Removal of Potassium and Iron in Low Grade Bauxite by a Calcination-Acid Leaching Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Analyzing and Characterizing Methods
3. Results and Discussion
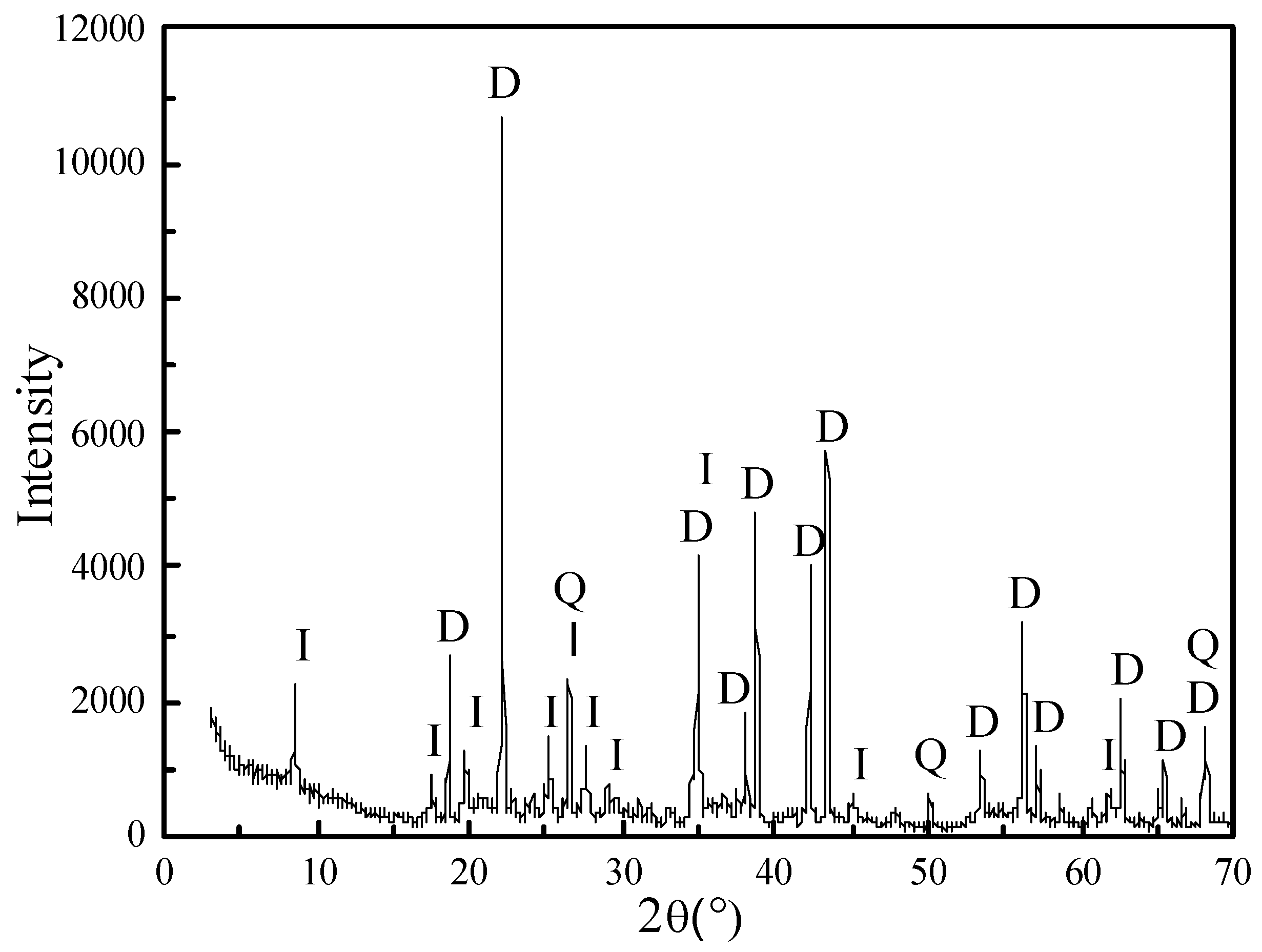
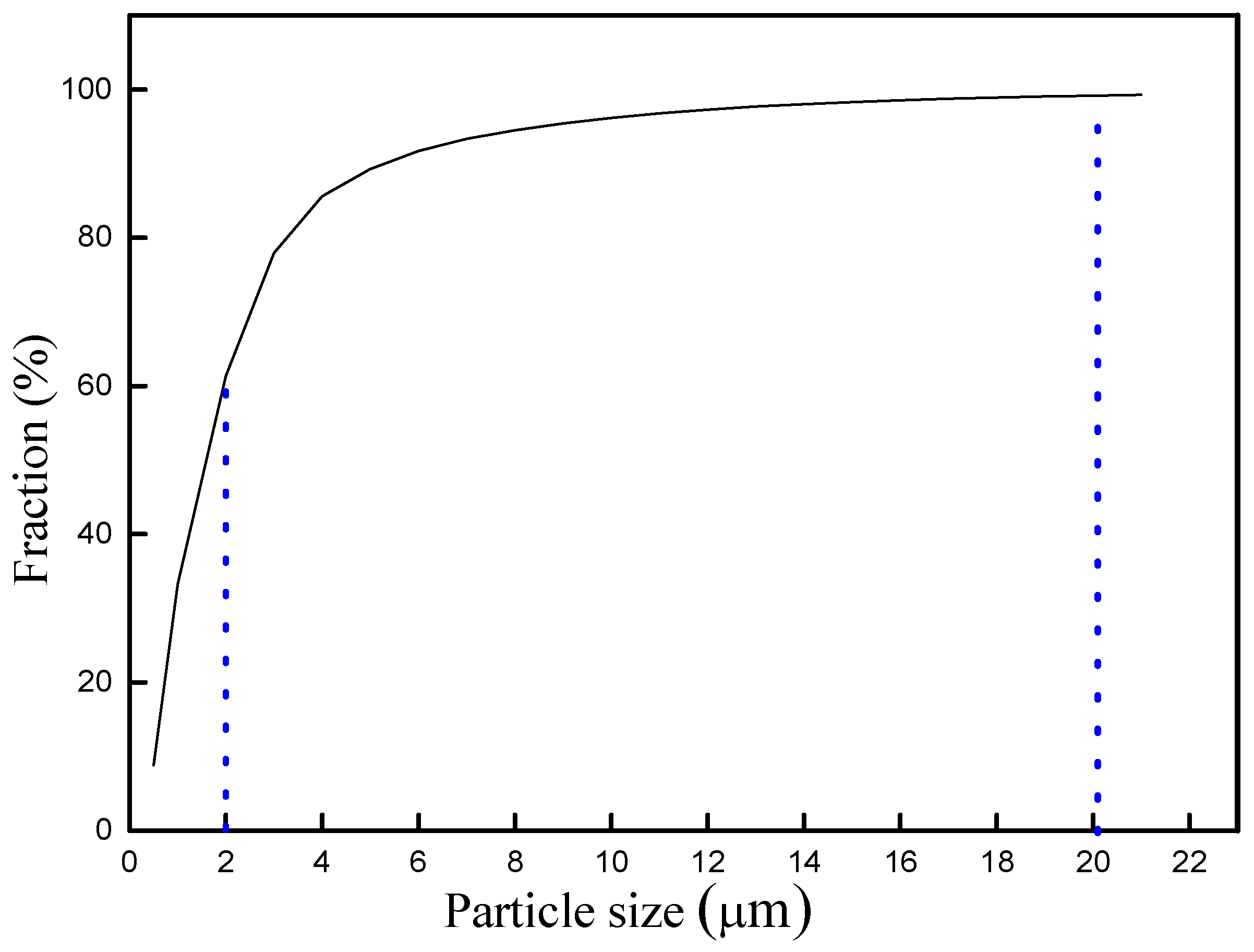
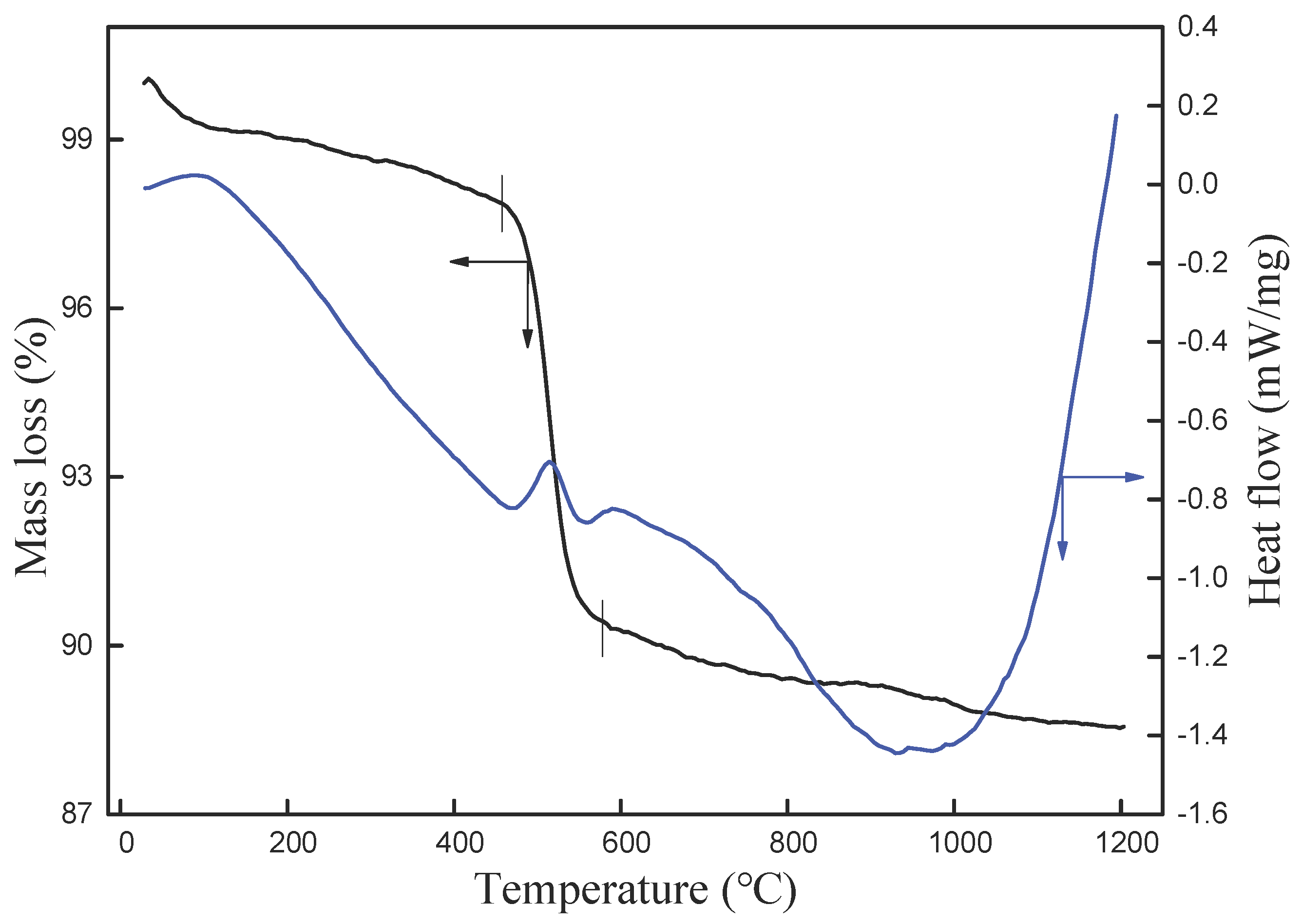
3.1. Analysis of Raw Material
3.2. Reaction Mechanism
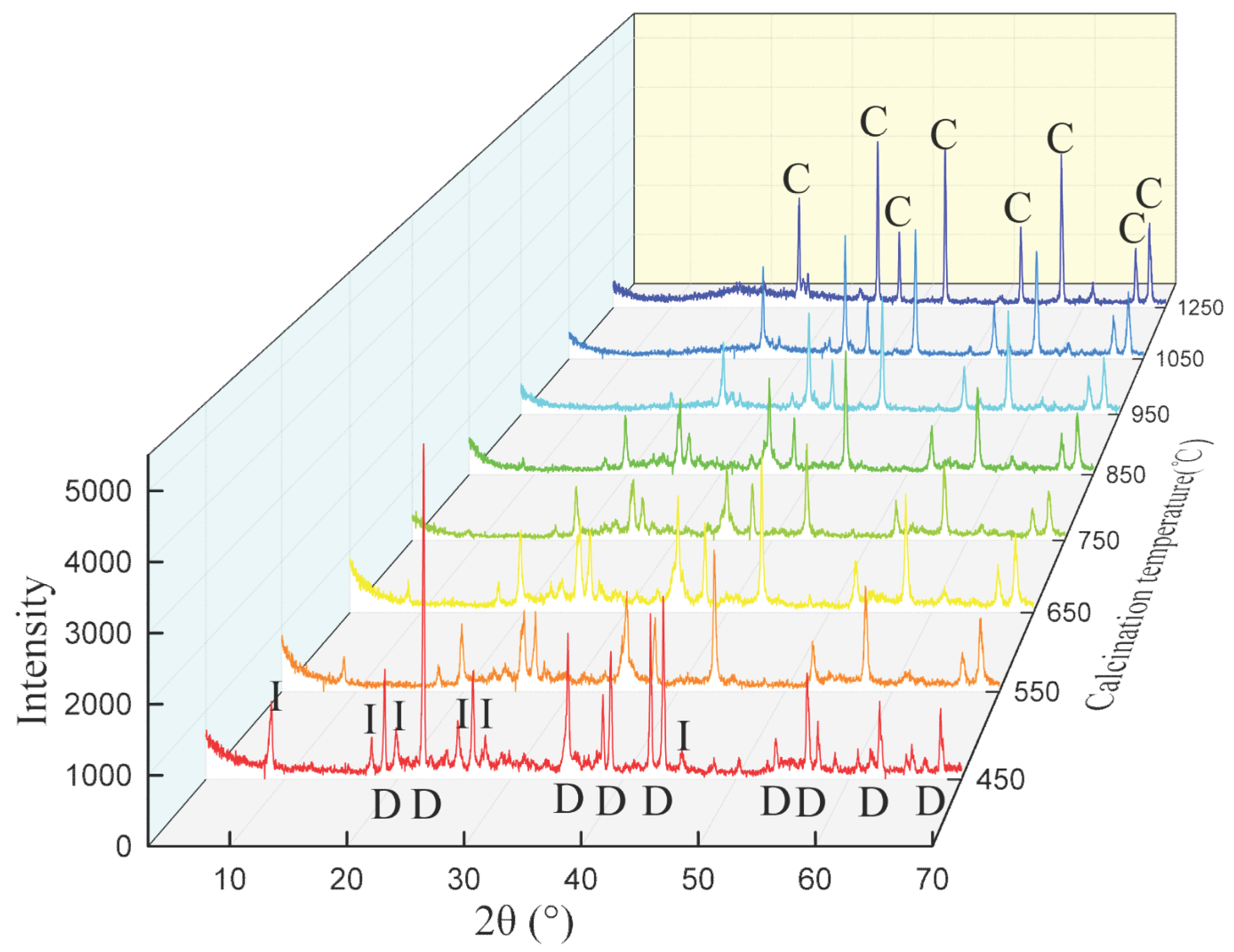
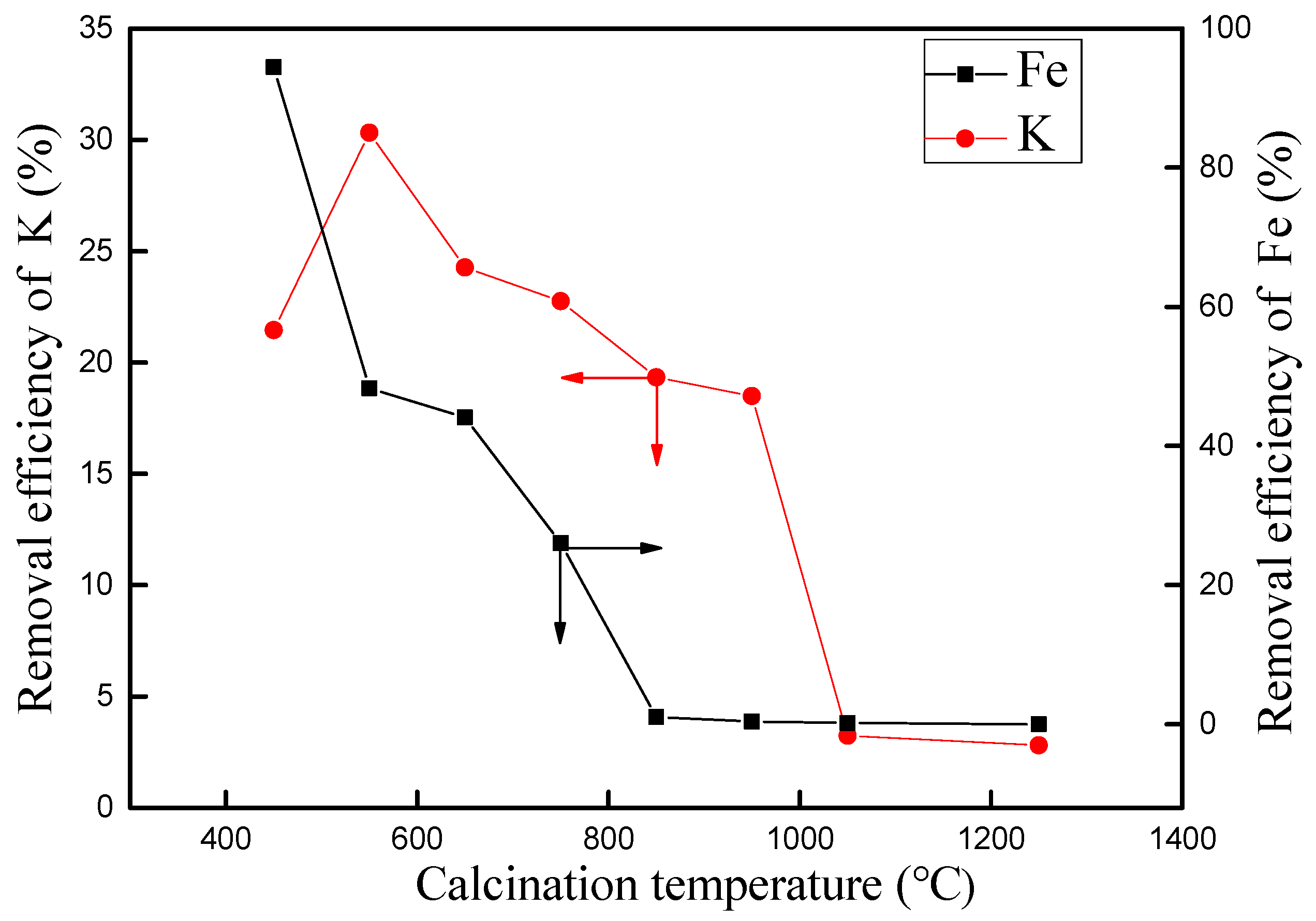
3.3. Effect of Calcination Temperature
3.4. Leaching Experiment Parameters
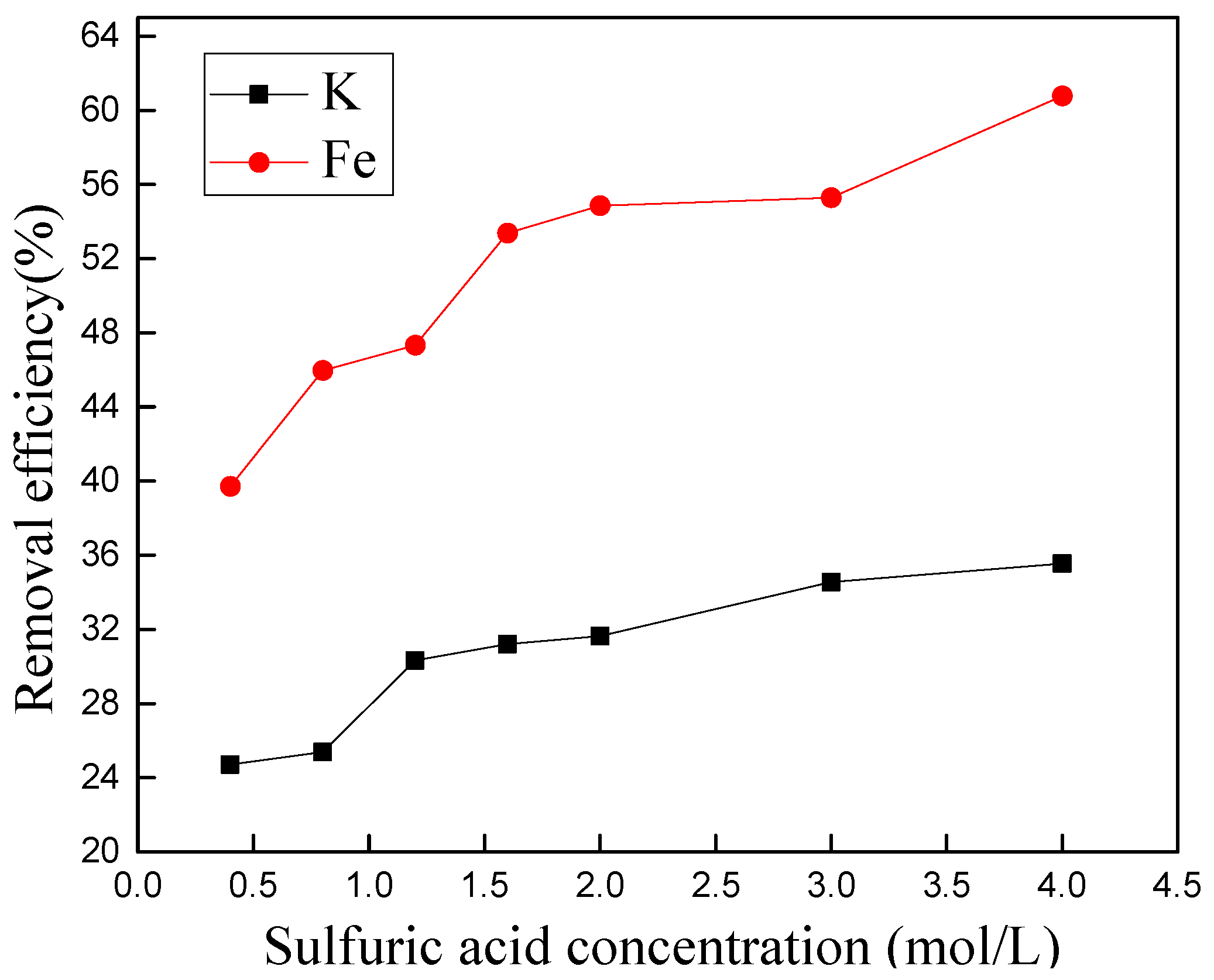
3.4.1. Effect of Sulfuric Acid Concentration
3.4.2. Effect of Reaction Temperature
3.4.3. Effect of Sulfuric Acid/Bauxite Ratio
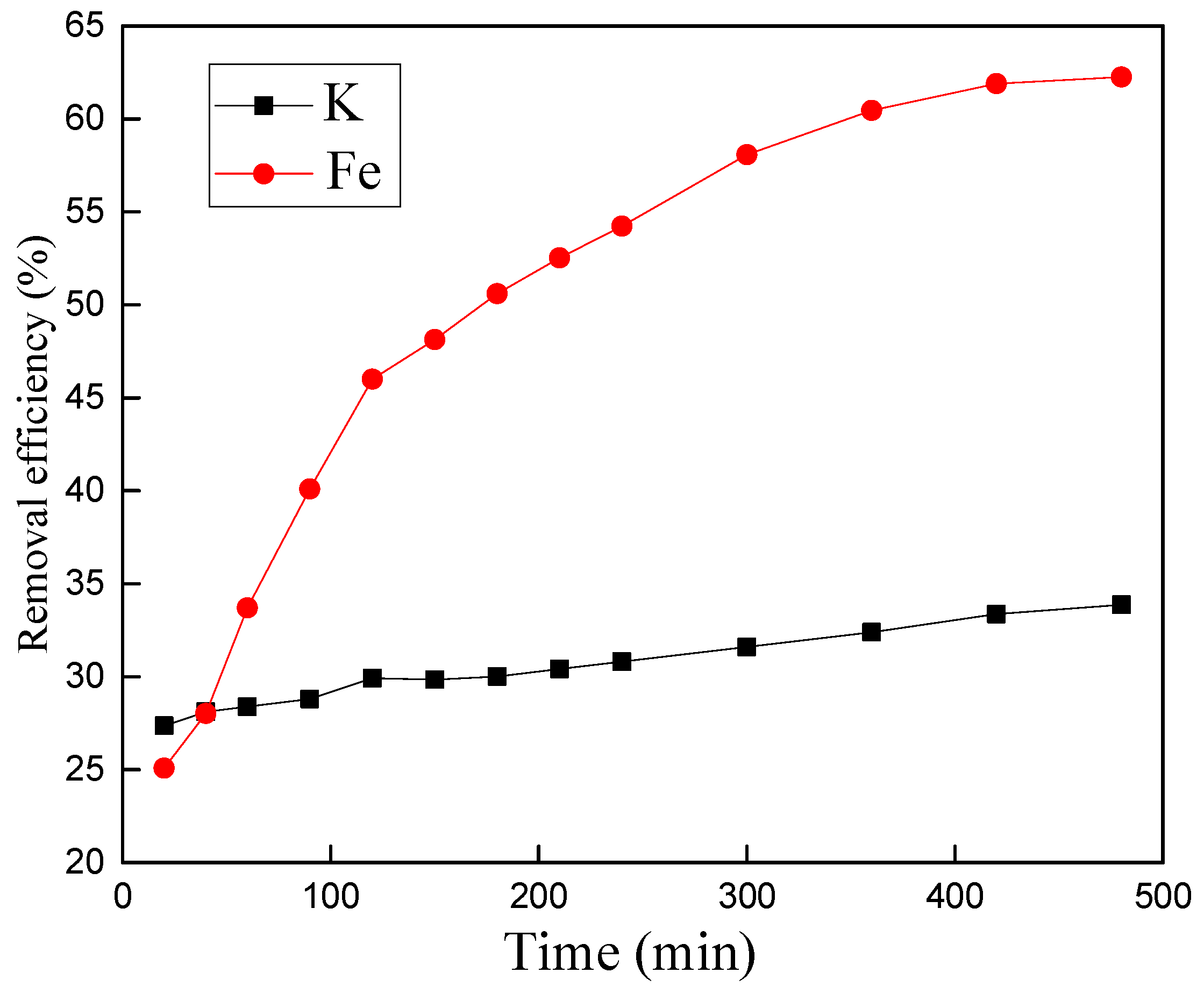
3.4.4. Effect of Reaction Time
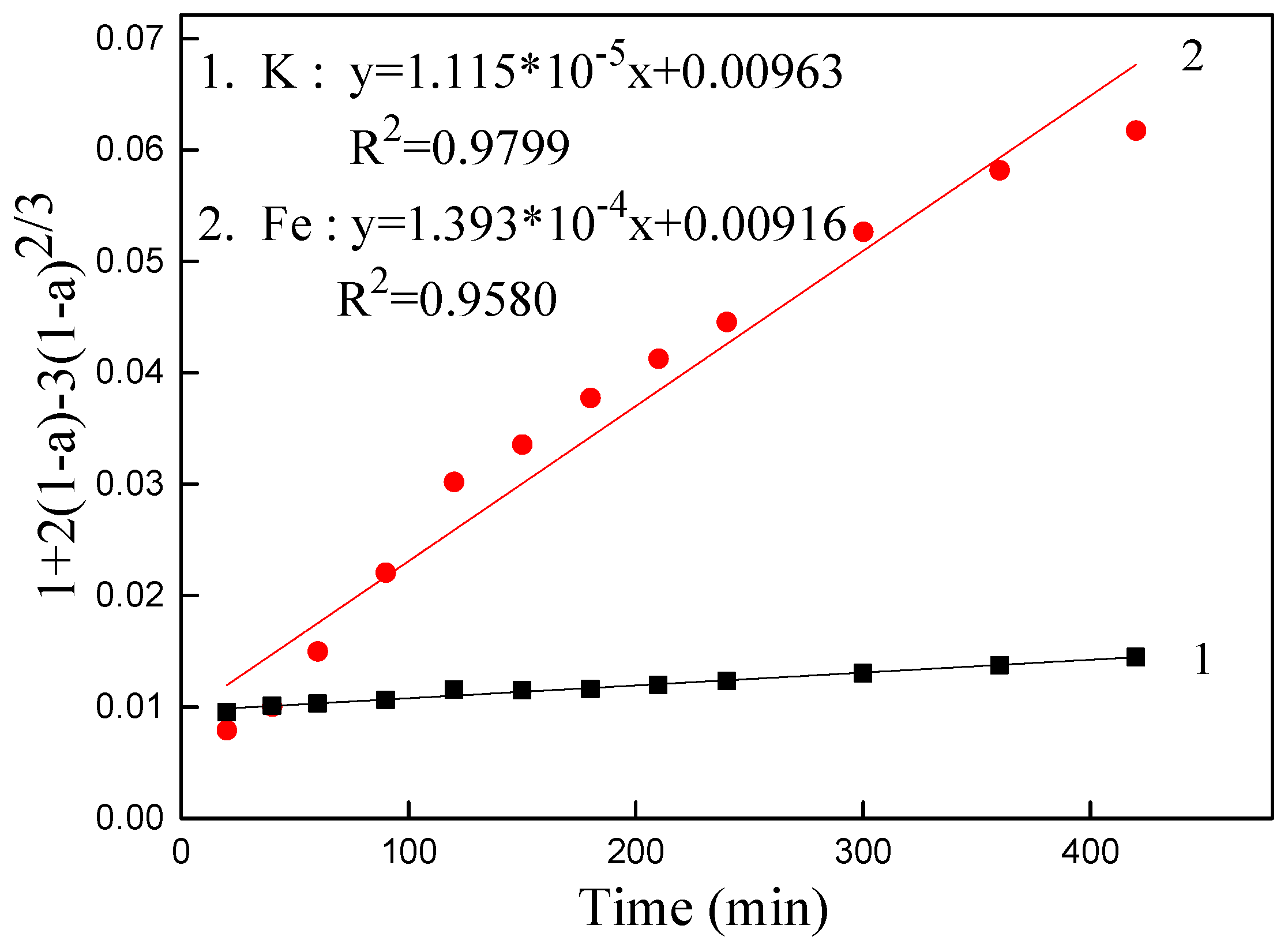
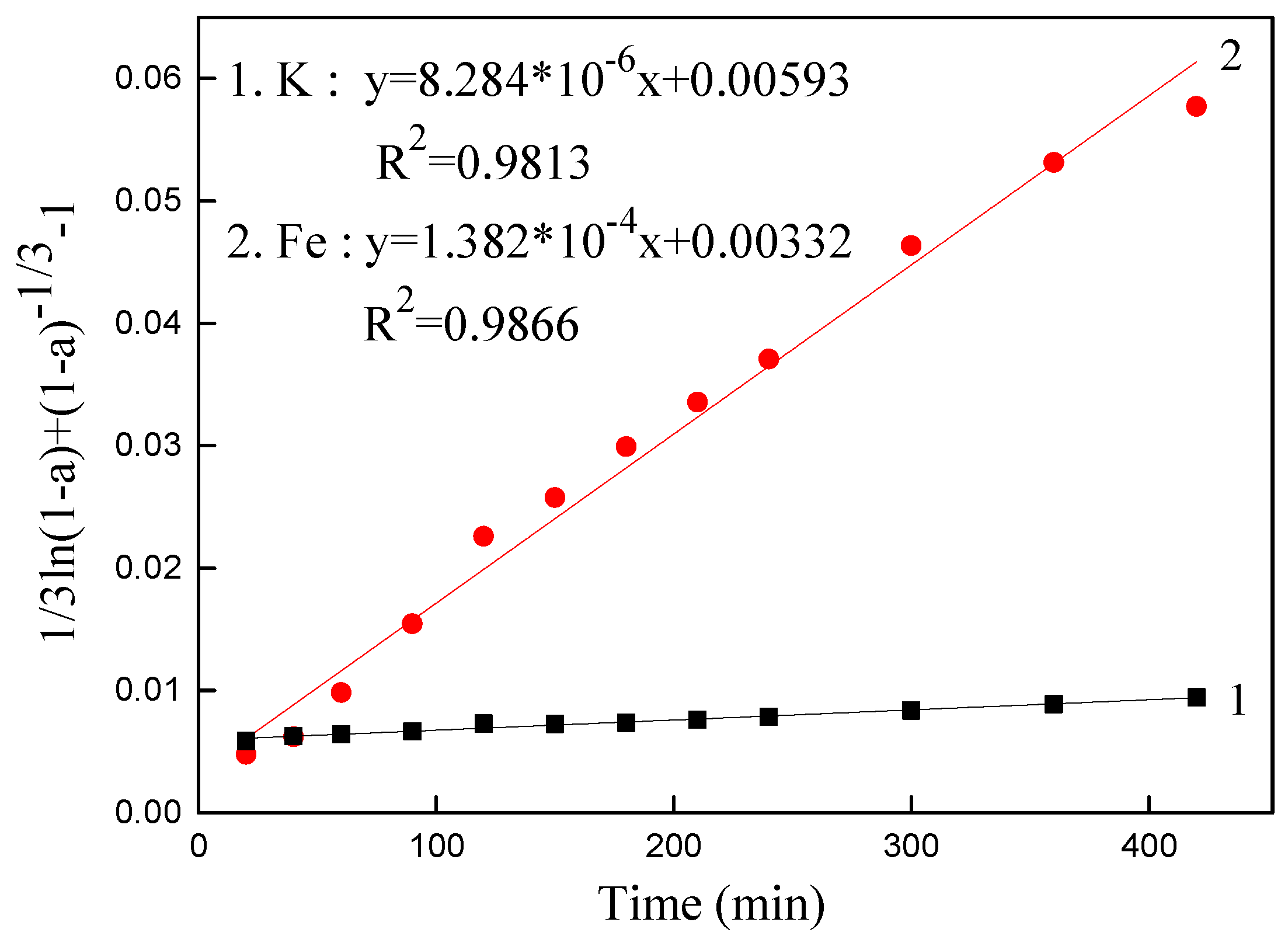
3.5. Kinetics Analysis
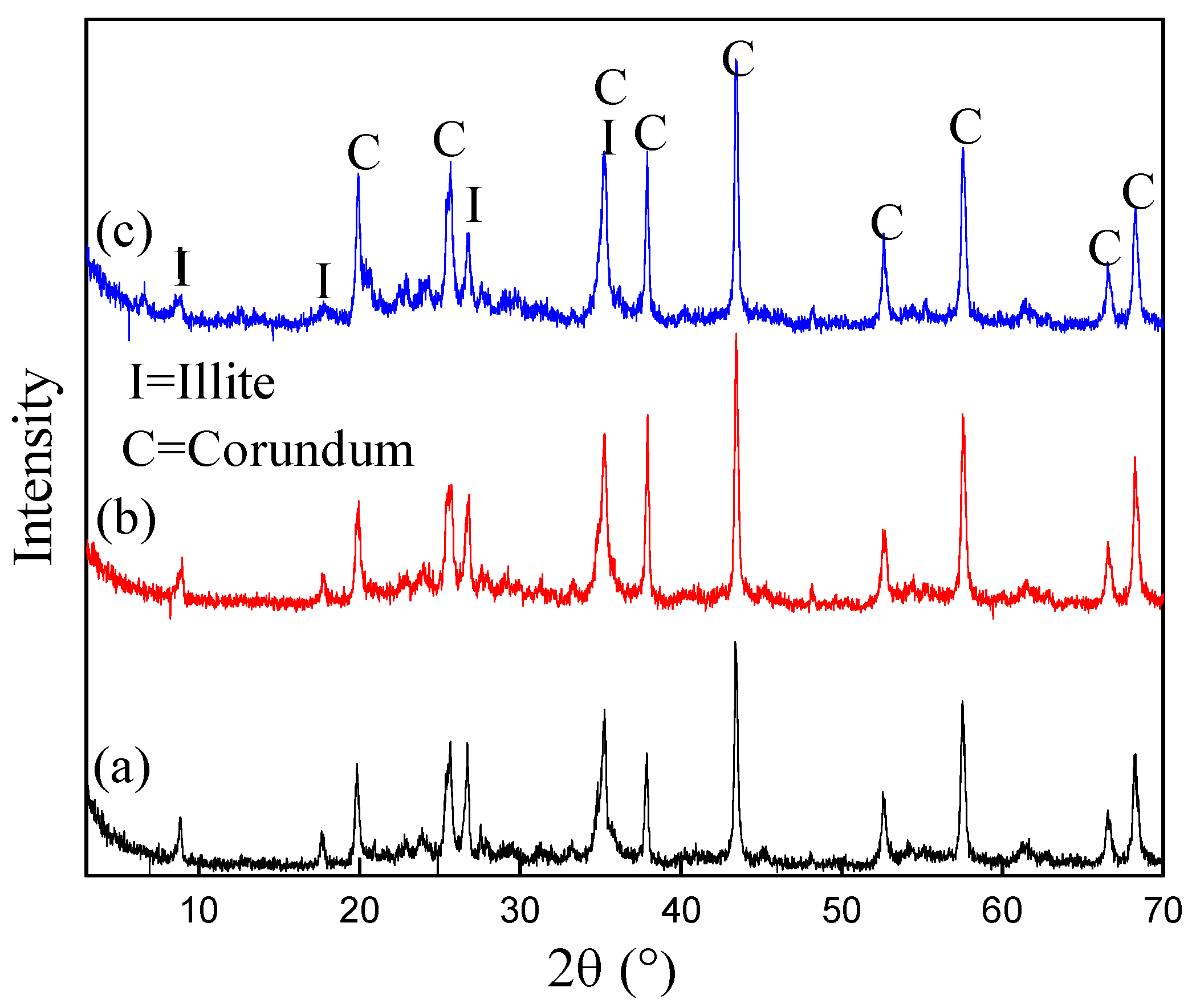
3.6. Characterization of Treated Bauxite
