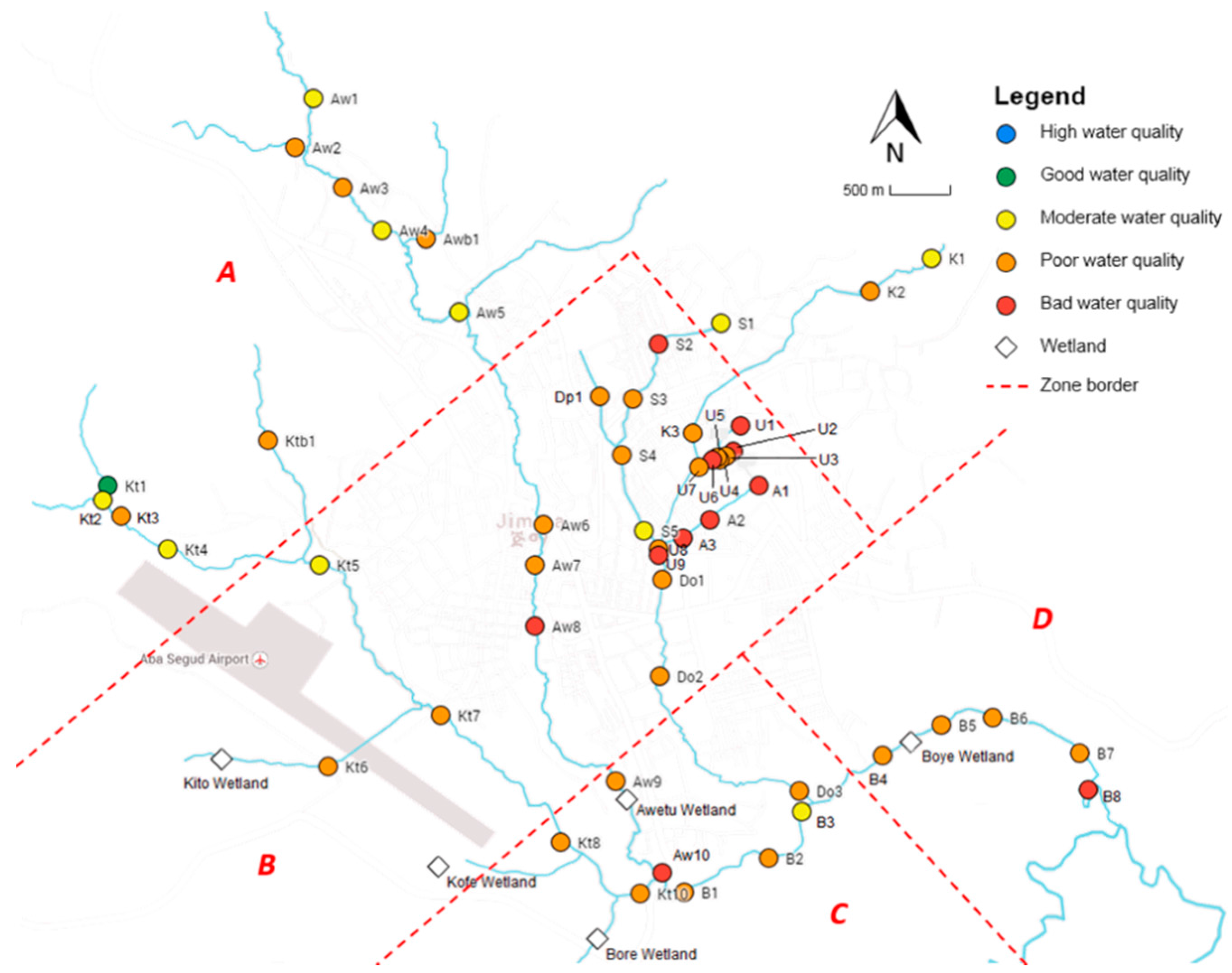
4.1. Water Quality
Our results show that the rivers in Jimma receive pollutants from different (diffuse and point) sources contributing to a significant decrease in dissolved oxygen along the rivers’ continuum. The upstream reaches are mainly characterized by agriculture and agro-pastoralism; thus, runoff from land is considered to be the major diffuse source of pollution in that area. Lencha & Moges [
36] showed that land with crop production in Jimma is more susceptible for erosion, and that changes in land use during the past years have increased the erosion potential. Deforestation, overgrazing, and cultivation of slopes unsuitable for agriculture significantly enhance land degradation in Jimma [
37]. Four catchments in Jimma with very high erosion susceptibility drain to the Gilgel Gibe hydropower dam, indicating the need for immediate actions to deal with erosion in order to protect the dam from siltation [
38]. The construction of soil bunds and biological stabilization with, e.g., elephant grass or
Sesbania sp, have proven to be efficient measures to reduce soil erosion in the Ethiopian highlands [
37]. Biological stabilization is an especially promising technique, as it also improves the availability of organics in the soil and provides food for cattle [
39].
