Salts Removal from Synthetic Solution-Potash Brine by Non-Planted Constructed Wetlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Brine Preparation
2.2. Instrumentation and Analysis
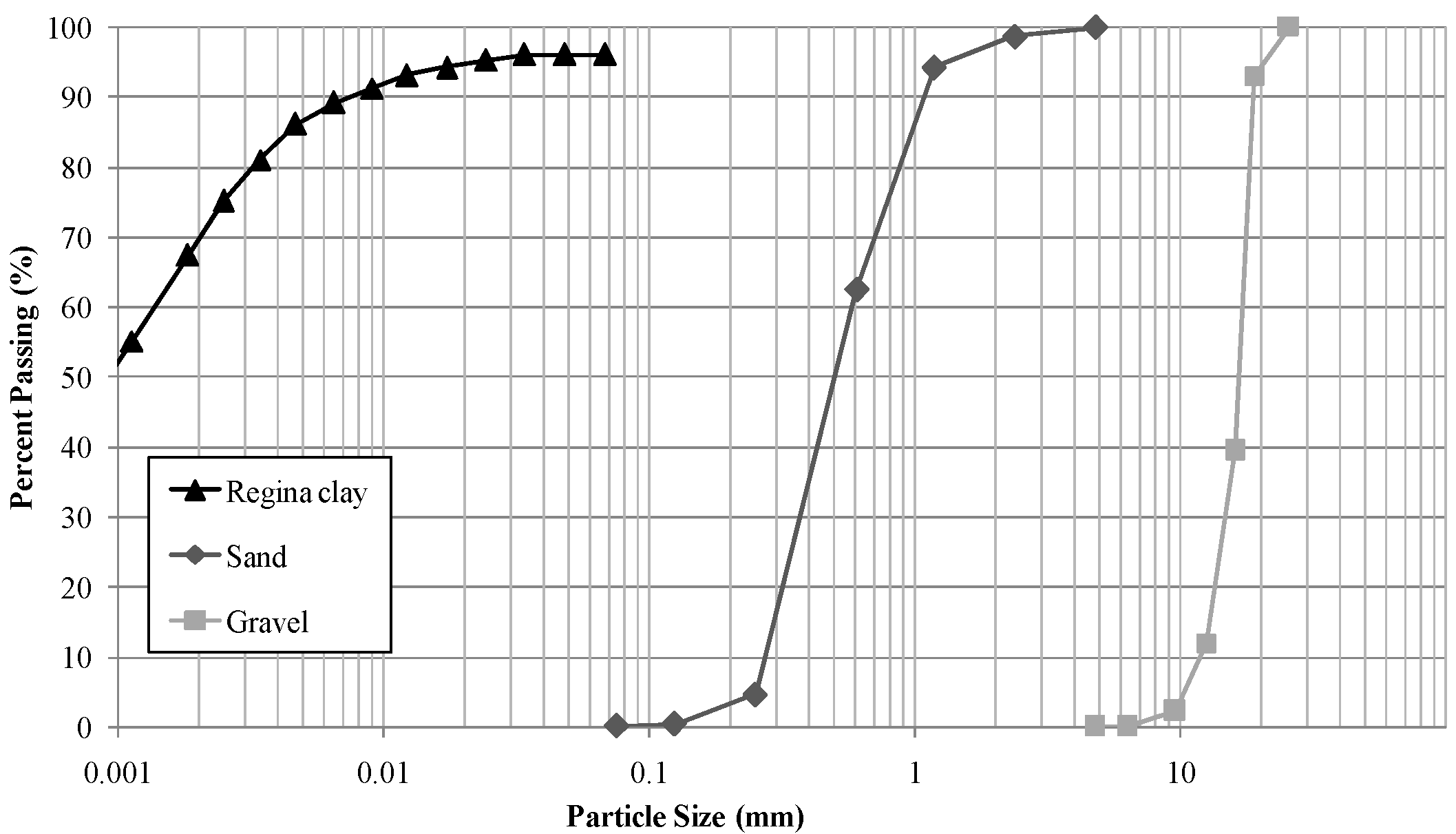
2.3. Experimental Cell Design and Multi-Layer Soils
2.4. Experiments
3. Results and Discussion
3.1. CW System Performance and Removal Efficiencies
3.2. Cation Removal
3.3. Chloride Ion Removal
3.4. Flushing Stage
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saskatchewan Power Corporation. An Operating Manual and Performance Evaluation Guide for the Biological Aspects of the Sask Power Constructed Wetlands; Lakshman, G., Ed.; Saskatchewan Power Corporation: Regina, SK, Canada, 1994. [Google Scholar]
- Saskatchewan Ministry of Agriculture Regulation of Intensive Livestock Operations in Saskatchewan. Available online: https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/livestock/livestock-and-the-environment/regulation-of-intensive-livestock-operations-in-saskatchewan (accessed on 1 February 2016).