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paz, S.P.A.; Angélica, R.S.; Kahn, H. Optimization of the reactive silica quantification method applied to Paragominas-type gibbsitic bauxites. Int. J. Miner. Process. 2017, 162, 10–15. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Guo, S.; Zhou, F. The influence of bauxite resource change in China on layout of world alumina industry. Light Met. 2014, 26, 1–5. [Google Scholar]
- Fan, Z.L.; Ma, Z.H. Countermeasures and safety evaluation on the use of foreign bauxite resources. China Min. Mag. 2010, 19, 13–15. [Google Scholar]
- Zhong, H.; Liu, G.Y.; Xia, L.Y.; Lu, Y.P.; Hu, Y.H.; Zhao, S.G.; Yu, X.Y. Flotation separation of diaspore from kaolinite, pyrophyllite and illite using three cationic collectors. Miner. Eng. 2008, 21, 1055–1061. [Google Scholar]
- Pan, S.X.; Wang, R.; Zhang, Z.Q.; Chu, G.S.; Wang, T.Z. Export and import situation of refractories and refractory raw materials in China. China Refract. 2000, 3, 11–15. [Google Scholar]
- Shi, G. Discussion on current situation of China’s refractories resources and development. Naihuo Cailiao 2007, 41, 63–67. [Google Scholar]