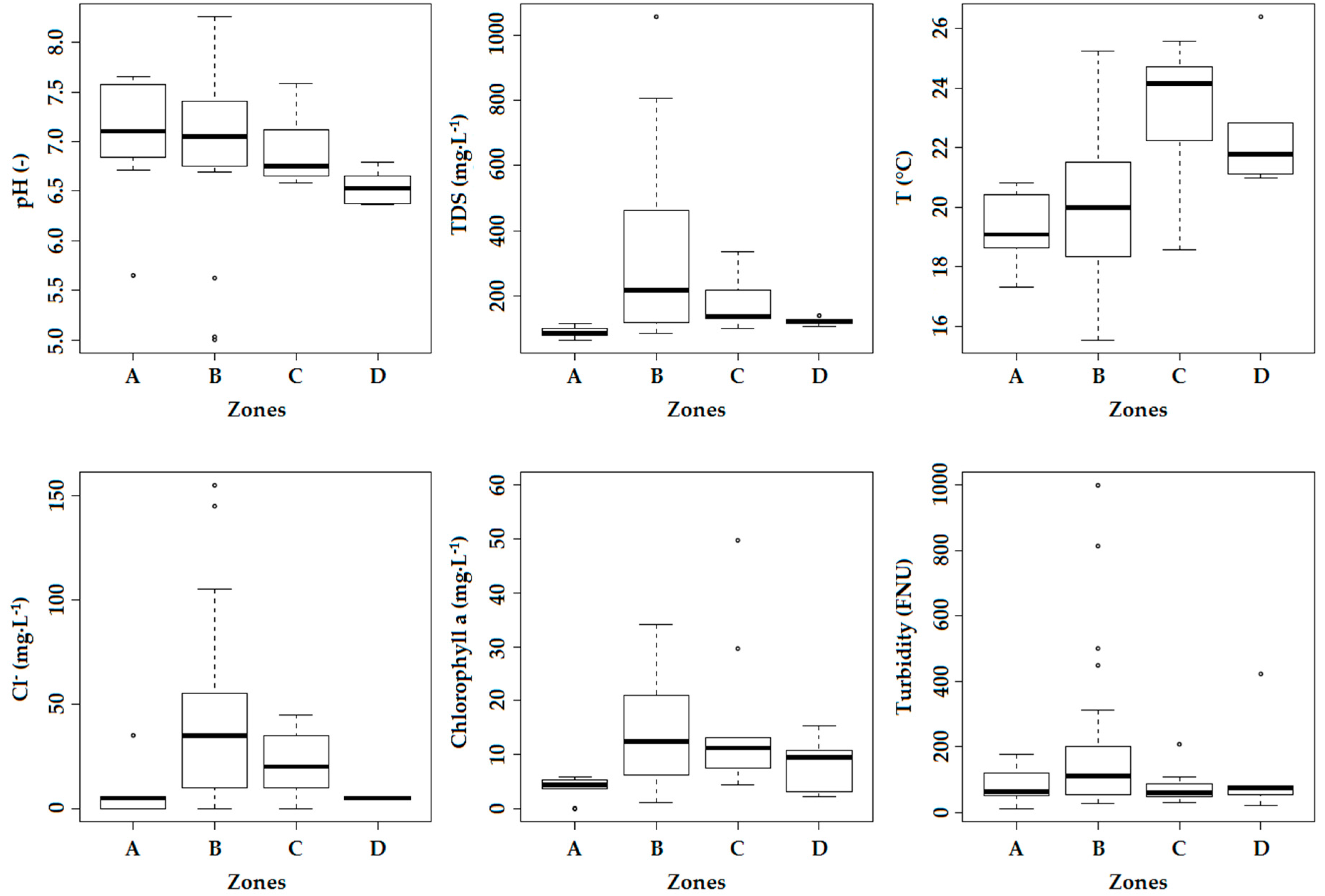
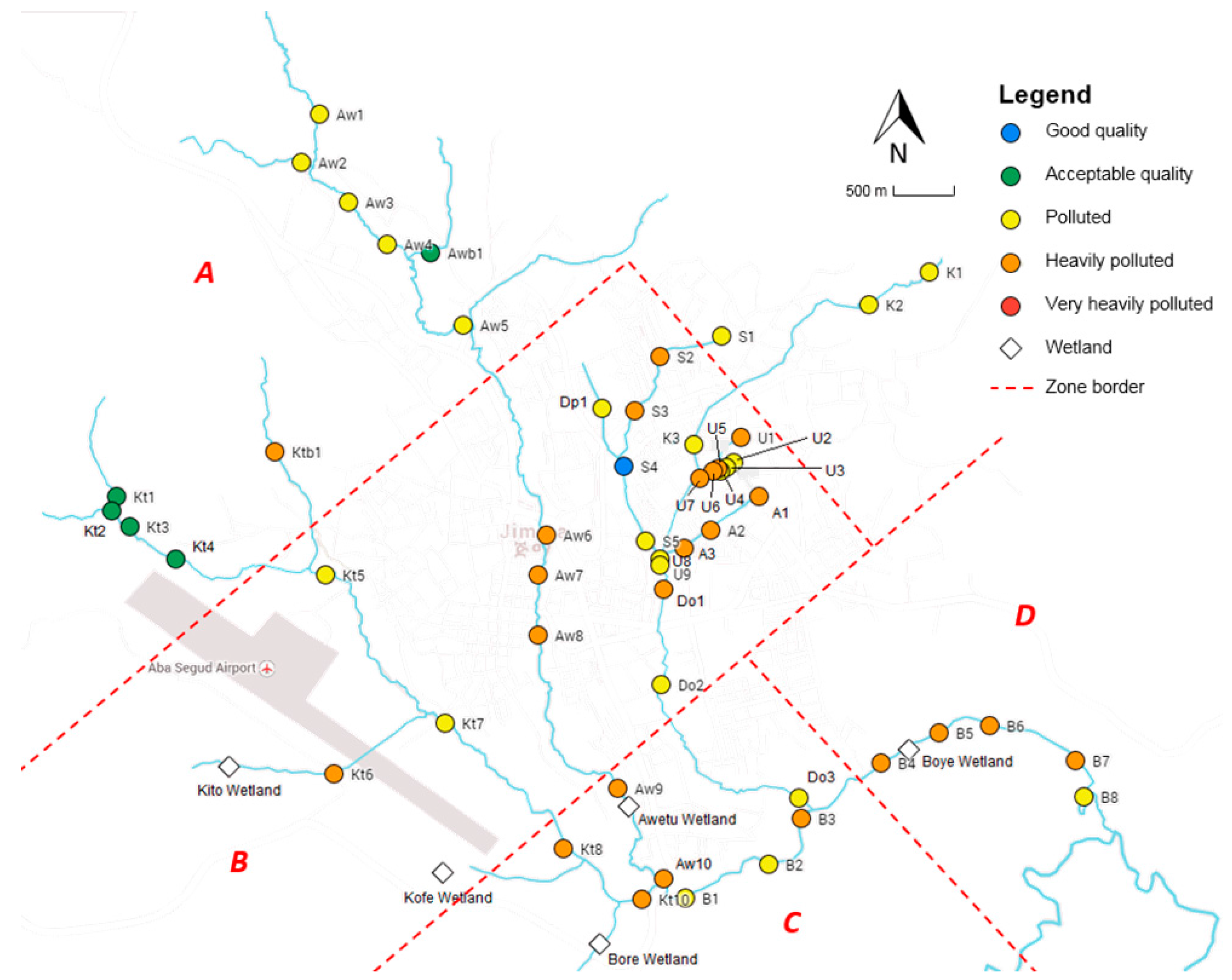
Erosion also promotes the input of nutrients into the rivers, and the frequent usage of pesticides (e.g., DDT, endosulfan, cypermethrin, and permethrin) is expected to have an adverse effect on the water quality. These sources of pollution contribute to the observed significant decrease in chemical and ecological water quality towards the mouth of the rivers. However, major pollution mainly occurs in the midstream reaches located in the urban area of Jimma, as most locations exceeding standards are situated there (78% for oPO
43−, 68% for TAN, 52% for DO, 82% for EC, 100% for Cl
−, and around 60% for TSS). The middle part of the rivers are, next to agriculture, impacted by a broader range of pollution sources: institutional, domestic and industrial wastewater discharge, solid waste (e.g., plastics) and its leachates, Jimma’s prison and the university campus, the newly built slaughterhouse, hotels, carwash and fuel stations, clinics, colleges, and industry (wood and coffee processing). The pollution is reflected by the significant decrease in oxygen, increase in nutrients (TAN and oPO
43−), leading to a higher abundance of algae (which require nitrogen and phosphorous for growth) and the increase in electrical conductivity and the related chloride concentration. The latter can be explained by the hide and skin processing of many retailers at different spots or by the usage of potassium-based fertilizers [
4]. There is, however, one location where the chemical quality is determined to be good: after the confluence of Seto and Dipo. The distinct abundantly present aquatic vegetation at this site probably explains the relative high level of DO resulting in relatively good chemical water quality. The site is, however, still characterized by poor ecological water quality, indicating that it is not only oxygen that is important to sustain a healthy macroinvertebrate community.
