The Biotechnological Applications of Recombinant Single-Domain Antibodies are Optimized by the C-Terminal Fusion to the EPEA Sequence (C Tag)
Abstract
:1. Introduction
2. Results and Discussion
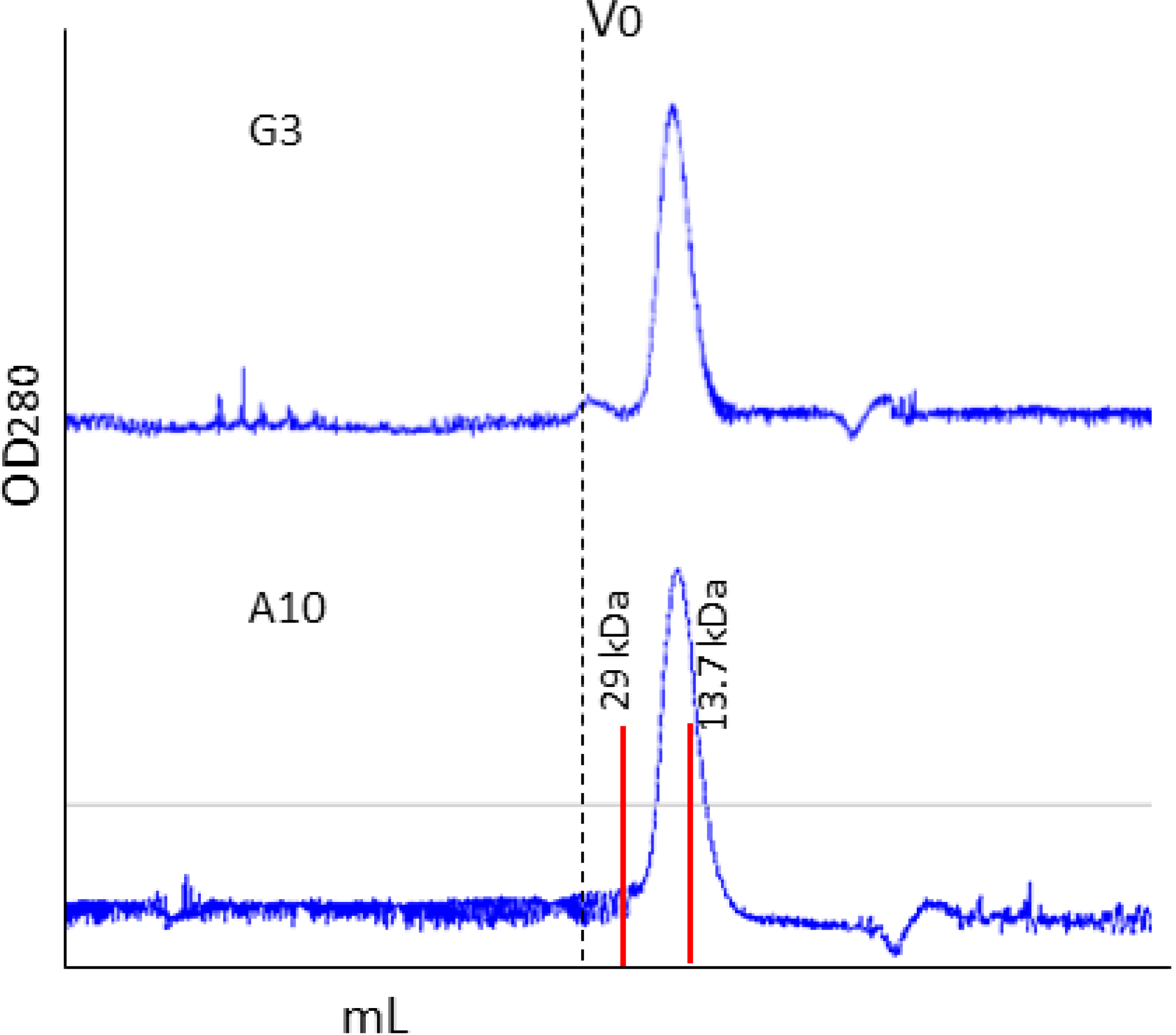
2.1. Affinity Purification Using Anti-C Tag Activated Resin
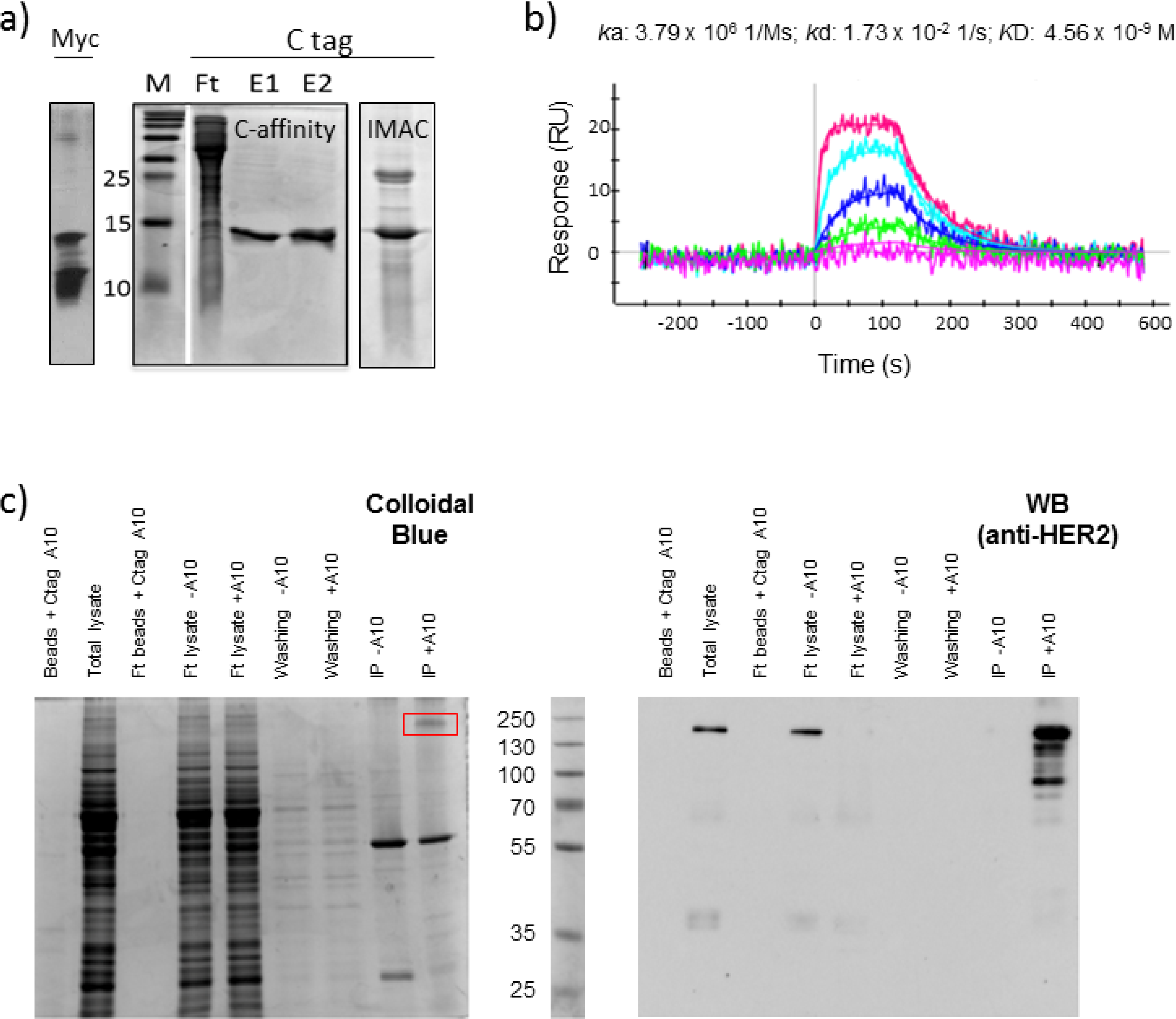

2.2. C Tag-Dependent Immunoprecipitation
2.3. Functional Validation of His-C Double-Tagged VHHs
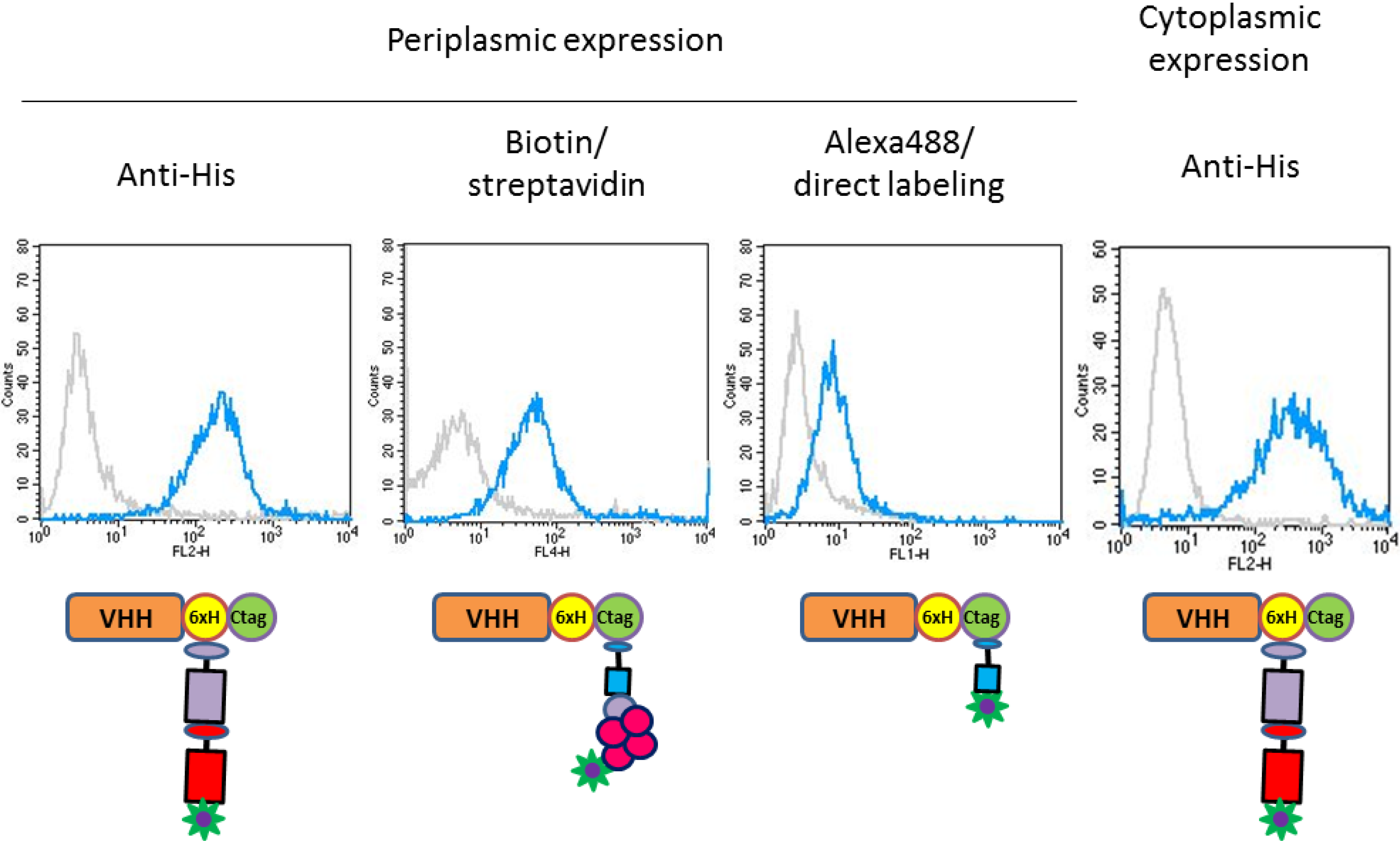

3. Experimental
3.1. Vector Preparation for Recombinant Antibody Expression
3.2. Antibody Production and Purification
3.3. Immunoprecipitation
3.4. FACS Analysis and Cell Imaging
3.5. Surface Plasmon Resonance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rigaut, G.; Shevchenko, A.; Rutz, B.; Wilm, M.; Mann, M.; Séraphin, B. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 1999, 17, 1030–1032. [Google Scholar] [CrossRef]
- Dümmler, A.; Lawrence, A.M.; de Marco, A. Simplified screening for the detection of soluble fusion constructs expressed in E. coli using a modular set of vectors. Microb. Cell Factories 2005, 4, 34. [Google Scholar] [CrossRef]
