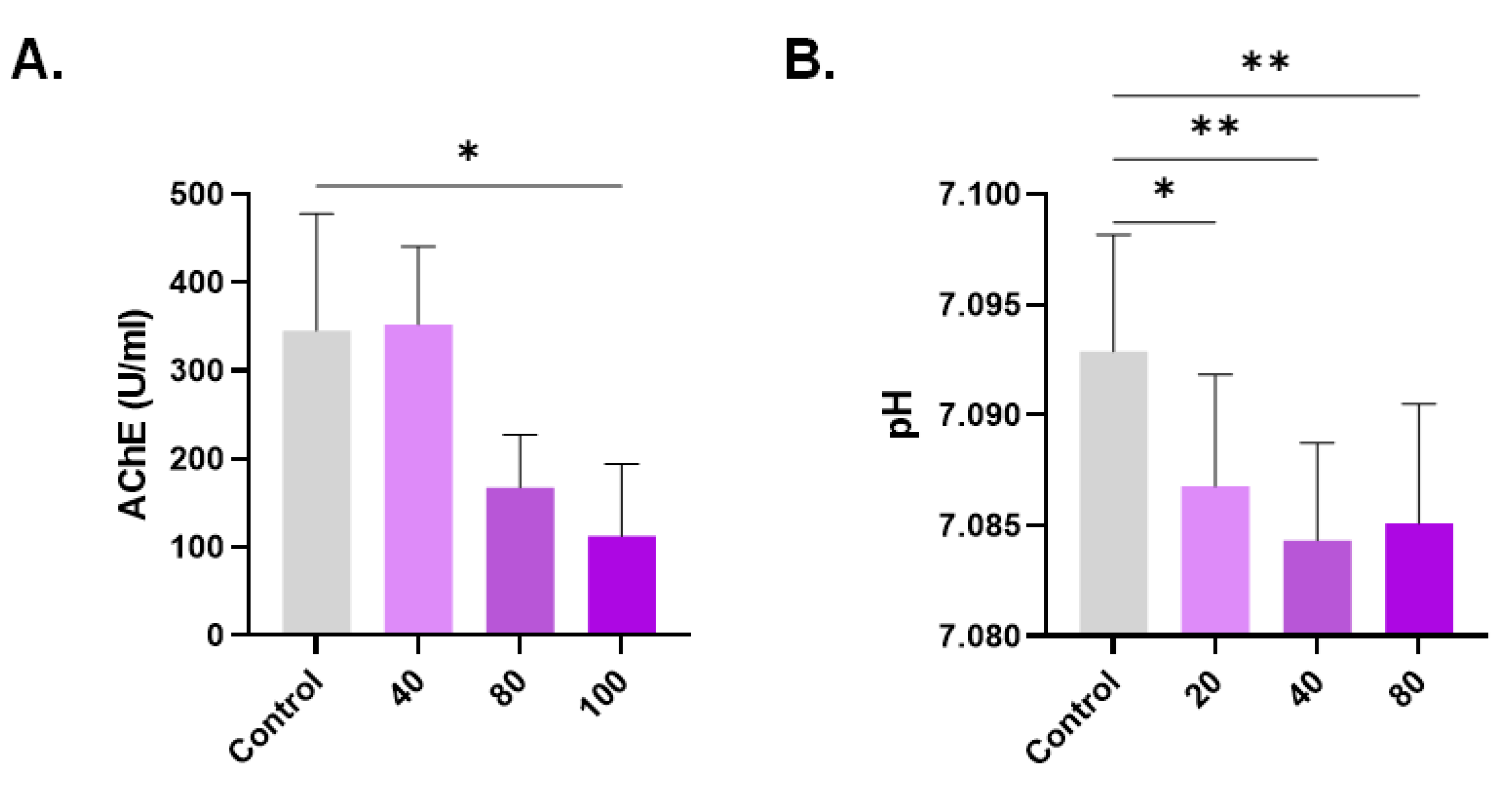
2.12. Acetylcholine Esterase (AChE) Activity
2.13. Extracellular Acidity
2.14. Intracellular Ca2+
2.15. Oxidative Stress
2.16. Systemic Toxicity
2.17. Statistical Analysis
3. Results
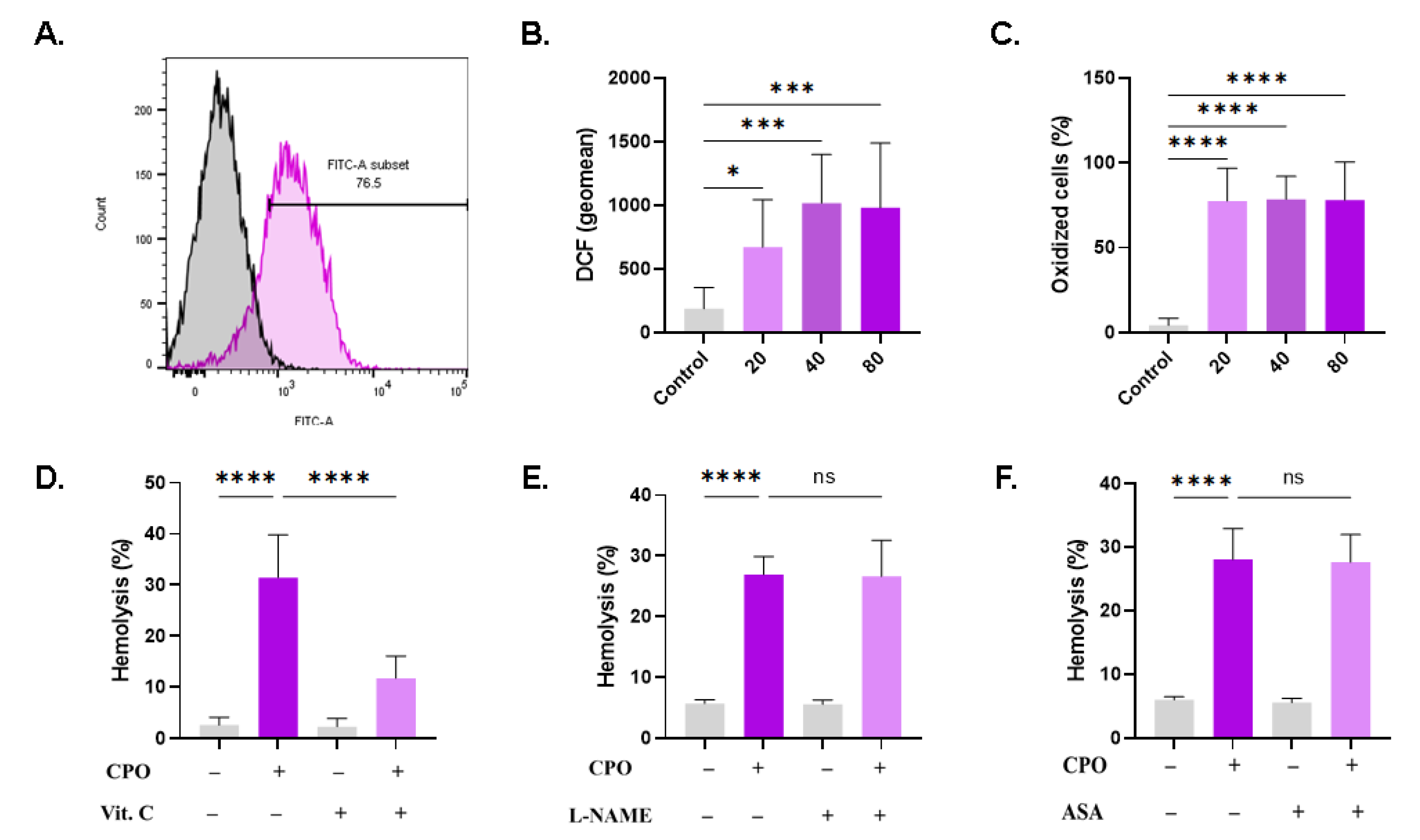
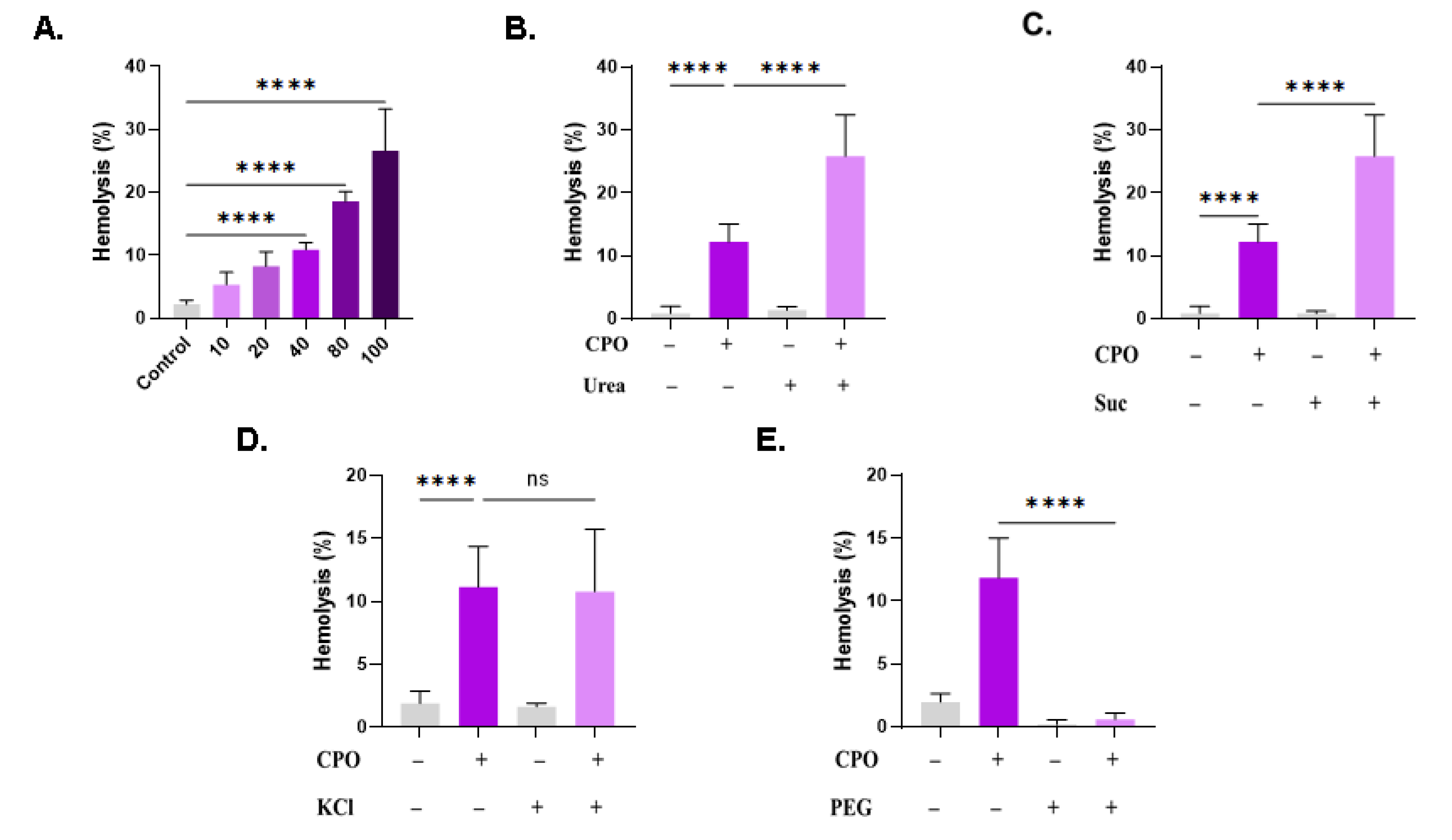
3.1. CPO Induces Concentration-Dependent Hemolysis
3.2. CPO Does Not Protect against Hypotonic Hemolysis
3.3. CPO-Induced Hemolysis Is Mediated through Multiple Signaling Pathways
3.4. CPO Causes Cell Shrinkage and Disrupted Morphology
3.5. CPO Triggers PS Externalization
3.6. Diminished AChE Activity and pH in Response to CPO
3.7. CPO Raises Cytosolic Ca2+
3.8. CPO Elicits Oxidative Stress
3.9. Effect of Modified Ringer Solutions on CPO Toxicity
3.10. CPO Causes Distinct Alterations in Whole Blood
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. beta-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, S.K.; Kim, K.D.; Nam, D.; Chung, W.S.; Jang, H.J.; Lee, S.G.; Shim, B.S.; Sethi, G.; Ahn, K.S. beta-Caryophyllene oxide potentiates TNFalpha-induced apoptosis and inhibits invasion through down-modulation of NF-kappaB-regulated gene products. Apoptosis 2014, 19, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef] [PubMed]
- Shabana, S.M.; Gad, N.S.; Othman, A.I.; Mohamed, A.F.; El-Missiry, M.A. beta-caryophyllene oxide induces apoptosis and inhibits proliferation of A549 lung cancer cells. Med. Oncol. 2023, 40, 189. [Google Scholar] [CrossRef]
- Visweshwar, N.; Jaglal, M.; Sokol, L.; Zuckerman, K. Chemotherapy-related anemia. Ann. Hematol. 2018, 97, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Bissinger, R.; Qadri, S.M.; Lang, F. Suicidal death of erythrocytes in cancer and its chemotherapy: A potential target in the treatment of tumor-associated anemia. Int. J. Cancer 2017, 141, 1522–1528. [Google Scholar] [CrossRef]
- Alghareeb, S.A.; Alfhili, M.A.; Fatima, S. Molecular Mechanisms and Pathophysiological Significance of Eryptosis. Int. J. Mol. Sci. 2023, 24, 5079. [Google Scholar] [CrossRef]
- Repsold, L.; Joubert, A.M. Eryptosis: An Erythrocyte’s Suicidal Type of Cell Death. BioMed Res. Int. 2018, 2018, 9405617. [Google Scholar] [CrossRef]
