Spatial and Temporal Dynamics of Potentially Toxic Cyanobacteria in the Riverine Region of a Temperate Estuarine System Altered by Weirs
Abstract
:1. Introduction
2. Materials and Methods
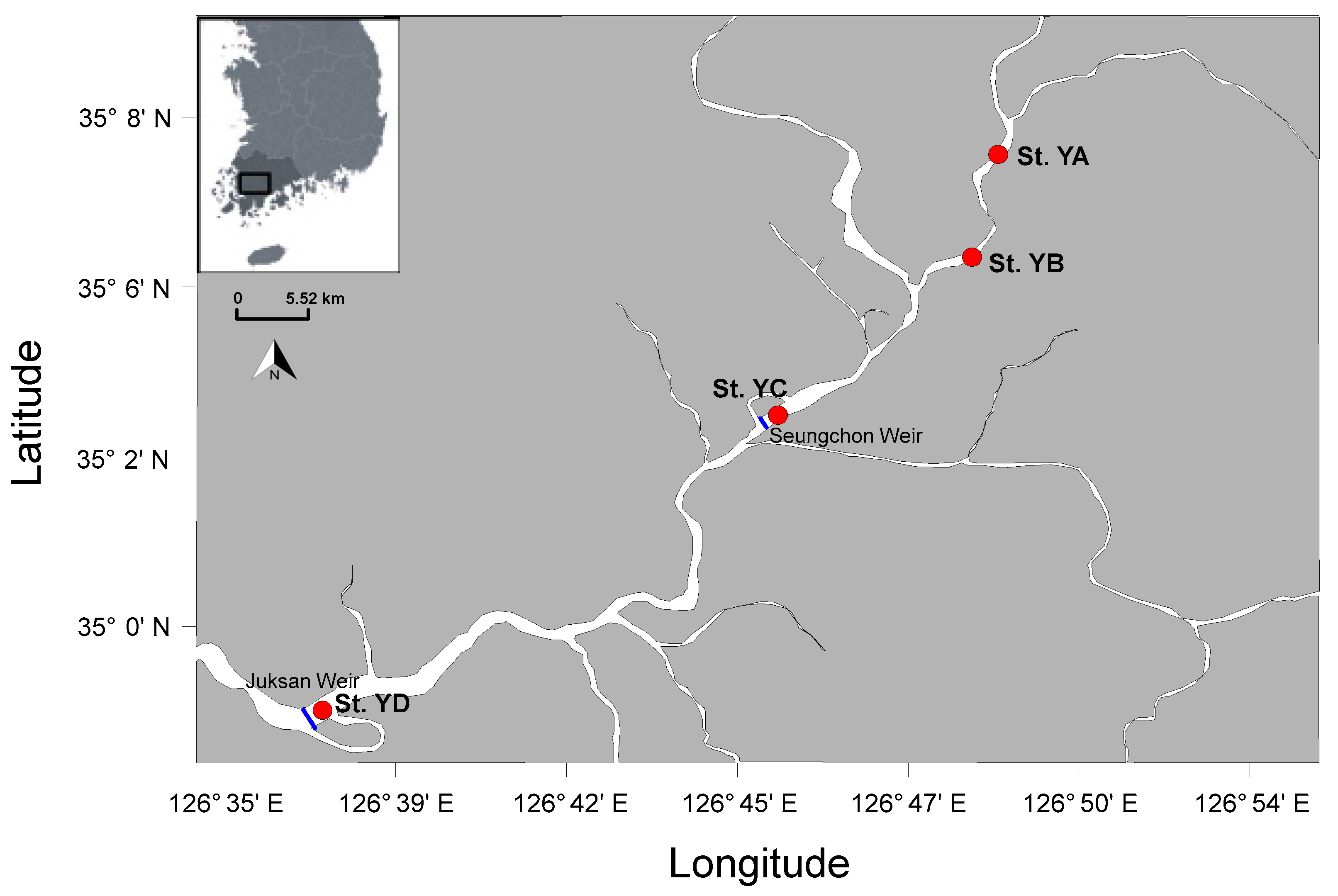
2.1. Study Site and Field Sampling
2.2. Determination of Physicochemical Parameters
2.3. Determination of Chlorophyll a
2.4. Determination of Zooplankton Abundance
2.5. Cyanobacteria Identification and Enumeration
2.6. Statistical Analyses
3. Results
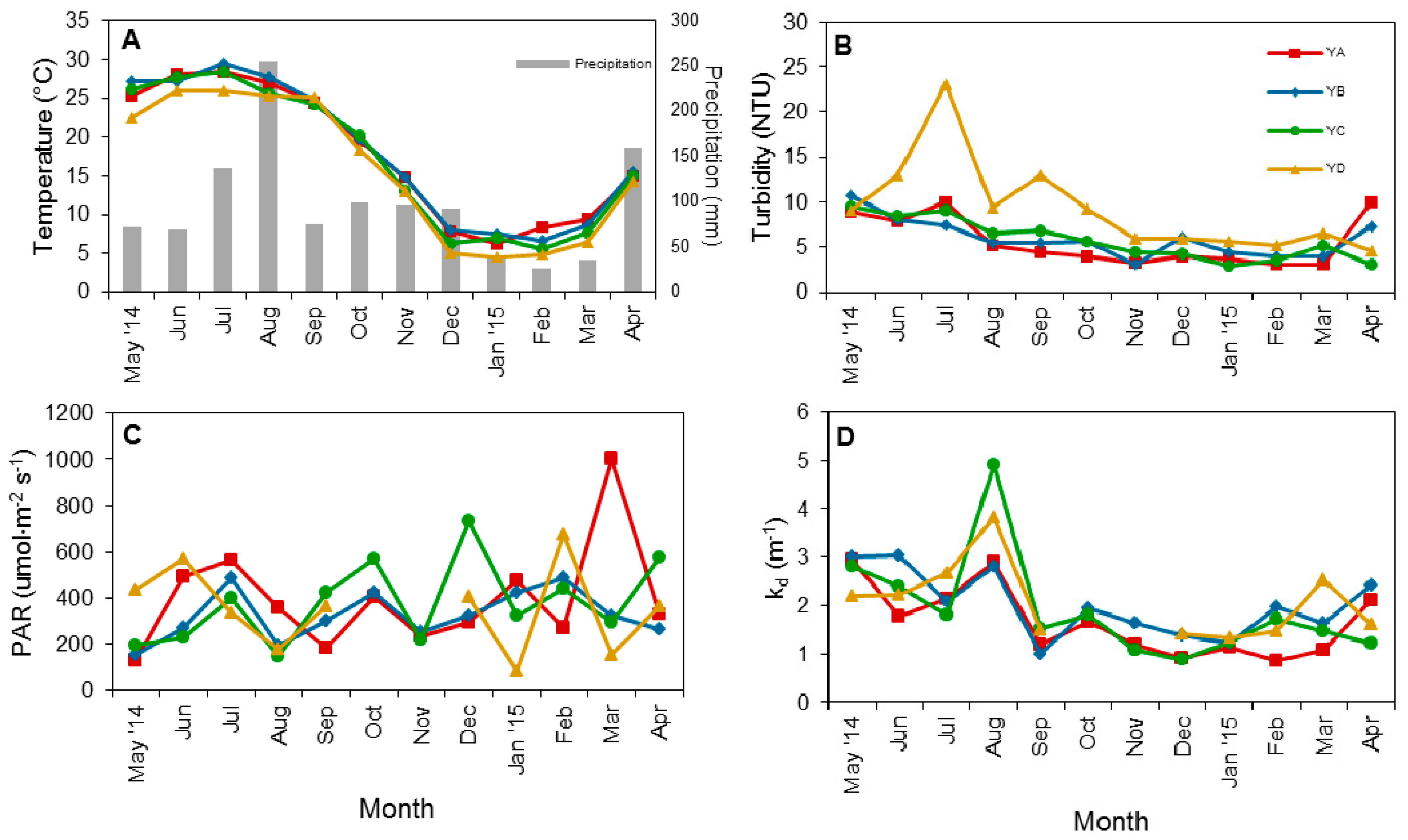
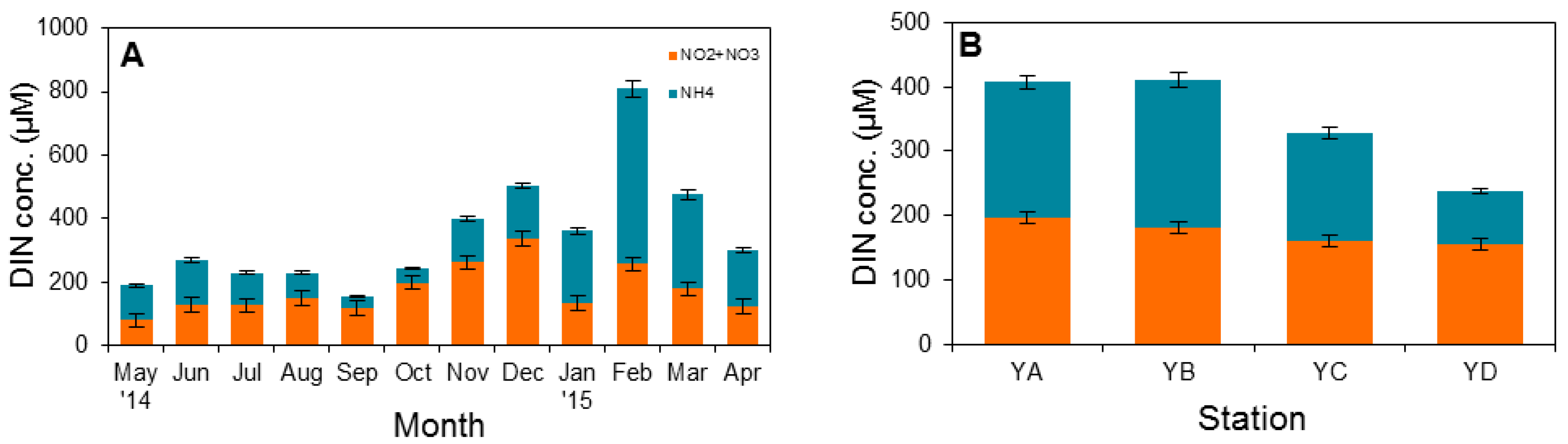
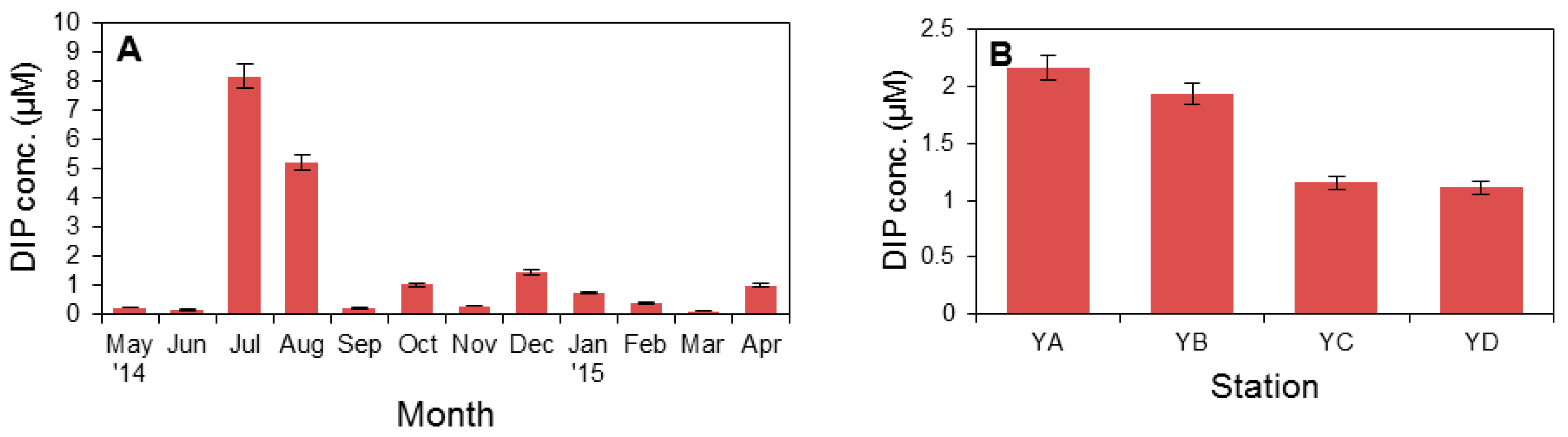
3.1. Physical Factors and Inorganic Nutrient Concentrations
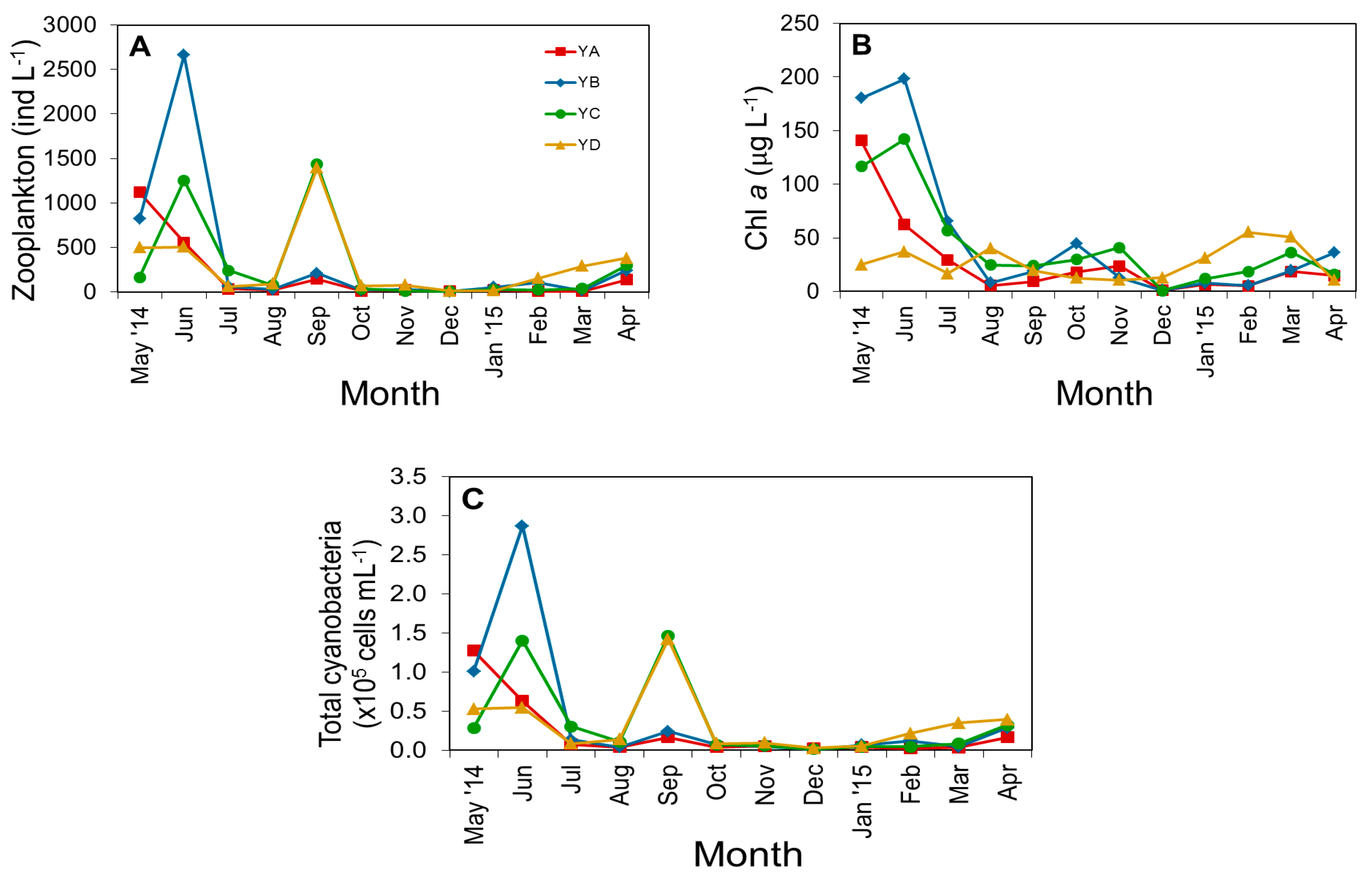
3.2. Biological Parameters
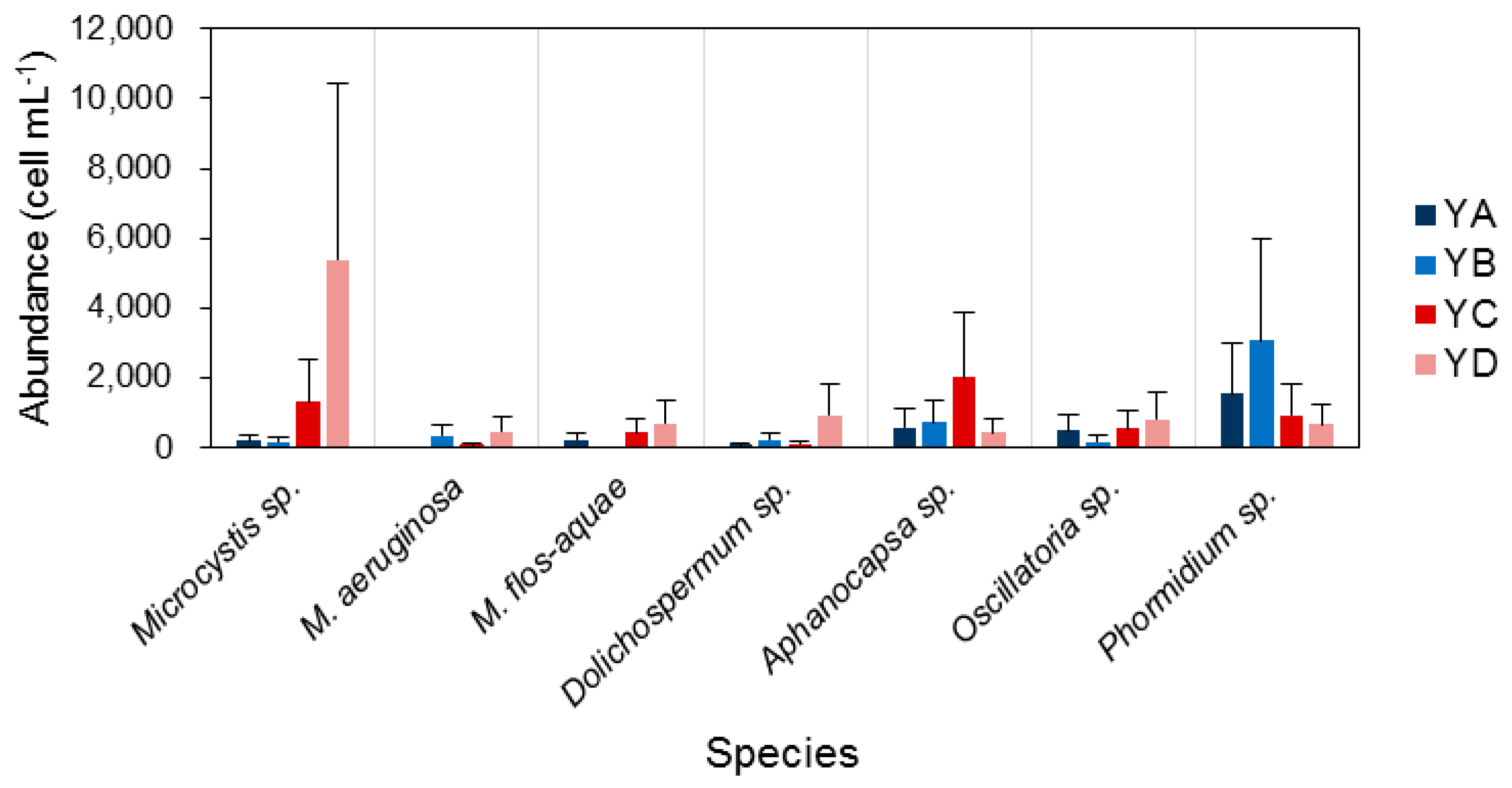
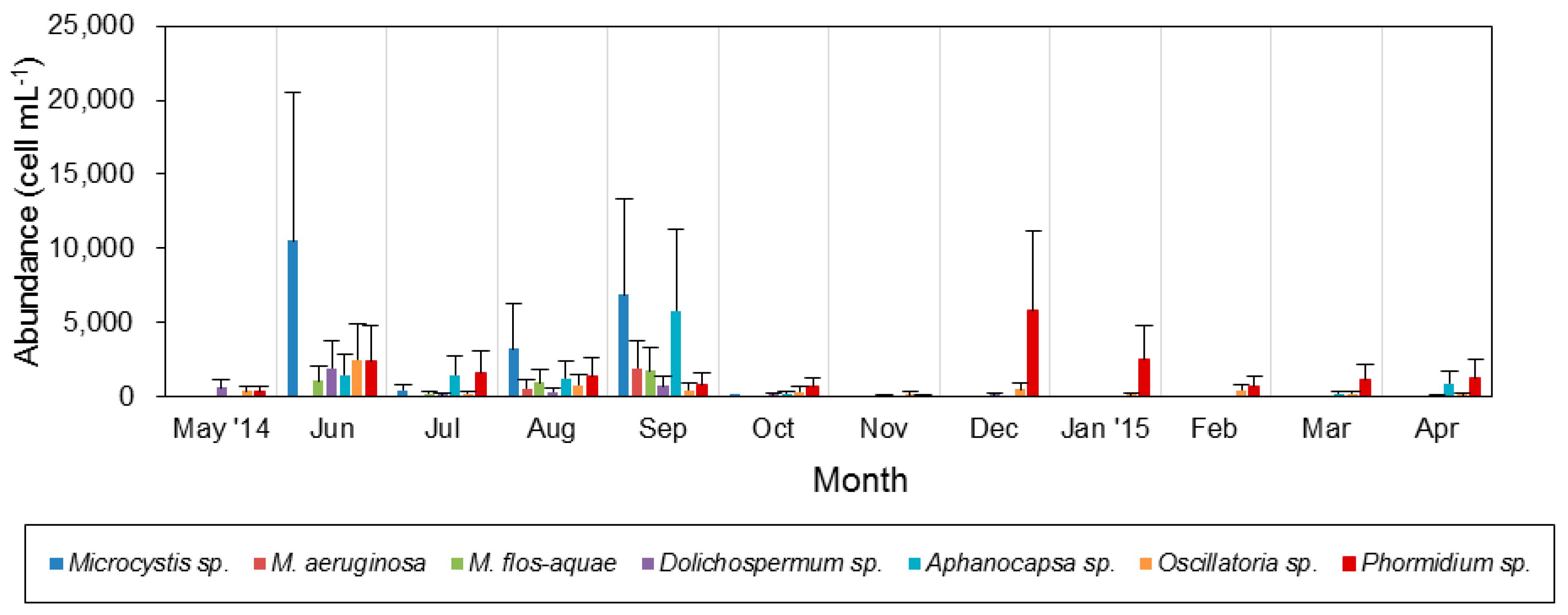
3.3. Spatiotemporal Variation of Potentially Toxic Cyanobacteria