- Fang, C.J.; Chang, Z.Y.; Feng, Q.M.; Xiao, W.; Yu, S.C.; Qiu, G.Z.; Wang, J. The Influence of Backwater Al3+ on Diaspore Bauxite Flotation. Minerals 2017, 7, 195. [Google Scholar]
- Li, X.B.; Liu, X.M.; Liu, G.H.; Peng, Z.H.; Liu, Y.X. Study and application of intensified sintering process for alumina production. Chin. J. Nonferr. Met. 2004, 14, 1031–1036. [Google Scholar]
- Lü, G.Z.; Zhang, T.A.; Li, B.; Dou, Z.H.; Zhang, W.G. Roasting Pretreatment of High-sulfur Bauxite. Chin. J. Process. Eng. 2008, 8, 892–896. [Google Scholar]
- Zhao, Q.; Miller, J.D.; Wang, X.M. Recent Developments in the Beneficiation of Chinese Bauxite. Miner. Process. Extr. Metall. Rev. 2010, 31, 111–119. [Google Scholar]
- Zhong, X.C. Strategic thoughts on innovative development of Chinese bauxites. Naihuo Cailiao 2009, 43, 241–243. [Google Scholar]
- Amrane, B.; Ouedraogo, E.; Mamen, B.; Djaknoun, S.; Mesrati, N. Experimental study of the thermo-mechanical behaviour of alumina-silicate refractory materials based on a mixture of Algerian kaolinitic clays. Ceram. Int. 2011, 37, 3217–3227. [Google Scholar] [CrossRef]