- Lang, F.; Lang, E.; Foller, M. Physiology and pathophysiology of eryptosis. Transfus. Med. Hemother. 2012, 39, 308–314. [Google Scholar] [CrossRef]
- Pretorius, E.; du Plooy, J.N.; Bester, J. A Comprehensive Review on Eryptosis. Cell. Physiol. Biochem. 2016, 39, 1977–2000. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.A.; Huober, J.; Bachmann, C.; Kempe, D.S.; Sobiesiak, M.; Akel, A.; Niemoeller, O.M.; Dreischer, P.; Eisele, K.; Klarl, B.A.; et al. Stimulation of erythrocyte phosphatidylserine exposure by paclitaxel. Cell. Physiol. Biochem. 2006, 18, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, H.; Foller, M.; Lang, F. Suicidal erythrocyte death triggered by cisplatin. Toxicology 2008, 249, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.; Tufarelli, V. In vitro antioxidant activities of resveratrol, cinnamaldehyde and their synergistic effect against cyadox-induced cytotoxicity in rabbit erythrocytes. Drug Chem. Toxicol. 2017, 40, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Behling-Kelly, E. Identifying Erythrocyte Injury in Toxicology Studies. Toxicol. Pathol. 2022, 50, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Lee, M.H. Flow Cytofluorometric Analysis of Molecular Mechanisms of Premature Red Blood Cell Death. Methods Mol. Biol. 2021, 2326, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Saebo, I.P.; Bjoras, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Alghareeb, S.A.; Alsughayyir, J.; Alfhili, M.A. Stimulation of Hemolysis and Eryptosis by α-Mangostin through Rac1 GTPase and Oxidative Injury in Human Red Blood Cells. Molecules 2023, 28, 6495. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Li, J.; Zhang, R.; Yu, C.; Zhou, Z.; Liu, J.; Liao, S.; Klionsky, D.J.; Kroemer, G.; et al. Identification of HPCAL1 as a specific autophagy receptor involved in ferroptosis. Autophagy 2023, 19, 54–74. [Google Scholar] [CrossRef]