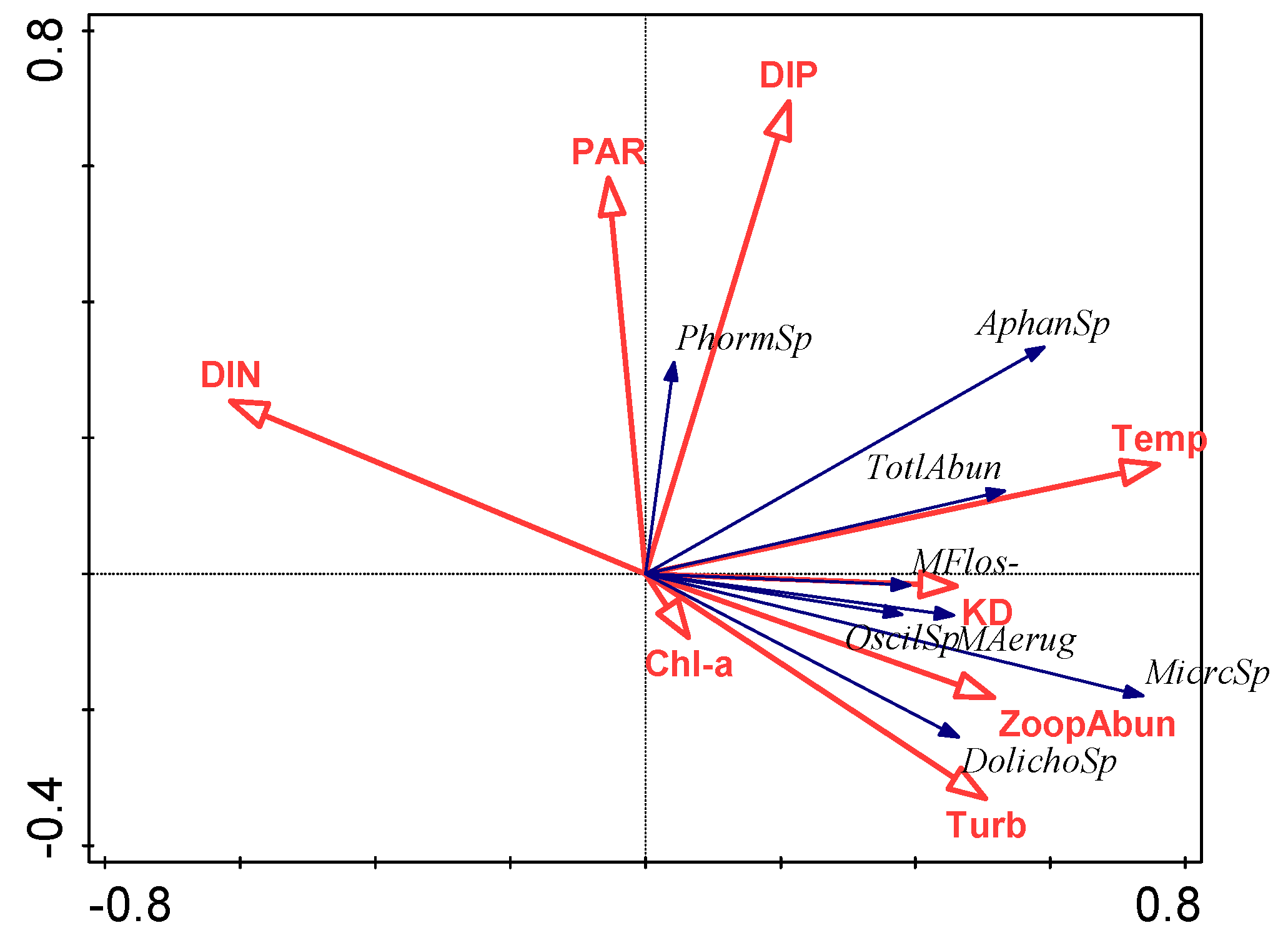
3.4. Relationship between Environmental Factors and Cyanobacterial Species
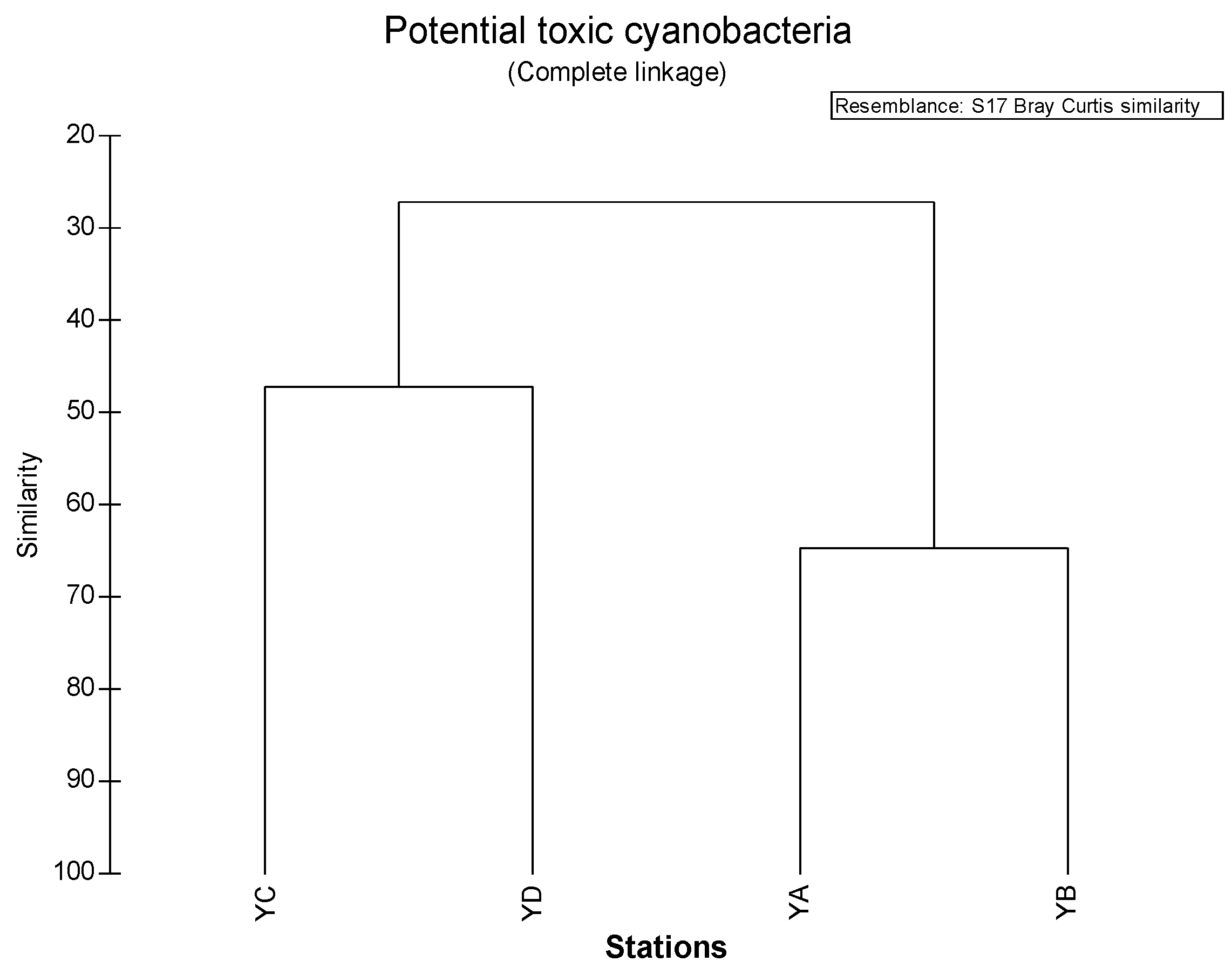
3.5. Similarity among Sampling Sites
4. Discussion
4.1. Temporal and Spatial Dynamics of Cyanobacteria
4.2. Effect of Environmental Variables on Cyanobacteria and Potentially Toxic Species
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Awramik, S. The oldest records of photosynthesis. Photosynth. Res. 1992, 33, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Nazifi, E.; Hirai, Y.; Wada, N.; Matsugo, S.; Sakamoto, T. The cyanobacterial UV-absorbing pigment scytonemin displays radical-scavenging activity. J. Gen. Appl. Microbiol. 2012, 58, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Klemer, A.; Cullen, J.; Mageau, M.; Hanson, K.; Sundell, R. Cyanobacterial Buoyancy Regulation: The paradoxical roles of carbon. J. Phycol. 1996, 32, 47–53. [Google Scholar] [CrossRef]
