Response of Vallisneria natans to Increasing Nitrogen Loading Depends on Sediment Nutrient Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling
2.3. Statistical Analyses
3. Results
3.1. Sediments
3.2. Nutrients
3.3. Phytoplankton and Periphyton
3.4. Macrophytes
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Mazzeo, N.; Meerhoff, M.; Branco, C.C.; Huszar, V.; Scasso, F. Lake restoration and biomanipulation in temperate lakes: Relevance for subtropical and tropical lakes. In Tropical Eutrophic Lakes: Their Restoration an Management; Vikram, R., Ed.; Science Publishers Inc.: Enfield, NH, USA, 2005; p. 533. [Google Scholar]
- Babourina, O.; Rengel, Z. Nitrogen removal from eutrophicated water by aquatic plants. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Sarvajeet, S.G., Guy, R.L., Walter, R., Eds.; Springer: Heidelberg, Germany, 2010; pp. 355–372. [Google Scholar]
- Beklioglu, M.; Altinayar, G.; Tan, C.O. Water level control over submerged macrophyte development in five shallow lakes of Mediterranean Turkey. Fund. Appl. Limnol. 2006, 166, 535–556. [Google Scholar] [CrossRef]
- Kadlec, R.H. Free surface wetlands for phosphorus removal: The position of the Everglades Nutrient Removal Project. Ecol. Eng. 2006, 27, 361–379. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1045. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.; Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Liu, Z.W. Nitrogen, macrophytes, shallow lakes and nutrient limitation: Resolution of a current controversy? Hydrobiologia 2013, 710, 3–21. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Meerhoff, M.; Lauridsen, T.L.; Jensen, J.P. Shallow lake restoration by nutrient loading reduction-some recent findings and challenges ahead. Hydrobiologia 2007, 584, 239–252. [Google Scholar] [CrossRef]
- James, C.; Fisher, J.; Russell, V.; Collings, S.; Moss, B. Nitrate availability and hydrophyte species richness in shallow lakes. Freshw. Biol. 2005, 50, 1049–1063. [Google Scholar] [CrossRef]