- Yelissev, A.; Zoubak, L.; Gawrisch, K. Usual of dual affinity tags for expression and purification of functional peripheral cannabinoid receptor. Protein Expr. Purif. 2007, 53, 153–163. [Google Scholar] [CrossRef]
- Bürckstümmer, T.; Bennett, K.L.; Preradovic, A.; Schütze, G.; Hantschel, O.; Superti-Furga, G.; Bauch, A. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat. Methods 2013, 12, 1013–1019. [Google Scholar]
- Dammeyer, T.; Timmis, K.N.; Tinnefeld, P. Broad host range vectors for expression of proteins with (Twin-) Strep-tag, His-tag and engineered, export optimized yellow fluorescent protein. Microb. Cell Fact. 2013, 12, 49. [Google Scholar] [CrossRef]
- Aliprandi, M.; Sparacio, E.; Pivetta, F.; Ossolengo, G.; Maestro, R.; de Marco, A. The availability of a recombinant anti-SNAP antibody in VHH format amplifies the application flexibility of SNAP-tagged proteins. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef]
- de Marco, A. The choice of appropriate tags improves the application effectiveness of the selected binders: The generation of user-friendly expression plasmids. Methods Mol. Biol. 2012, 911, 507–522. [Google Scholar]
- Veggiani, G.; de Marco, A. Improved quantitative and qualitative production of single-domain intrabodies mediated by the co-expression of Erv1p sulfhydryl oxidase. Protein Expr. Purif. 2011, 79, 111–114. [Google Scholar] [CrossRef]