- Lopez-Rodas, V.; Maneiro, E.; Costas, E. Adaptation of cyanobacteria and microalgae to extreme environmental changes derived from anthropogenic pollution. Limnetica 2006, 25, 403–410. [Google Scholar]
- Su, M.; Yu, J.; Pan, S.; An, W.; Yang, M. Spatial and temporal variations of two cyanobacteria in the mesotrophic Miyun reservoir, China. J. Environ. Sci. 2014, 26, 289–298. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Yang, Z.; Wei, L.; Yang, W.; Chen, C.; Kong, F. Spatial and seasonal shifts in bloom-forming cyanobacteria in Lake Chaohu: Patterns and driving factors. Phycol. Res. 2015, 64, 44–55. [Google Scholar] [CrossRef]
- Bormans, M.; Ford, P.; Fabbro, L. Spatial and temporal variability in cyanobacterial populations controlled by physical processes. J. Plankton Res. 2005, 27, 61–70. [Google Scholar] [CrossRef]
- Harris, J.; Vinobaba, P.; Kularatne, R.; Kankanamge, C. Spatial and temporal distribution of cyanobacteria in Batticaloa Lagoon. J. Environ. Sci. 2016, 47, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.; Skulberg, O.; Utkilen, H. Chapter 2: Cyanobacteria in the environment. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; World Health Organization (WHO): Geneva, Switzerland, 1999. [Google Scholar]
- Paerl, H.; Hall, N.; Calandrino, E. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.; Paerl, H.; Howarth, R.; Boesch, D.; Seitzinger, S.; Havens, K.; Lancelot, C.; Likens, G. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Suurnakki, S.; Gomez-Saez, G.V.; Rantala-Ylinen, A.; Jokela, J.; Fewer, D.P.; Sivonen, K. Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers of these compounds. Water Res. 2015, 68, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Juttner, F.; Watson, S. Biochemical and ecological control of geosmin and 2-Methylisoborneol in source waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Bi, Y.; Deng, Y.; He, Z.; Wu, L.; Nostrand, J.; Shi, Z.; Li, J.; Xi, W.; Hu, Z.; et al. Impacts of the Three Gorges Dam on microbial structure and potential function. Sci. Rep. 2015, 5, 8605. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.; Wood, S.; Ryan, K. Spatial and temporal variability in Phormidium mats and associated anatoxin-a and homoanatoxin-a in two New Zealand rivers. Aquat. Microb. Ecol. 2011, 64, 69–79. [Google Scholar] [CrossRef]
- Miranda, R.; Oscoz, J.; Leunda, P.; Garcia-Fresca, C.; Escala, M.C. Effects of weir construction on fish population structure in the River Erro (North of Spain). Ann. Limnol. Int. J. Limnol. 2005, 41, 7–13. [Google Scholar] [CrossRef]
- McCarthy, B.; Gawne, B.; Meredith, S.; Roberts, J.; Williams, D. Effects of Weirs in the Mallee Tract of the River Murray; MDFRC Technical Report; Murray-Darling Freshwater Research Centre: Wodonga, Australia, 2004. [Google Scholar]
- Thackeray, S. Plankton: A Guide to their Ecology and Monitoring for Water Quality. J. Fish Biol. 2010, 76, 1869–1870. [Google Scholar] [CrossRef]
- Swan River Trust. Seasonal nutrient dynamics in the canning river and estuary, 1995–98. River Sci. 2002, 2, 1–12. [Google Scholar]
- Chan, T.; Hamilton, D.; Robson, B.; Hodges, B.; Dallimore, C. Impacts of hydrological changes on phytoplankton succession in the Swan River, Western Australia. Estuaries 2002, 25, 1406–1415. [Google Scholar] [CrossRef]
- Characteristics and Control of Algal Blooms in the Youngsan and Sumjin River Basins; Korea Ministry of Environment: Sejong, Korea, 2015.