- Cheng, H. Influence of K2O on Sintering-Synthesizing Mullite with Bauxite. Chin. Ceram. 2013, 49, 48–50. [Google Scholar]
- Sadik, C.; Amrani, I.E.; Albizane, A. Recent advances in silica-alumina refractory: A review. J. Asian Ceram. Soc. 2014, 2, 83–96. [Google Scholar] [CrossRef]
- Stjernberg, J.; Ion, J.C.; Antti, M.L.; Nordin, L.O.; Lindblom, B.; Odén, M. Extended studies of degradation mechanisms in the refractory lining of a rotary kiln for iron ore pellet production. J. Eur. Ceram. Soc. 2012, 32, 1519–1528. [Google Scholar] [CrossRef]
- Reddy, B.R.; Mishra, S.K.; Banerjee, G.N. Kinetics of leaching of a gibbsitic bauxite with hydrochloric acid. Hydrometallurgy 1999, 51, 131–138. [Google Scholar] [CrossRef]
- Zhao, A.C.; Zhang, T.A.; Lü, G.Z.; Dou, Z.H. Study on the leaching rules of aluminum and ferrum from high iron bauxite by acid leaching at low temperature. J. Funct. Mater. 2012, 43, 105–108. [Google Scholar]
- Hu, W.T.; Wang, H.J.; Liu, X.W.; Sun, C.Y.; Duan, X.Q. Restraining Sodium Volatilization in the Ferric Bauxite Direct Reduction System. Minerals 2016, 6, 31. [Google Scholar] [CrossRef]
- Papassiopi, N.; Vaxevanidou, K.; Paspaliaris, I. Effectiveness of iron reducing bacteria for the removal of iron from bauxite ores. Miner. Eng. 2010, 23, 25–31. [Google Scholar] [CrossRef]
- Ma, D.Y. Preparation of Mullite-Based Complex Refractory from Bauxite Tailings. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Gao, Z.X.; Li, G.P. On the classification of Chinese bauxites. J. Chin. Ceram. Soc. 1984, 2, 115–122. [Google Scholar]
- Zheng, L.; Yang, Y.; Ma, H.W.; Liu, M.T.; Ma, X. Recovery of magnesium and potassium from biotite by sulfuric acid leaching and alkali precipitation with ammonia. Hydrometallurgy 2015, 157, 188–193. [Google Scholar]
- Yuan, B.; Li, C.; Liang, B.; Lü, L.; Yue, H.R.; Sheng, H.Y.; Ye, L.P.; Xie, H.P. Extraction of potassium from K-feldspar via the CaCl2 calcination route. Chin. J. Chem. Eng. 2015, 23, 1557–1564. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Ma, J.Y.; Qin, Y.H.; Zhou, J.F.; Li, Y.; Wu, Z.K.; Wang, T.L.; Wang, W.G.; Wang, C.W. Ultrasound-assisted leaching of potassium from phosphorus-potassium associated ore. Hydrometallurgy 2016, 166, 237–242. [Google Scholar] [CrossRef]
- Ma, H.W.; Yang, J.; Su, S.Q.; Liu, M.T.; Zheng, H.; Wang, Y.B.; Qi, H.B.; Zhang, P.; Yao, W.G. 20 Years Advances in Preparation of Potassium Salts from Potassic Rocks: A Review. Acta Geol. Sin-Engl. 2015, 89, 2058–2071. [Google Scholar]
- Drits, V.A.; Zviagina, B.B. Trans-vacant and cis-vacant 2:1 layer silicates: Structural features, identification, and occurrence. Clays Clay Miner. 2009, 57, 405–415. [Google Scholar] [CrossRef]
- Yang, H.M.; Yang, W.G.; Hu, Y.H.; Qiu, G.Z. Kinetics analysis of thermal decomposition reaction of diaspore. Chin. J. Nonferr. Met. 2003, 55, 9750–9757. [Google Scholar]
- Wattanasiriwech, D.; Srijan, K.; Wattanasiriwech, S. Vitrification of illitic clay from Malaysia. Appl. Clay Sci. 2009, 43, 57–62. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhong, X.C. The effects of potassium oxide and iron oxide additions on the phase composition of sintered bauxites (DK type). J. Chin. Ceram. Soc. 1984, 12, 77–84. [Google Scholar]
- He, D.S.; Feng, Q.M.; Zhang, G.F.; Long, S.S.; Ou, L.M.; Lu, Y.P. Effect of roasting on dissolution behavior of illite in acid solution. J. Cent. South Univ. 2011, 42, 1533–1537. [Google Scholar]
- Bibi, I.; Singh, B.; Silvester, E. Dissolution of illite in saline–acidic solutions at 25 °C. Geochim. Cosmochim. Acta 2011, 75, 3237–3249. [Google Scholar] [CrossRef]
- Çalban, T.; Kaynarca, B.; Kuşlu, S.; Çolak, S. Leaching kinetics of Chevreul’s salt in hydrochloric acid solutions. J. Ind. Eng. Chem. 2014, 20, 1141–1147. [Google Scholar] [CrossRef]
- Dickinson, C.F.; Heal, G.R. Solid–liquid diffusion controlled rate equations. Thermochim. Acta 1999, 340, 89–103. [Google Scholar] [CrossRef]
- Lin, B.Y.; Hu, L. Raw Materials of Refractory; Metallurgical Industry Press: Beijing, China, 2015; pp. 137–166. ISBN 978-7-5024-7038-8. [Google Scholar]