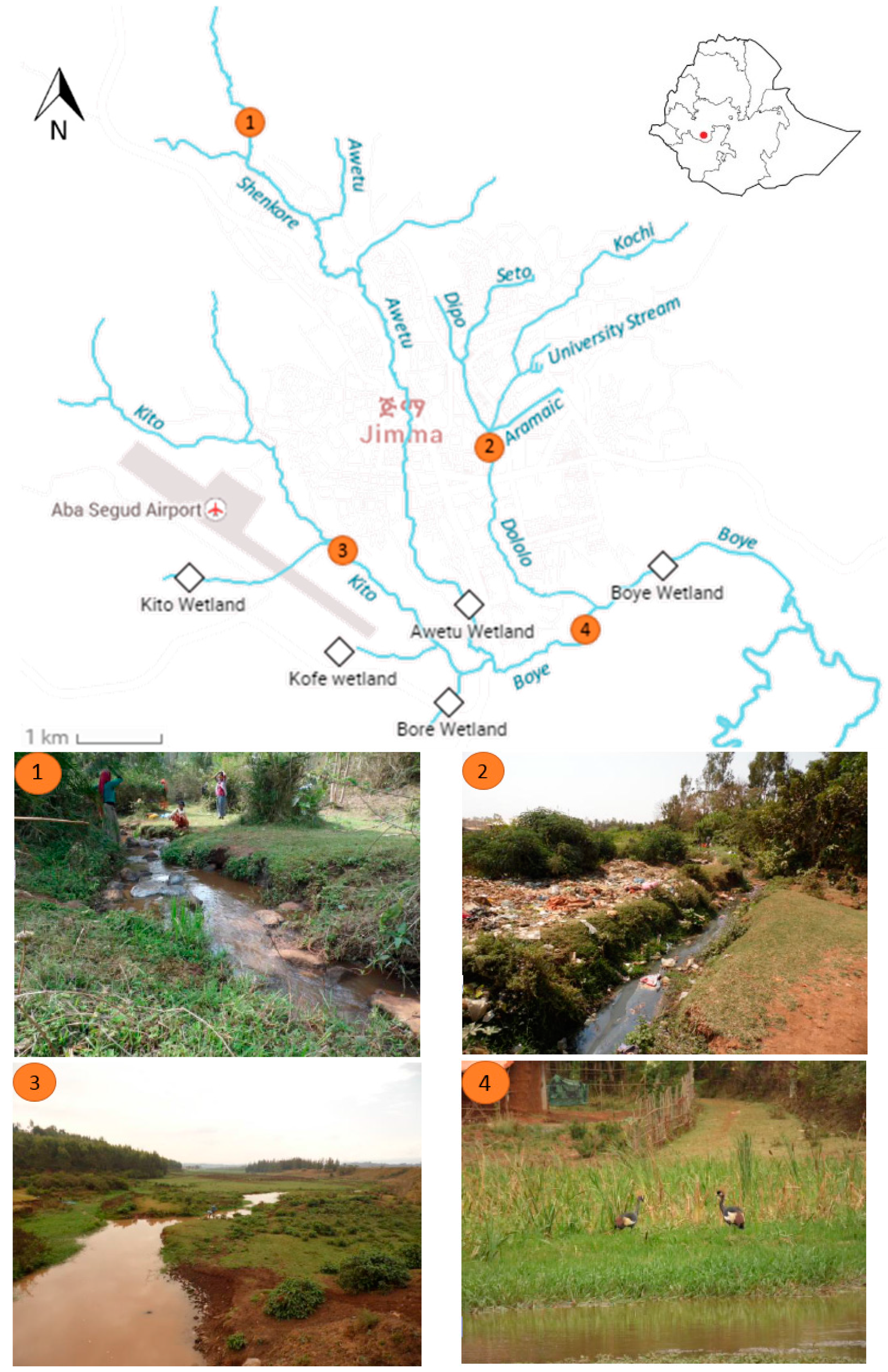
At the technology campus of Jimma University, the water is heavily polluted probably due to the discharge of wastewater originating from the malfunctioning WSP. The relative high nitrate concentration (only 23% of all sampling locations is higher than the measured value of 2.05 mg·L
−1) and relative low DO level (40%) are an indication of the bad functioning of this wastewater treatment system. This is due to the fact that the construction of the WSP is not yet finished because of complaints (odor issues) of local communities. Other major point sources of pollution found in this study are Jimma’s prison and Jimma University. The discharge of organic pollutants into the river is reflected by the strongly increased EC (and Cl
−), the drop in DO (due to nitrification and biological oxidation), and, linked with this, a decline in chemical water quality (from polluted to heavily polluted). Additionally, significant higher amounts of TAN are detected after the discharge point, indicating that wastewater contributes most to the pollution of the rivers. Jimma’s prison has a septic tank to treat its wastewater, which is located approximately 100 m away from the river Seto. During sampling, the septic tank was not functional, as it was under maintenance. This led to a direct discharge of the untreated wastewater into the river, explaining the considerable decrease in water quality after the prison facility. If the septic tank is designed and operated properly, a reduced impact on the riverine environment can be expected. However, a subsurface flow of groundwater contaminated by the effluent can still pollute the river [
40].
Surprisingly, the water quality did not change due to the newly built slaughterhouse and the dry coffee processing plant, which were assumed to be important point sources of pollution. Additionally, no significant changes were observed in nutrient status and the level of DO, indicating their limited impact on the freshwater environment. However, this does not mean that the current business management protects the river from pollution. The WSP of the new abattoir was not functional and did not treat the effluent from the factory, indicating its potential of polluting Awetu upstream. The research from Haddis
et al. [
4] proved that the abattoir and dry coffee processing plants discharge high amounts of organic waste, depleting oxygen levels. The authors found that BOD
5 was 11 times higher after the investigated abattoir and 37 times after the dry coffee processing plant, while oxygen levels decreased with a factor 3.5 and 4.5, respectively. Consequently, wastewater treatment is indispensable to minimize deterioration of the natural environment resulting from human activities. Having this in mind, the WSP of the new abattoir needs to be employed and optimized.