- Barker, T.; Hatton, K.; O’Connor, M.; Connor, L.; Moss, B. Effects of nitrate load on submerged plant biomass and species richness: Results of a mesocosm experiment. Fund. Appl. Limnol. 2008, 173, 89–100. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R. Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Zhao, S.T.; Yin, L.Y.; Chang, F.Y.; Olsen, S.; Søndergaard, M.; Jeppesen, E.; Li, W. Response of Vallisneria spinulosa (Hydrocharitaceae) to contrasting nitrogen loadings in controlled lake mesocosms. Hydrobiologia 2015, 766, 1–9. [Google Scholar] [CrossRef]
- Gonzales, S.; María, A.; Jeppesen, E.; Gomà, J.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.; Landkildehus, F. Does high nitrogen loading prevent clear-water conditions in shallow lakes at moderately high phosphorus concentrations? Freshw. Biol. 2005, 50, 27–41. [Google Scholar]
- Cao, T.; Xie, P.; Ni, L.Y.; Wu, A.P.; Zhang, M.; Wu, S.K.; Smolders, A.J.P. The role of NH4+ toxicity in the decline of the submersed macrophyte V. natans in lakes of the Yangtze River basin, China. Mar. Freshw. Res. 2007, 58, 581–587. [Google Scholar] [CrossRef]
- Nimptsch, J.; Pflugmacher, S. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere 2007, 66, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Jeppesen, E. The response of Vallisneria spinulosa (Hydrocharitaceae) to different loadings of ammonia and nitrate at moderate phosphorus concentration: A mesocosm approach. Freshw. Biol. 2008, 53, 2321–2330. [Google Scholar]
- Özkan, K.; Jeppesen, E.; Johansson, L.S.; Beklioglu, M. The response of periphyton and submerged macrophytes to nitrogen and phosphorus loading in shallow warm lakes: A mesocosm experiment. Freshw. Biol. 2010, 55, 463–475. [Google Scholar] [CrossRef]
- Gao, Y.X.; Zhu, G.W.; Qin, B.Q.; Pang, Y.; Gong, Z.J.; Zhang, Y.L. Effect of ecological engineering on the nutrient content of surface sediments in Lake Taihu, China. Ecol. Eng. 2009, 35, 1624–1630. [Google Scholar] [CrossRef]