- AMEC. Environmental Impact Statement City of Regina Expansion of Fleet Street Solid Waste Disposal and Recovery Facility; City of Regina, Public Works Division: Regina, SK, Canada, 2009. [Google Scholar]
- Azam, S.; Shah, I.; Raghunandan, M.E.; Ito, M. Study on swelling properties of an expansive soil deposit in Saskatchewan, Canada. Bull. Eng. Geol. Environ. 2013, 72, 25–35. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Dawi, N.H.; Tawfik, A. Non-Vegetated Constructed Wetland with Graded Sand Bed System for Hazardous Landfill Leachate Treatment and Heavy Metals Removal. Available online: http://webcache.googleusercontent.com/search?q=cache:Vw6DoYE6zpsJ:waset.org/pdf/books/%3Fid%3D40018%26pageNumber%3D1407+&cd=1&hl=en&ct=clnk&gl=ca (accessed on 22 March 2016).
- Yesiller, N.; Miller, C.J.; Inci, G.; Yaldo, K. Desiccation and cracking behavior of three compacted landfill liner soils. Eng. Geol. 2000, 57, 105–121. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Li, Y.; Ruan, X. Effects of leachate infiltration and desiccation cracks on hydraulic conductivity of compacted clay. Water Sci. Eng. 2015, 8, 151–157. [Google Scholar] [CrossRef]
- MDH Engineering Solution Corp. Environment Impact Statement Mosaic Potash Belle Plaine Expansion Main Document; MDH Engineering Solution Corp.: Saskatoon, SK, Canada, 2009. [Google Scholar]
- ASTM COMPASS® 04.02. Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates; ASTM C136—06; ASTM COMPASS: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM COMPASS® 04.08. Standard Test Method for Particle-Size Analysis of Soils; ASTM D422—63(2007); ASTM COMPASS: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Hoffmann, H.; Platzer, C.; Winker, M.; Muench, E. Von Technology Review of Constructed Wetlands—Subsurface Flow Constructed Wetlands for Greywater and Domestic Wastewater Treatment; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH: Eschborn, Germany, 2011. [Google Scholar]
- Ito, M. Effect of seasonal climate on volume change behaviour of Regina clay. Masters Abst. Int. 2009, 49, 2. [Google Scholar]
- ASTM COMPASS® 04.08. Standard Test Methods for Laboratory Determination of Water (Moisture) Content of Soil and Rock by Mass; ASTM D2216—10; ASTM COMPASS: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ASTM COMPASS® 04.08. Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils; ASTM D4318—10; ASTM COMPASS: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Bergaya, F.; Lagaly, G.; Vayer, M. Cation and Anion Exchange. In Developments in Clay Science; Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Chapter 12.10; Volume 1, pp. 979–1001. [Google Scholar]
- Holtz, R.D. An Introduction to Geotechnical Engineering, 2nd ed.; Pearson: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Giese, R.F. Colloid and Surface Properties of Clays and Related Minerals; Surfactant Science Series; M. Dekker: New York, NY, USA, 2002. [Google Scholar]