- Yeongsan River Flood Control Office. Available online: www.yeongsanriver.go.kr (accessed on 4 June 2016).
- Kang, J.H.; Lee, S.W.; Cho, K.H.; Seo, S.J.; Cha, S.M.; Kim, J.H. Linking land-use type and stream water quality using spatial data of fecal indicator bacteria and heavy metals in the Yeongsan river basin. Water Res. 2010, 44, 4143–4157. [Google Scholar] [CrossRef] [PubMed]
- Hötzel, G.; Croome, R. A Phytoplankton Methods Manual for Australian Freshwaters; Land and Water Resources Research and Development Corporation (LWRRDC): Canberra, Australia, 1999. [Google Scholar]
- Swineheart, D. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Kolzau, S.; Wiedner, C.; Rucker, J.; Kohler, J.; Kohler, A.; Dolman, A. Seasonal Patterns of Nitrogen and Phosphorus Limitation in Four German Lakes and the Predictability of Limitation Status from Ambient Nutrient Concentrations. PLoS ONE 2014, 9, e96065. [Google Scholar] [CrossRef] [PubMed]
- Arar, E.J.; Collins, G.B. In Vitro Determination of Chlorophyll a and Phaeophytin in Marine and Freshwater Algae by Fluorescence. In Methods for the Determination of Chemical Substances of Marine and Estuarine Environmental Matrices, 2nd ed.; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1997. [Google Scholar]
- Voigt, M.; Koste, W. Rotatoria: Die Räderiere Mitteleuropas, ein Bestimmungswerk: Überordnung Monogononta; Bornträger: Stuttgart, Germany, 1978. (In German) [Google Scholar]
- Smirnov, N.N.; Timms, B.V. A Revision of the Australian Cladocera (Crustacea); The Australian Museum: Sydney, Australia, 1983; Volume 1, pp. 1–132. [Google Scholar]
- Einsle, U. Crustacea, Copepoda: Calanoida und Cyclopoida; Gustav Fischer Verlag: Stuttgart, Germany, 1993. (In German) [Google Scholar]
- Protocol for Phytoplankton Sampling. Protocols Manual for Water Quality Sampling in Canada. Canadian Council of Ministers of the Environment: Canada, 2011. Available online: http://www.ccme.ca/files/Resources/water/water_quality/protocols_document_e_final_101.pdf (accessed on 10 July 2017).
- Hirose, H.M.; Akiyama, T.; Imahori, K.; Kasaki, H.; Kumano, S.; Kobayasi, H.; Takahashi, E.; Tsumura, T.; Hirano, M.; Yamagishi, T. Illustrations of the Japanese Freshwater Algae; Uchidarokakuho Publ Co., Ltd.: Tokyo, Japan, 1977. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota Teil 2: Oscillatoriales; Budel, B., Krienitz, L., Gartner, G., Schagerl, M., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2005; p. 750. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota Teil 2 Chroococcales; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 1998; Volume 19, p. 548. [Google Scholar]
- Wetzel, R. Limnology: Lake and River Ecosystems, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Graham, J.; Loftin, K.; Ziegler, A.; Meyer, M. Cyanobacteria in Lakes and Reservoirs: Toxin and Taste-and-Odor Sampling Guidelines; U.S. Geological Survey: Reston, VA, USA, 2008.
- Zakeel, M.; Weerasinghe, P.; Kumari, B.; Wickremasinghe, H. Spatial and temporal dynamics and parameters of growth of toxic cyanobacteria in Nuwara wewa and Nachchaduwa wewa in Sri Lanka. Am. J. Environ. Prot. 2015, 4, 23–28. [Google Scholar] [CrossRef]
- Guidelines for Safe Recreational Water Environments Volume 1 Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2003.
- Lehman, P.W.; Kendall, C.; Guerin, M.A.; Young, M.B.; Silva, S.R.; Boyer, G.L.; Teh, S.J. Characterization of the Microcystis bloom and its Nitrogen Supply in the San Francisco Estuary using Stable Isotopes. Estuar. Coasts 2015, 38, 165–178. [Google Scholar] [CrossRef]
- Sellner, K.G.; Lacouture, R.V.; Parrish, C.R. Effects of increasing salinity on a cyanobacteria bloom in the Potomac River estuary. J. Plankton Res. 1988, 10, 49–61. [Google Scholar] [CrossRef]
- Paerl, H.W.; Bland, P.T.; Blackwell, J.H.; Bowles, N.D. The Effects of Salinity on the Potential of a Blue-Green Algal (Microcystis aeruginosa) Bloom in the Neuse Estuary; N.C. Working Paper 84-1; UNC Sea Grant College Program: Raleigh, NC, USA, 1984. [Google Scholar]
- Baxa, D.; Kurobe, T.; Ger, K.; Lehman, P.; Teh, S. Estimating the abundance of toxic Microcystis in the San Francisco Estuary using quantitative real-time PCR. Harmful Algae 2010, 9, 342–349. [Google Scholar] [CrossRef]
- Walsby, A.E.; McAllister, G.K. Buoyancy Regulation by Microcystis in Lake Okaro. N. Z. J. Mar. Freshw. Res. 1987, 21, 521–524. [Google Scholar] [CrossRef]
- John, J.; Kemp, A. Cyanobacterial Blooms in the Wetlands of the Perth region, Taxonomy and Distribution: An Overview. J. R. Soc. West. Aust. 2006, 89, 51–56. [Google Scholar]
- Stanne, S.; Panetta, R.; Forist, B. The Hudson: An Illustrated Guide to the Living River; Rutgers University Press: New Brunswick, NJ, USA, 1996. [Google Scholar]
- Sin, Y.; Lee, E.; Lee, Y.; Shin, K. The river-estuarine continuum of nutrients and phytoplankton communities in an estuary physically divided by a sea dike. Estuar. Coast Shelf Sci. 2015, 163, 279–289. [Google Scholar] [CrossRef]
- Teixeira, L.M.; Bohnenberger, J.; Rodrigues, L.; Schulz, U.; Marques, D.; Crossetti, L. Temporal variability determines phytoplankton structure over spatial organization in a large shallow heterogeneous subtropical lake. Inland Waters 2016, 6, 325–335. [Google Scholar]
- Kihwele, E.S.; Lugomela, C.; Howell, K.M.; Nonga, H.E. Spatial and Temporal Variations in the Abundance and Diversity of Phytoplankton in Lake Manyara, Tanzania. Int. J. Innov. Stud. Aquat. Biol. Fish 2015, 1, 1–14. [Google Scholar]
- Bryanskaya, A.V.; Malup, T.; Lazareva, E.; Peltek, S. The role of environmental factors for the composition of microbial communities of saline lakes in the Novosibirsk region (Russia). BMC Microbiol. 2016, 16, S4. [Google Scholar] [CrossRef] [PubMed]
- Graneli, E.; Turner, T. Ecology of Harmful Algae; Springer: Berlin, Germany, 2007. [Google Scholar]
- Simulation Results of the Residence Time of Water in the Four Major Rivers Report; Korean National Institute of Environmental Research: Incheon, Korea, 2013.