- Wang, S.R.; Jin, X.C.; Jiao, L.X.; Wu, F.C. Response in root morphology and nutrient contents of Myriophyllum spicatum to sediment type. Ecol. Eng. 2009, 35, 1264–1270. [Google Scholar] [CrossRef]
- Ni, L.Y. Effects of water column nutrient enrichment on the growth of Potamogeton maackianus A. Been . J. Aquat. Plant Manag. 2001, 39, 83–87. [Google Scholar]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506–509, 135–145. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E.; Møller, P.H. Seasonal dynamics in the concentrations and retention of phosphorus in shallow Danish lakes during recovery. Aquat. Ecosyst. Health 2002, 5, 19–29. [Google Scholar] [CrossRef]
- Duan, H.T.; Ma, R.H.; Xu, X.F.; Kong, F.X.; Zhang, S.X.; Kong, W.J.; Hao, J.Y.; Shang, L.L. Two-decade reconstruction of algal blooms in China’s Lake Taihu. Environ. Sci. Technol. 2009, 43, 3522–3528. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.Q.; Zhu, G.W.; Gao, G.; Zhang, Y.L.; Wei, L.; Paerl, H.W.; Carmichael, W.W. A drinking water crisis in Lake Taihu, China: Linkage to climatic variability and lake management. Environ. Manag. 2010, 45, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Qu, J.T.; Xu, Q.J.; Hu, X.Z.; Wang, Z.M.; Zhao, W.D. Distribution of nitrogen in water, soil and sediment in water/land ecotone of returning fishpond to lake area on northern Gonghu Bay. J. Agro-Environ. Sci. 2014, 33, 2234–2241. [Google Scholar]
- Zhao, K.; Li, Z.G.; Wei, H.N.; Zhang, J.; Ma, J.Y.; Wang, G.X. The distribution of aquatic vegetation in Gonghu Bay, Lake Taihu, 2012. J. Lake Sci. 2015, 27, 421–428. [Google Scholar]