- Huang, X.-J.; Choi, Y.-K.; Im, H.-S.; Yarimaga, O.; Yoon, E.; Kim, H.-S. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Cheung, A.K.; Yang, A.K.; Ngai, B.H.; Yu, S.S.; Gao, M.; Lau, P.M.; Kong, S.K. Quantitative detection of eryptosis in human erythrocytes using tunable resistive pulse sensing and annexin-V-beads. Analyst 2015, 140, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Mischitelli, M.; Jemaa, M.; Almasry, M.; Faggio, C.; Lang, F. Stimulation of Suicidal Erythrocyte Death by Rottlerin. Cell. Physiol. Biochem. 2016, 40, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Pai, S.H. Influences of hypercholesterolemia on red cell indices and erythrocyte sedimentation rate in elderly persons. Clin. Chim. Acta 2004, 341, 117–121. [Google Scholar] [CrossRef]
- Pohanka, M.; Hrabinova, M.; Kuca, K.; Simonato, J.P. Assessment of acetylcholinesterase activity using indoxylacetate and comparison with the standard Ellman’s method. Int. J. Mol. Sci. 2011, 12, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Alyousef, A.M.; Alsughayyir, J. Tamoxifen induces eryptosis through calcium accumulation and oxidative stress. Med. Oncol. 2023, 40, 333. [Google Scholar] [CrossRef]
- Alzoubi, K.; Alktifan, B.; Oswald, G.; Fezai, M.; Abed, M.; Lang, F. Breakdown of phosphatidylserine asymmetry following treatment of erythrocytes with lumefantrine. Toxins 2014, 6, 650–664. [Google Scholar] [CrossRef]
- Yeung, K.W.; Lau, P.M.; Tsang, H.L.; Ho, H.P.; Kwan, Y.W.; Kong, S.K. Extracellular Histones Induced Eryptotic Death in Human Erythrocytes. Cell. Physiol. Biochem. 2019, 53, 229–241. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Alsughayyir, J. Metabolic exhaustion and casein kinase 1alpha drive deguelin-induced premature red blood cell death. Xenobiotica 2023, 53, 445–453. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strzadala, L.; Szumny, A. beta-caryophyllene and beta-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Lang, F.; Qadri, S.M. Mechanisms and significance of eryptosis, the suicidal death of erythrocytes. Blood Purif. 2012, 33, 125–130. [Google Scholar] [CrossRef]
- Ataga, K.I.; Staffa, S.J.; Brugnara, C.; Stocker, J.W. Haemoglobin response to senicapoc in patients with sickle cell disease: A re-analysis of the Phase III trial. Br. J. Haematol. 2021, 192, e129–e132. [Google Scholar] [CrossRef] [PubMed]