| Syn. Brine | NaCl:KCl | Dissociation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+, mg/L | K+, mg/L | Cl−, mg/L | ||||||||
| Concentration | mg/L:mg/L | Eq | ISE | sd | Eq | ISE | sd | Eq | ISE | Sd |
| C1 | DI water | 0 | 1.9 | 1.3 | 0 | 0.4 | 0.3 | 0 | 1 | 0.7 |
| C2 | 75:7.5 | 37.5 | 28.1 | 6.7 | 3.8 | 4.9 | 0.8 | 41.3 | 38.6 | 1.9 |
| C3 | 150:15 | 75 | 56.9 | 12.8 | 7.5 | 8.2 | 0.5 | 82.5 | 81.2 | 0.9 |
| C4 | 300:30 | 105 | 117.8 | 22.8 | 15 | 17.3 | 1.6 | 165 | 171.6 | 4.6 |
| ISEs | Reference Electrode | pH/Ion 3400i | Conditioning | Storing | Standard Solution (STD) | Calibration | |
|---|---|---|---|---|---|---|---|
| Series (mg/L) | Slope Check (mV) | ||||||
| Type 10-205-3064Na | R-503/D | Required | 103 mg/L of STD Na+ | 1 mg/L of STD Na+ | 10 g/L Na+(NaCl) | 10, 102, 103 | 56–58 |
| K-800 | Not required | Required | 103 mg/L of STD K+ | 1 mg/L of STD K+ | 10 g/L K+ (KCl) | 10, 102, 103 | 56–58 |
| Cl-800 | Not required | Required | 103 mg/L of STD Cl− | 1 mg/L of STD Cl− | 10 g/L Cl− (NaCl) | 10, 102, 103 | 56–58 |
| Parameter | Unit | Value | Test Method |
|---|---|---|---|
| Field dry density | g/cm3 | 1.37 | [13] |
| Specific gravity, Gs | - | 2.71 | [13] |
| Dry density, ɤd | g/cm3 | 1.02 | ɤd = ɤ/(1 + w) |
| Bulk density, ɤ, Db | g/cm3 | 1.4 | Core Method |
| Particle density, Dρ | g/cm3 | 2.21 | Core Method |
| Porosity, ɳ | - | 0.4 | ɳ = 1 − (Db/Dρ) |
| Bed volume | cm3 | 2740 | ɳ = VV/VT, VV = BV |
| Water content, w | % | 37 | [14] |
| Liquid limit, LL | % | 74.5 | [15] |
| Plastic limit, PL | % | 28.3 | [15] |
| Dimension of CW cells (length × width) | cm2 | 62 × 38 |
| Batch | Operational | Feeding | Cleaning | HRT | Effluent Sampling | ||
|---|---|---|---|---|---|---|---|
| Experiments | Formats | Cells | Stage (Day) | Stage (Day) | (h) | Frequency | Total |
| B 1, 2, 3 | 16-day | 4 | 13 | 3 | 24 | at 24 h | 192 |
| Ion | Day | Influent Concentration | Outflow Concentration | Removal Efficiency |
|---|---|---|---|---|
| (mg/L) | (mg/L) | (%) | ||
| Na+ | 1 | 117.8 ± 7.6 | 64.5 ± 87.1 | 45 |
| K+ | 8 | 4.9 ± 3.2 | 0.3 ± 3.1 | 92 |
| Cl− | 1 | 171.6 ± 15.4 | 86.2 ± 195 | 50 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chairawiwut, W.; McMartin, D.W.; Azam, S. Salts Removal from Synthetic Solution-Potash Brine by Non-Planted Constructed Wetlands. Water 2016, 8, 113. https://doi.org/10.3390/w8040113
Chairawiwut W, McMartin DW, Azam S. Salts Removal from Synthetic Solution-Potash Brine by Non-Planted Constructed Wetlands. Water. 2016; 8(4):113. https://doi.org/10.3390/w8040113
Chicago/Turabian StyleChairawiwut, Warawut, Dena W. McMartin, and Shahid Azam. 2016. "Salts Removal from Synthetic Solution-Potash Brine by Non-Planted Constructed Wetlands" Water 8, no. 4: 113. https://doi.org/10.3390/w8040113
APA StyleChairawiwut, W., McMartin, D. W., & Azam, S. (2016). Salts Removal from Synthetic Solution-Potash Brine by Non-Planted Constructed Wetlands. Water, 8(4), 113. https://doi.org/10.3390/w8040113