- Ma, R.H.; Duan, H.T.; Gu, X.H.; Zhang, S.X. Detecting aquatic vegetation changes in Taihu Lake, China using multi-temporal satellite imagery. Sensors 2008, 8, 3988–4005. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Paerl, H.W.; Qin, B.Q.; Zhu, G.W.; Guang, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Jin, X.C.; Tu, Q. The Standard Methods for Observation and Analysis in Lake Eutrophication, 2nd ed.; Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Southeastern Psychological Association. Analytical Methods for Water and Wasterwater Monitor, 4th ed.; Chinese Enviromental Science Press: Beijing, China, 2002. [Google Scholar]
- Shi, H.R. Agricultural and Chemical Analysis for Soil; Chinese Agriculture Press: Beijing, China, 1994. [Google Scholar]
- Novozamsky, I.; Houba, V.J.G.; Temminghoff, E.; Lee, J.J. Determination of ‘total’ N and ‘total’ P in a single soil digest. Neth. J. Agric. Sci. 1984, 32, 322–324. [Google Scholar]
- Cao, T.; Ni, L.Y.; Xie, P. Acute biochemical responses of a submersed macrophyte, Potamogeton crispus L., to high ammonium in an aquarium experiment. J. Freshw. Ecol. 2004, 19, 279–284. [Google Scholar]
- Silveira, M.J.; Harthman, V.C.; Michelan, T.S.; Souza, L.A. Anatomical development of roots of native and non-native submerged aquatic macrophytes in different sediment types. Aquat. Bot. 2016, 133, 24–27. [Google Scholar] [CrossRef]
- Lorenzen, B.; Brix, H.; Mendelssohn, I.A.; Mckee, K.L.; Miao, S.L. Growth, biomass allocation and nutrient use efficiency in Cladium jamaicense and Typha domingensis as affected by phosphorus and oxygen availability. Aquat. Bot. 2001, 70, 117–133. [Google Scholar] [CrossRef]
- Chen, R.L.; Barko, J.W. Effects of freshwater macrophytes on sediment chemistry. J. Freshw. Ecol. 1988, 4, 279–289. [Google Scholar] [CrossRef]
- Baldy, V.; Trémolières, M.; Andrieu, M.; Belliard, J. Changes in phosphorus content of two aquatic macrophytes according to water velocity, trophic status and time period in hardwater streams. Hydrobiologia 2007, 575, 343–351. [Google Scholar] [CrossRef]
- Gerloff, G.C. Evaluating nutrient supplies for the growth of aquatic plants in natural waters. In Eutrophication: Causes, Consequences, Correctives; Proceedings of A Symposium; National Academy of Sciences: Washington, DC, USA, 1969; pp. 537–555. [Google Scholar]