| Sample | Al2O3 (%) | SiO2 (%) | TiO2 (%) | Fe2O3 (%) | K2O (%) | Na2O (%) | CaO (%) | MgO (%) |
|---|---|---|---|---|---|---|---|---|
| Treated | 58.17 | 20.56 | 1.82 | 1.78 | 2.09 | 0.07 | 0.09 | 0.15 |
| Raw | 57.34 | 18.22 | 2.32 | 3.81 | 3.23 | 0.26 | 0.64 | 0.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Cao, Y.; Jiang, Y.; Han, G.; Fan, G.; Chang, L. Removal of Potassium and Iron in Low Grade Bauxite by a Calcination-Acid Leaching Process. Minerals 2018, 8, 125. https://doi.org/10.3390/min8040125
Li Z, Cao Y, Jiang Y, Han G, Fan G, Chang L. Removal of Potassium and Iron in Low Grade Bauxite by a Calcination-Acid Leaching Process. Minerals. 2018; 8(4):125. https://doi.org/10.3390/min8040125
Chicago/Turabian StyleLi, Zhuang, Yijun Cao, Yuanli Jiang, Guihong Han, Guixia Fan, and Luping Chang. 2018. "Removal of Potassium and Iron in Low Grade Bauxite by a Calcination-Acid Leaching Process" Minerals 8, no. 4: 125. https://doi.org/10.3390/min8040125