- Romo, S.; Soria, J.; Fernandez, F.; Ouahid, Y.; Baron-Sola, A. Water residence time and the dynamics of toxic cyanobacteria. Freshw. Biol. 2013, 58, 513–522. [Google Scholar] [CrossRef]
- Lurling, M.; Eshetu, F.; Faassen, E.; Kosten, S.; Huszar, V. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Konopka, A.; Brock, T. Effect of temperature on Blue-Green Algae (Cyanobacteria) in Lake Mendota. Appl. Environ. Microbiol. 1978, 36, 572–576. [Google Scholar] [PubMed]
- McAllister, T. Environmental Factors That Promote Phormidium Blooms in Canterbury Rivers; Waterways Centre for Freshwater Management: Christchurch, New Zealand, 2014. [Google Scholar]
- Korea Ministry of Environment. Status of cyanobacterial blooms and management in Korea. In Proceedings of the International Symposium on Freshwater Ecology, Chuncheon, Korea, 16 October 2015. [Google Scholar]
- Dziallas, C.; Grossart, H.P. Increasing oxygen radicals and water temperature select for toxic Microcystis sp. PLoS ONE 2011, 6, e25569. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Chang, K.; Nakano, S. Growth Responses of Harmful Algal Species Microcystis (Cyanophyceae) under Various Environmental Conditions. In Interdisciplinary Studies on Environmental Chemistry—Environmental Research in Asia; TerraPub: Tokyo, Japan, 2009; pp. 269–275. [Google Scholar]
- Davis, T.; Berry, D.; Boyer, G.; Gobler, C. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Elser, J.; Marzolf, E.; Goldman, C. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: A review and critique of experimental enrichments. Can. J. Fish Aquat. Sci. 1990, 47, 1468–1477. [Google Scholar] [CrossRef]
- Jia, J.; Shi, W.; Chen, Q.; Lauridsen, T. Spatial and temporal variations reveal the response of zooplankton to cyanobacteria. Harmful Algae 2017, 64, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Pander, J.; Geist, J. The effects of weirs on structural stream habitat and biological communities. J. Appl. Ecol. 2011, 48, 1450–1461. [Google Scholar] [CrossRef]
- Krstulović, N.; Šolić, M.; Marasović, I. Relationship between bacteria, phytoplankton, and heterotrophic nanoflagellates along the trophic gradient. Helgol. Mar. Res. 1998, 51, 433. [Google Scholar] [CrossRef]
| Statistic | Axis 1 |
|---|---|
| Eigenvalues | 0.3528 |
| Explained variation (cumulative) | 32.34 |
| Gradient length | 2.34 |
| Environmental Variable | Contribution % | P-adj |
|---|---|---|
| Temperature | 45.2 | 0.008 |
| DIN | 32.1 | 0.008 |
| Zooplankton abundance | 22.0 | 0.028 |
| Variables | Control (Mean ± SD) * | Treatment (Mean ± SD) * | p-Values (One-Tailed) |
|---|---|---|---|
| Environmental | |||
| Temperature | 17.97 ± 8.42 | 16.61 ± 8.73 | 0.00026 |
| DIN | 409.27 ± 223.73 | 284.0 ± 149.56 | 9.0386 × 10−5 |
| Turbidity | 5.80 ± 2.36 | 7.52 ± 4.23 | 0.01451 |
| Zooplankton abundance | 264 ± 570 | 298 ± 428 | 0.01098 |
| Toxic cyanobacteria | |||
| Microcystis sp. | 164 ± 477 | 3331 ± 9234 | 0.00752 |
| Phormidium sp. | 2300 ± 3867 | 776 ± 1000 | 0.00324 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malazarte, J.M.; Lee, H.; Kim, H.-W.; Sin, Y. Spatial and Temporal Dynamics of Potentially Toxic Cyanobacteria in the Riverine Region of a Temperate Estuarine System Altered by Weirs. Water 2017, 9, 819. https://doi.org/10.3390/w9110819
Malazarte JM, Lee H, Kim H-W, Sin Y. Spatial and Temporal Dynamics of Potentially Toxic Cyanobacteria in the Riverine Region of a Temperate Estuarine System Altered by Weirs. Water. 2017; 9(11):819. https://doi.org/10.3390/w9110819
Chicago/Turabian StyleMalazarte, Jacqueline Martha, Hakyoung Lee, Hyun-Woo Kim, and Yongsik Sin. 2017. "Spatial and Temporal Dynamics of Potentially Toxic Cyanobacteria in the Riverine Region of a Temperate Estuarine System Altered by Weirs" Water 9, no. 11: 819. https://doi.org/10.3390/w9110819
APA StyleMalazarte, J. M., Lee, H., Kim, H.-W., & Sin, Y. (2017). Spatial and Temporal Dynamics of Potentially Toxic Cyanobacteria in the Riverine Region of a Temperate Estuarine System Altered by Weirs. Water, 9(11), 819. https://doi.org/10.3390/w9110819