- Rapetti-Mauss, R.; Soriani, O.; Vinti, H.; Badens, C.; Guizouarn, H. Senicapoc: A potent candidate for the treatment of a subset of hereditary xerocytosis caused by mutations in the Gardos channel. Haematologica 2016, 101, e431–e435. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, L.J.; Sapkota, G.P. Functions and regulation of the serine/threonine protein kinase CK1 family: Moving beyond promiscuity. Biochem. J. 2020, 477, 4603–4621. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.; Onishchenko, A. Casein kinase 1alpha mediates eryptosis: A review. Apoptosis 2023, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Janovska, P.; Normant, E.; Miskin, H.; Bryja, V. Targeting Casein Kinase 1 (CK1) in Hematological Cancers. Int. J. Mol. Sci. 2020, 21, 9026. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, A.; Persson, J.L. Induction of apoptosis by staurosporine involves the inhibition of expression of the major cell cycle proteins at the G(2)/m checkpoint accompanied by alterations in Erk and Akt kinase activities. Anticancer. Res. 2009, 29, 2893–2898. [Google Scholar] [PubMed]
- Akinaga, S.; Nomura, K.; Gomi, K.; Okabe, M. Effect of UCN-01, a selective inhibitor of protein kinase C, on the cell-cycle distribution of human epidermoid carcinoma, A431 cells. Cancer Chemother. Pharmacol. 1994, 33, 273–280. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, Q.; Huang, Q.; Cheng, J. Targeting Protein Kinase C for Cancer Therapy. Cancers 2022, 14, 1104. [Google Scholar] [CrossRef]
- Ma, D.; Wang, P.; Fang, Q.; Yu, Z.; Zhou, Z.; He, Z.; Wei, D.; Yu, K.; Lu, T.; Zhang, Y.; et al. Low-dose staurosporine selectively reverses BCR-ABL-independent IM resistance through PKC-alpha-mediated G2/M phase arrest in chronic myeloid leukaemia. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S208–S216. [Google Scholar] [CrossRef]
- McMahon, T.J. Red Blood Cell Deformability, Vasoactive Mediators, and Adhesion. Front. Physiol. 2019, 10, 1417. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Stivison, E.A.; Hod, E.A.; Spitalnik, S.L.; Cowan, P.J.; Randis, T.M.; Ratner, A.J. Human-specific bacterial pore-forming toxins induce programmed necrosis in erythrocytes. mBio 2014, 5, e01251-14. [Google Scholar] [CrossRef]