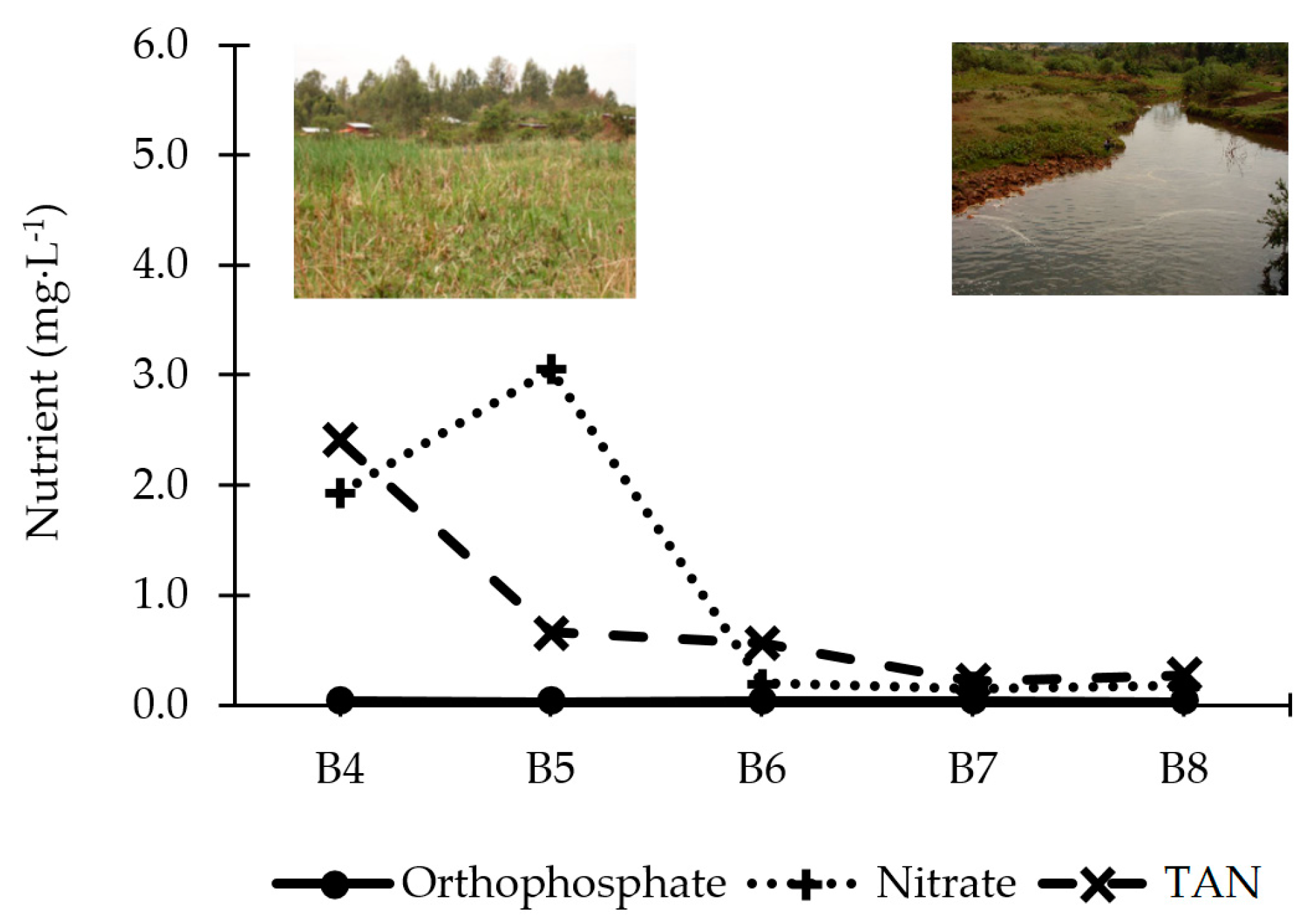
South of Jimma, the highly polluted rivers flow together to the natural wetland of Boye, which is expected to purify the water. However, our results indicate that the ecological and chemical water quality does not significantly improve in Zone D. Nonetheless, there is some evidence that indicates its purification potential. Firstly, a decrease in nitrogen levels is observed along the wetland (
Figure 10). This pattern is also verified by Teferi
et al. [
13], who constructed a physicochemical profile for Boye pond in 2005. The consumption of these nutrients leads to proliferation of vegetation in the wetland and prohibits downstream areas from becoming overgrown by vegetation. Compared to the standards in
Table 3, nutrient levels are sufficiently low to protect the natural environment. Secondly, the concentration of fecal and total coliforms is reduced by 68.8% and 91.9% after the water has passed Boye wetland, indicating its removal potential of pathogens.