- Li, K.Y.; Liu, Z.W.; Gu, B.H. Compensatory growth of a submerged macrophyte (Vallisneria spiralis) in response to partial leaf removal: Effects of sediment nutrient levels. Aquat. Ecol. 2010, 44, 701–707. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Zhu, Z. Growth response of the submerged macrophyte Myriophyllum spicatum to sediment nutrient levels and water-level fluctuations. Aquat. Biol. 2012, 17, 295–303. [Google Scholar]
- Wang, S.; Zhao, H.; Wang, J.; Yang, S.; Jiao, L.; Jin, X. The effects of organic matter on the release kinetics of nitrogen with different forms in the lake sediments. Acta Sci. Circumst. 2012, 32, 332–340. [Google Scholar]
- Xiang, S.L.; Zhu, M.Y.; Zhu, G.W.; Xu, H. Pollution characteristics of nitrogen and phosphorus in sediment of eastern bays of Lake Taihu with aquatic macrophytes. Acta Sedimentol. Sin. 2014, 32, 1083–1088. [Google Scholar]
- Zhu, G.W.; Qin, B.Q.; Gao, G. Direct evidence of phosphorus outbreak release from sediment to overlying water in a large shallow lake caused by strong wind wave disturbance. Chinese Sci. Bull. 2005, 50, 577–582. [Google Scholar] [CrossRef]
- Scheffer, M.; Portielje, R.; Zambrano, L. Fish facilitate wave resuspension of sediment. Limnol. Oceanogr. 2003, 48, 1920–1926. [Google Scholar] [CrossRef]
- Hu, K.M.; Wang, S.; Pang, Y. Suspension-sedimentation of sediment and release amount of internal load in Lake Taihu. J. Lake Sci. 2014, 26, 191–199. [Google Scholar]
- Severe Situayion of Algae Blooms in Lake Taihu, No Panic with Scientific Methods. Available online: http://xh.xhby.net/mp1/html/2008–04/21/content_6810351.htm (accessed on 21 April 2008).
- Zhu, G.R.; Cao, T.; Zhang, M.; Ni, L.Y.; Zhang, X.L. Fertile sediment and ammonium enrichment decrease the growth and biomechanical strength of submersed macrophyte Myriophyllum spicatum in an experiment. Hydrobiologia 2014, 727, 109–120. [Google Scholar] [CrossRef]