- McCaig, W.D.; Hodges, A.L.; Deragon, M.A.; Haluska, R.J., Jr.; Bandyopadhyay, S.; Ratner, A.J.; Spitalnik, S.L.; Hod, E.A.; LaRocca, T.J. Storage Primes Erythrocytes for Necroptosis and Clearance. Cell. Physiol. Biochem. 2019, 53, 496–507. [Google Scholar] [CrossRef]
- Johnston, A.; Wang, Z. Necroptosis: MLKL Polymerization. J. Nat. Sci. 2018, 4, e513. [Google Scholar]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. MLKL: Functions beyond serving as the Executioner of Necroptosis. Theranostics 2021, 11, 4759–4769. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Guo, D.; Wang, F.C.; Ding, W.Y. Necrosulfonamide (NSA) protects intervertebral disc degeneration via necroptosis and apoptosis inhibition. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2683–2691. [Google Scholar] [CrossRef]
- Yang, W.; Tao, K.; Wang, Y.; Huang, Y.; Duan, C.; Wang, T.; Li, C.; Zhang, P.; Yin, Y.; Gao, J.; et al. Necrosulfonamide ameliorates intestinal inflammation via inhibiting GSDMD-medicated pyroptosis and MLKL-mediated necroptosis. Biochem. Pharmacol. 2022, 206, 115338. [Google Scholar] [CrossRef]
- Ueda, S.; Chen-Yoshikawa, T.F.; Tanaka, S.; Yamada, Y.; Nakajima, D.; Ohsumi, A.; Date, H. Protective effect of necrosulfonamide on rat pulmonary ischemia-reperfusion injury via inhibition of necroptosis. J. Thorac. Cardiovasc. Surg. 2022, 163, e113–e122. [Google Scholar] [CrossRef]
- Pretorius, E. Erythrocyte deformability and eryptosis during inflammation, and impaired blood rheology. Clin. Hemorheol. Microcirc. 2018, 69, 545–550. [Google Scholar] [CrossRef]
- Saldanha, C. Human Erythrocyte Acetylcholinesterase in Health and Disease. Molecules 2017, 22, 1499. [Google Scholar] [CrossRef]
- Gupta, S.; Belle, V.S.; Kumbarakeri Rajashekhar, R.; Jogi, S.; Prabhu, R.K. Correlation of Red Blood Cell Acetylcholinesterase Enzyme Activity with Various RBC Indices. Indian J. Clin. Biochem. 2018, 33, 445–449. [Google Scholar] [CrossRef]