Fecal contamination of the rivers is a major problem in Jimma, which can considerably affect human health. The assessed rivers are water resources for irrigation and domestic activities, exposing humans to the exceedingly high concentrations of fecal and total coliforms. The major sources of microbial contamination are practices of open defecation, livestock breeding, and the discharge of untreated wastewater [
41]. We suspect that, in the upstream areas, cattle is responsible for the fecal contamination, as agriculture is the main activity, while, in the midstream reaches, the poor sanitation mainly pollutes the water with coliforms. This could have been verified by DNA analysis techniques (e.g., qPCR), but this was beyond the scope of the present study.
4.2. Water Quality Improvement Options
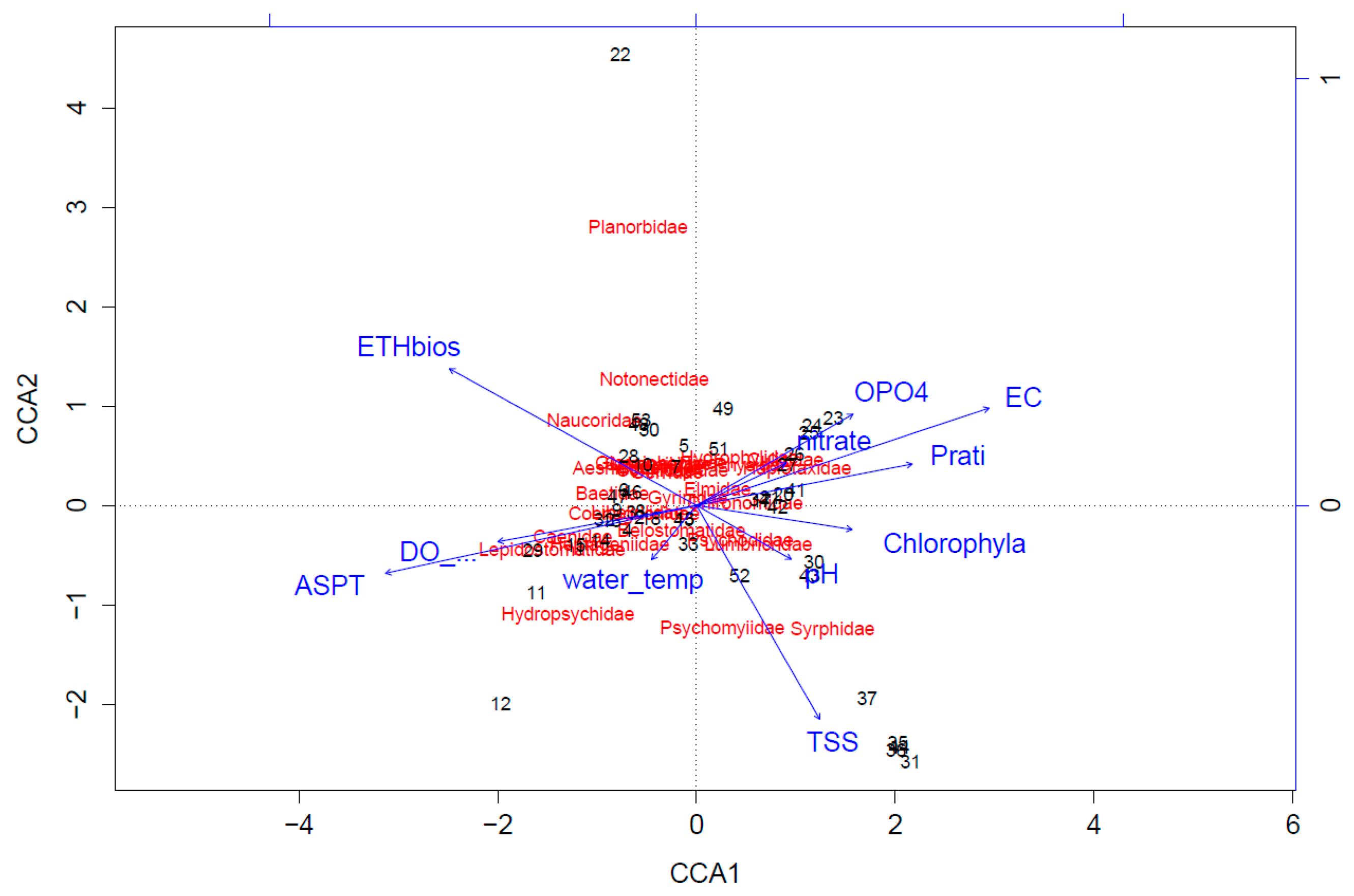
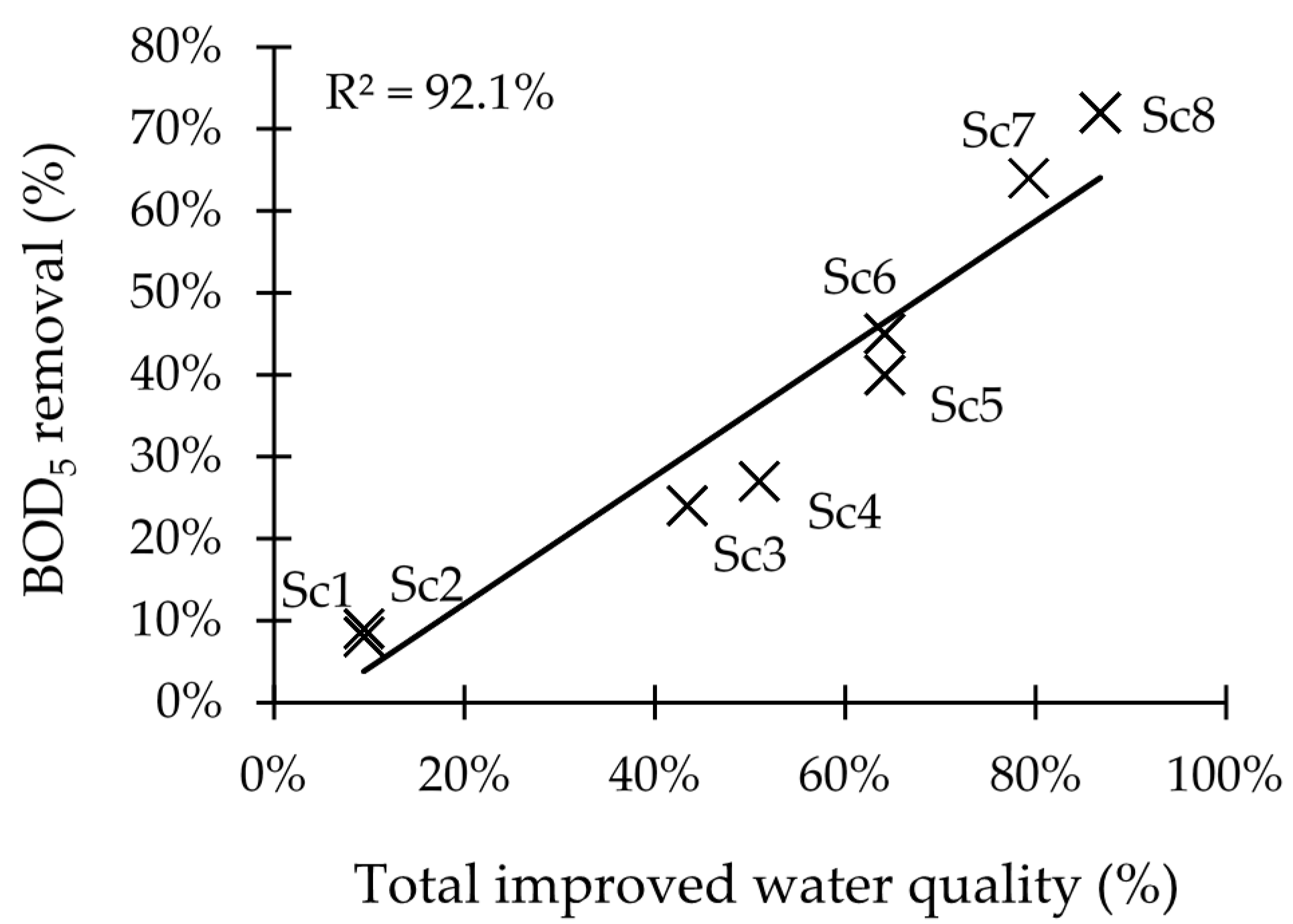
In order to reduce human-induced pollution (e.g., organics, nutrients, and chemicals) and to improve the water quality, it is imperative to tackle the major issues. There is an urgent need to have a proper sanitation infrastructure. The scenario analysis reveals that more locations show better chemical water quality if less organics enter the river ecosystem due to reduced oxygen consumption by biological reactions. This will also have a positive effect on the ecological water quality (ETHbios) according to the CCA plot. The results from this model should be seen as indicative, as it only considers dissolved oxygen and organics while surface water quality depends on several other water characteristics such as nutrient status, pH, heavy metals, EC, turbidity, minerals, temperature, hydromorphology, and biological factors [
42]. Nonetheless, efficient protection of the freshwater environment from organic pollution can be achieved via connecting more people to the sewerage system (increase α) that transports sewage to WWTPs with high removal efficiencies (increase ε). After that, the implementation of WWTPs to treat the wastewater from Jimma University, the WSP at Jimma University technology campus, and the prison facility, which are the major point sources of pollution in this research, could lead to a significant improvement of the chemical water quality of the adjacent freshwater ecosystems, indicating the necessity to treat this type of wastewater.