- Sperinde, J.; Jin, X.; Banerjee, J.; Penuel, E.; Saha, A.; Diedrich, G.; Huang, W.; Leitzel, K.; Weidler, J.; Ali, SM.; et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin. Cancer Res. 2010, 16, 4226–4235. [Google Scholar] [CrossRef]
- Monegal, A.; Ami, D.; Martinelli, C.; Huang, H.; Aliprandi, M.; Capasso, P.; Francavilla, C.; Ossolengo, G.; de Marco, A. Immunological applications of single domain llama recombinant antibodies isolated from a naïve library. Protein Eng. Des. Sel. 2009, 22, 273–280. [Google Scholar] [CrossRef]
- Sala, E.; de Marco, A. Screening optimized protein purification protocols by coupling small-scale expression and mini-size exclusion chromatography. Protein. Expr. Purif. 2010, 74, 231–235. [Google Scholar] [CrossRef]
- Shimada, M.; Chen, X.; Cvrk, T.; Hilfiker, H.; Parfenova, M.; Segre, GV. Purification and characterization of a receptor for human parathyroid hormone and parathyroid hormone-related peptide. J. Biol. Chem. 2002, 277, 31774–31780. [Google Scholar]
- Locatelli-Hoops, S.C.; Gorshkova, I.; Gawrisch, K.; Yeliseev, A.A. Expression, surface immobilization, and characterization of functional recombinant cannabinoid receptor CB2. Biochim. Biophys. Acta 2013, 1834, 2045–2056. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Djender, S.; Beugnet, A.; Schneider, A.; De Marco, A. The Biotechnological Applications of Recombinant Single-Domain Antibodies are Optimized by the C-Terminal Fusion to the EPEA Sequence (C Tag). Antibodies 2014, 3, 182-191. https://doi.org/10.3390/antib3020182
Djender S, Beugnet A, Schneider A, De Marco A. The Biotechnological Applications of Recombinant Single-Domain Antibodies are Optimized by the C-Terminal Fusion to the EPEA Sequence (C Tag). Antibodies. 2014; 3(2):182-191. https://doi.org/10.3390/antib3020182
Chicago/Turabian StyleDjender, Selma, Anne Beugnet, Aurelie Schneider, and Ario De Marco. 2014. "The Biotechnological Applications of Recombinant Single-Domain Antibodies are Optimized by the C-Terminal Fusion to the EPEA Sequence (C Tag)" Antibodies 3, no. 2: 182-191. https://doi.org/10.3390/antib3020182
APA StyleDjender, S., Beugnet, A., Schneider, A., & De Marco, A. (2014). The Biotechnological Applications of Recombinant Single-Domain Antibodies are Optimized by the C-Terminal Fusion to the EPEA Sequence (C Tag). Antibodies, 3(2), 182-191. https://doi.org/10.3390/antib3020182