- Baek, M.W.; Cho, H.S.; Kim, S.H.; Kim, W.J.; Jung, J.Y. Ascorbic Acid Induces Necrosis in Human Laryngeal Squamous Cell Carcinoma via ROS, PKC, and Calcium Signaling. J. Cell. Physiol. 2017, 232, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; D’Arpa, D.; Conti, S.; Giaccone, V.; Pintaudi, A.M.; Livrea, M.A. Melatonin protects human red blood cells from oxidative hemolysis: New insights into the radical-scavenging activity. J. Pineal Res. 1999, 27, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Allegra, M.; D’Arpa, D.; Butera, D.; Livrea, M.A. Reaction of melatonin with hemoglobin-derived oxoferryl radicals and inhibition of the hydroperoxide-induced hemoglobin denaturation in red blood cells. J. Pineal Res. 2001, 31, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Lang, K.S.; Myssina, S.; Lang, P.A.; Tanneur, V.; Kempe, D.S.; Mack, A.F.; Huber, S.M.; Wieder, T.; Lang, F.; Duranton, C. Inhibition of erythrocyte phosphatidylserine exposure by urea and Cl. Am. J. Physiol. Renal Physiol. 2004, 286, F1046–F1053. [Google Scholar] [CrossRef]
- Virzi, G.M.; Mattiotti, M.; Clementi, A.; Milan Manani, S.; Battaglia, G.G.; Ronco, C.; Zanella, M. In Vitro Induction of Eryptosis by Uremic Toxins and Inflammation Mediators in Healthy Red Blood Cells. J. Clin. Med. 2022, 11, 5329. [Google Scholar] [CrossRef]
- Oscarson, D.W.; Van Scoyoc, G.E.; Ahlrichs, J.L. Effect of poly-2-vinylpyridine-N-oxide and sucrose on silicate-induced hemolysis of erythrocytes. J. Pharm. Sci. 1981, 70, 657–659. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghareeb, S.A.; Alfhili, M.A.; Alsughayyir, J. Stimulation of Hemolysis and Eryptosis by β-Caryophyllene Oxide. Life 2023, 13, 2299. https://doi.org/10.3390/life13122299
Alghareeb SA, Alfhili MA, Alsughayyir J. Stimulation of Hemolysis and Eryptosis by β-Caryophyllene Oxide. Life. 2023; 13(12):2299. https://doi.org/10.3390/life13122299
Chicago/Turabian StyleAlghareeb, Sumiah A., Mohammad A. Alfhili, and Jawaher Alsughayyir. 2023. "Stimulation of Hemolysis and Eryptosis by β-Caryophyllene Oxide" Life 13, no. 12: 2299. https://doi.org/10.3390/life13122299