Regarding wastewater treatment technologies, the conventional activated sludge system (CAS) has proven to be very efficient, but from a financial point of view it is more interesting to invest in waste stabilization ponds (WSP) and constructed wetlands (CW) in developing countries. According to Mburu
et al. [
27], the total cost ranges from 12 to 33 €·PE
−1·year
−1, of which the highest cost is related to land purchase, whereas the cost for operating a CAS is at least three times higher. A disadvantage of the proposed systems is the area they require: 4 to 25 times more than a CAS does [
27]. Mara [
43] state that a CW requires more land and is thus more expensive than a WSP, making the latter a more attractive technology. Next to cost and land use, the performance of the treatment systems need to be taken into consideration. Mburu
et al. [
27], for example, tested the performance of a WSP and a horizontal subsurface flow constructed wetland (HSSF-CW) in Juja town (Kenya). The authors reported removal efficiencies of 91% for BOD
5 and TSS, 76% for COD, 56% for NH
4+-N, and 21% for TP (total phosphorous), leading to the conclusion that the amount of organics is more reduced in the treatment system than the nutrient level. This is even more pronounced in the results of the unplanted HSSF-CW: 83% reduction for BOD
5, 77% for COD, 71% for TSS, and 13% for the TP. The model used for the scenario analyses in this research predicts the chemical water quality based on this high removal efficiency of organics, explaining the strong decrease in oxygen Prati values for the point sources of pollution. However, the discharge of nutrients also has a substantial influence on the water quality, as this may lead to eutrophication, indicating the importance of implementing this factor in the model. This may be particularly important near Jimma University and the prison facility, as the nutrients level in the river increase strongly after passing these point sources of pollution. Additionally, the ecological water quality is affected by the nutrient status. Stronger reduction in nutrients will not only be beneficial for the chemical water quality, but will have a positive effect on the macroinvertebrate community and will probably lead to better ecological water quality [
42]. In general, the performance mainly depends on the type of macrophytes used, system configuration, pollutant loading, hydrologic regime, and temperature [
44]. Regarding the latter, the tropical climate ensures relatively high temperatures and radiation, allowing year-round plant growth in CW and an increase in the microbiological activity which improves the pollutant removal efficiency [
45]. WSPs and CWs are also known to reduce the level of pathogens significantly, making it a promising technology to reduce the fecal contamination of the assessed rivers [
46,
47,
48]. This was observed in our study for the natural wetland of Boye, indicating the importance of these ecosystems for ecosystem services. A final advantage of WSPs and CWs is the high performance reliability, meaning that the probability of malfunctioning is low once they are properly designed, built, operated, and maintained [
49]. We recommend treating wastewater first in a WSP before it goes to a CW in order to prevent overloading of the latter [
50]. Finally, the combined system should be designed in such a way that the water from the rivers in Jimma can be used at least for agricultural and domestic activities in a safe way.
Diffuse sources of pollution are more difficult to address than point sources. In Jimma, there are already some initiatives that deal with solid waste. Containers are placed in overcrowded residential and commercial areas and are emptied by the municipality. Additionally, a few microscale enterprises collect waste from door to door. Afterwards, the garbage is dumped at a site on the road from Jimma to Seka near Kito Wetland (
Figure 11). Getahun
et al. [
51] estimated that only 25% of the community uses the municipal containers, and private waste collection applies to only 2% of the households. The majority of the population (51%) still randomly dumps their waste or incinerates it at an open area (22%).
An improvement of the current waste management is indispensable for sustainable development. Firstly, environmental awareness of the community should be increased. This can be achieved via sensitization of people (e.g., signboards, group discussions, media, posters, and education) and reinforcement of environmental laws, the lack of which currently impedes correct waste management. In general, collaboration between public and private sectors is essential [
51]. Next to NGOs and the Ethiopian government, Jimma University is perceived as a centre of excellence regarding this matter [
52]. It is Ethiopia’s first innovative community-oriented education institution of higher learning. Teaching, research, extension, and public services are the core tasks of the university. Practical training and education has been given through community-based education (CBE). Students involved in this program learn to assess environmental issues in the community for which an action plan has to be developed. This plan is implemented via mobilization of the community and participation of private and governmental sectors [
53].
Secondly, as waste is inevitable, collection and waste treatment should be reconsidered and optimized. In the Waste Framework Directive 2008/98/EC [
54], incineration without gas treatment and uncontrolled dumping are detrimental for the environment and should thus be avoided. On the other hand, recycling, composting, and waste incineration with energy recovery are technologies that are very promising in that they treat waste in a sustainable way. Incineration facilities with energy recovery and gas treatment, which are used in many developed countries, imply a relative high financial start-up and operational capital, making it difficult to successfully implement in developing countries [
55]. On the contrary, as 30% of the generated waste in Jimma is recyclable and 54% is biodegradable, there is a high potential for material recovery and conversion of waste into an economic valuable product through composting and recycling [
51]. However, there are some important factors impeding the implementation of these technologies. According to Troschinetz & Mihelcic [
56], socio-economic status is surprisingly not the limiting factor, but personnel education, waste collection and segregation, and government finances are considered to be the three biggest barriers to recycling in developing countries.