- Wang, L.Z. Response of Potamogeton crispus root characteristics to sediment heterogeneity. Acta Ecol. Sin. 2013, 33, 282–286. [Google Scholar] [CrossRef]
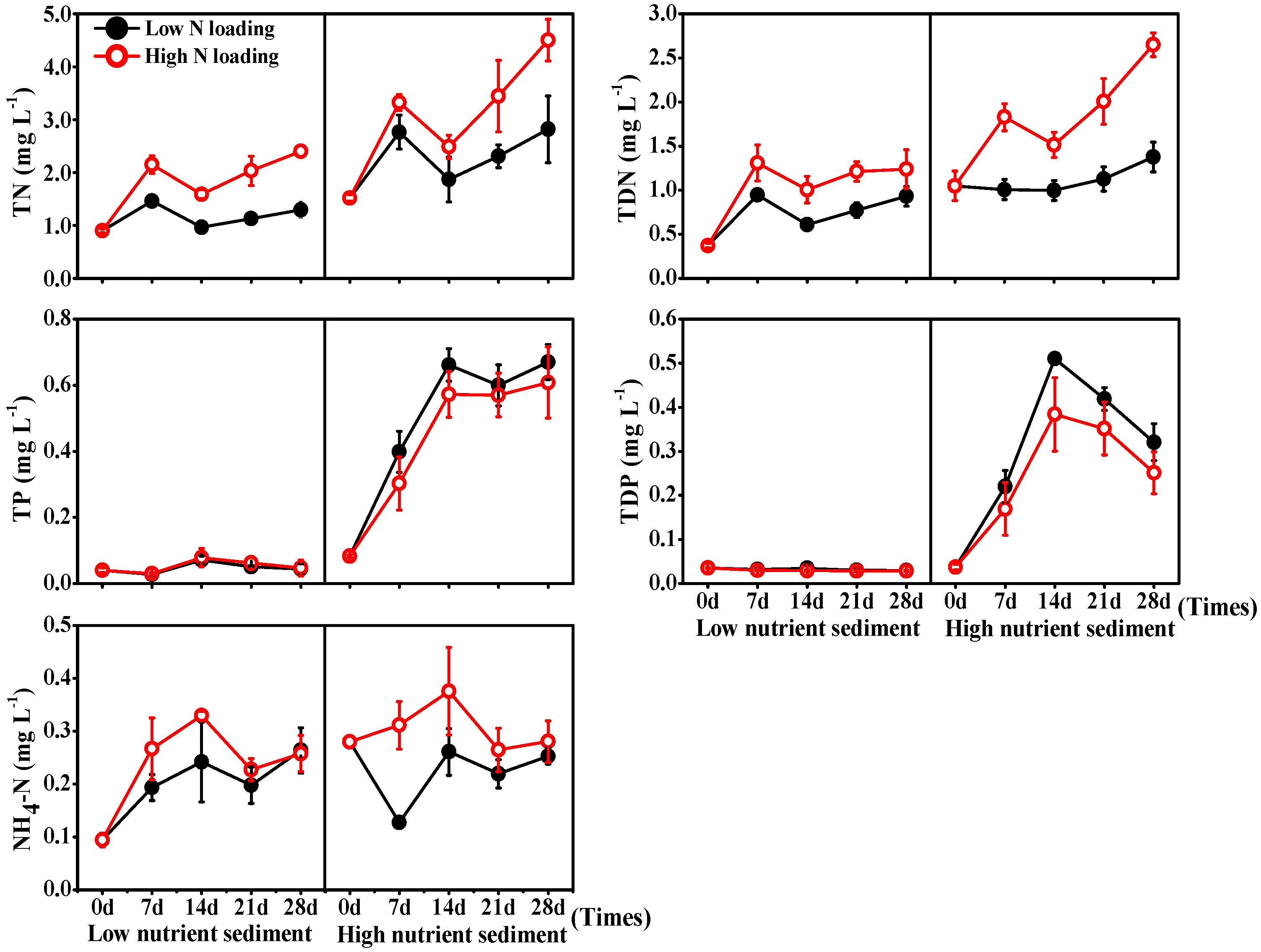
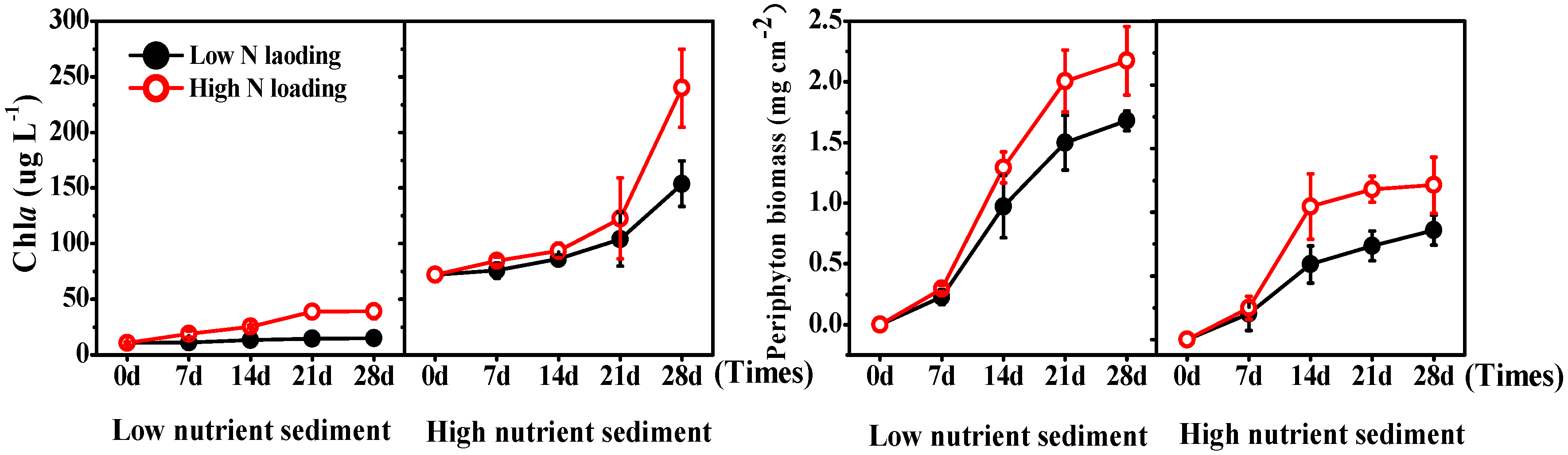
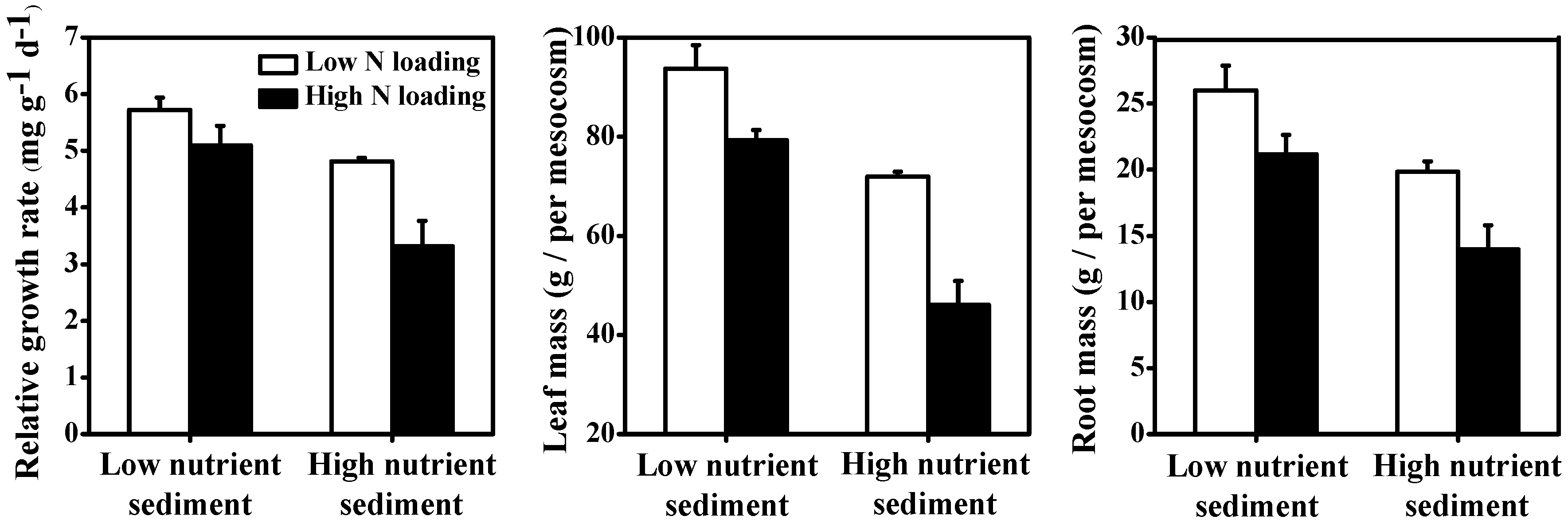
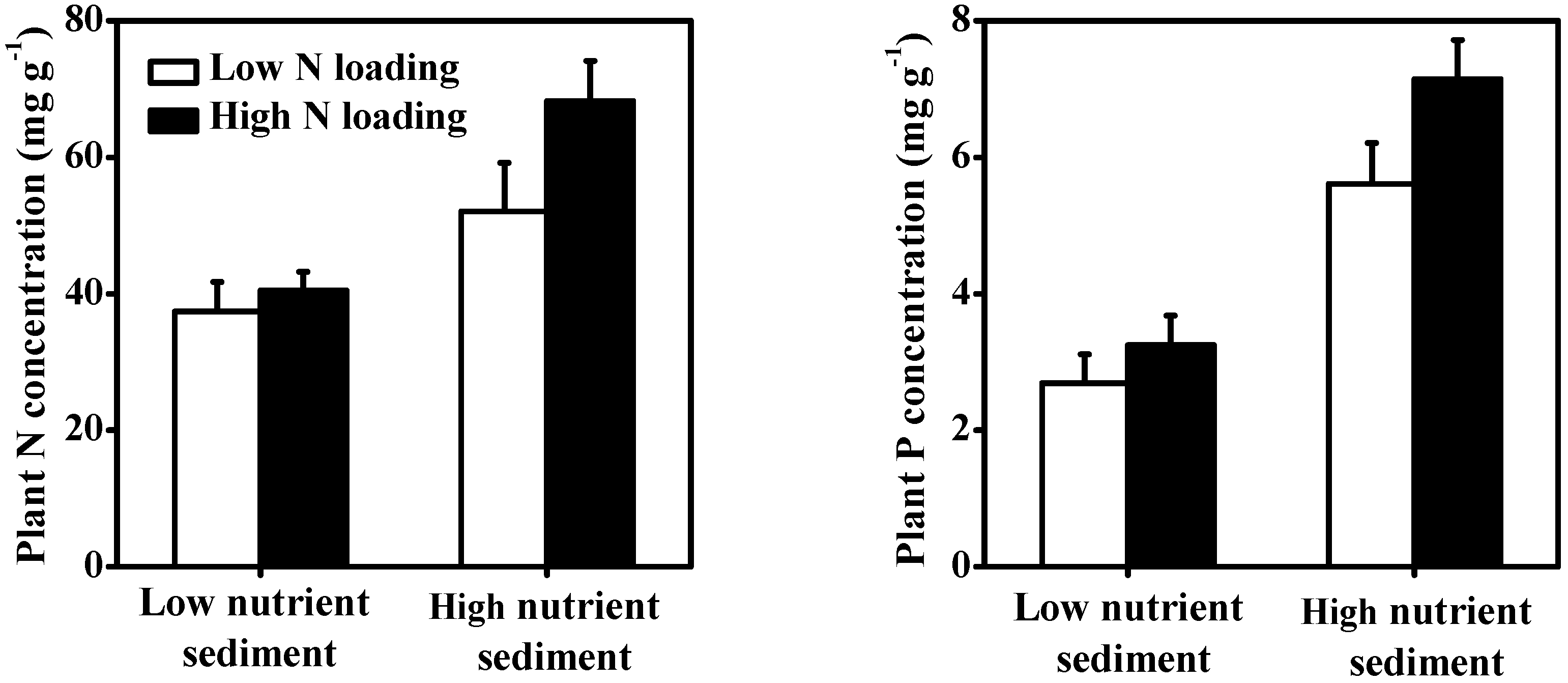
| Sediment Type | Total Nitrogen (mg∙g−1) | Total Phosphorus (mg∙g−1) | Organic Matter (%) |
|---|---|---|---|
| High nutrient sediment | 4.36 ± 0.09 | 1.6 ± 0.06 | 10.68 ± 0.03 |
| Low nutrient sediment | 1.09 ± 0.05 | 0.40 ± 0.04 | 5.39 ± 0.04 |
| p | <0.01 | <0.01 | <0.01 |
| Dependent Variables | Sediment Type (S) | Nitrogen Loading (N) | S × N | |||
|---|---|---|---|---|---|---|
| P | SS (%) | P | SS (%) | P | SS (%) | |
| Relative growth rate | <0.001 | 53.27% | <0.001 | 33.02% | 0.013 | 5.70% |
| Leaf mass | <0.001 | 32.53% | <0.001 | 60.67% | 0.018 | 2.65% |
| Root mass | <0.001 | 55.33% | <0.001 | 35.50% | ns | 0.30% |
| Plant N content | <0.001 | 67.37% | 0.003 | 13.86% | 0.028 | 6.40% |
| Plant P content | <0.001 | 84.58% | ns | 7.95% | 0.030 | 5.32% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Xu, Z.; Jin, H.; Ning, X.; He, H.; Yu, J.; Jeppesen, E.; Li, K. Response of Vallisneria natans to Increasing Nitrogen Loading Depends on Sediment Nutrient Characteristics. Water 2016, 8, 563. https://doi.org/10.3390/w8120563
Gu J, Xu Z, Jin H, Ning X, He H, Yu J, Jeppesen E, Li K. Response of Vallisneria natans to Increasing Nitrogen Loading Depends on Sediment Nutrient Characteristics. Water. 2016; 8(12):563. https://doi.org/10.3390/w8120563
Chicago/Turabian StyleGu, Jiao, Zenghong Xu, Hui Jin, Xiaoyu Ning, Hu He, Jinlei Yu, Erik Jeppesen, and Kuanyi Li. 2016. "Response of Vallisneria natans to Increasing Nitrogen Loading Depends on Sediment Nutrient Characteristics" Water 8, no. 12: 563. https://doi.org/10.3390/w8120563






