Acid Water Neutralization Using Microbial Fuel Cells: An Alternative for Acid Mine Drainage Treatment
Abstract
:1. Introduction
2. Materials and Methods
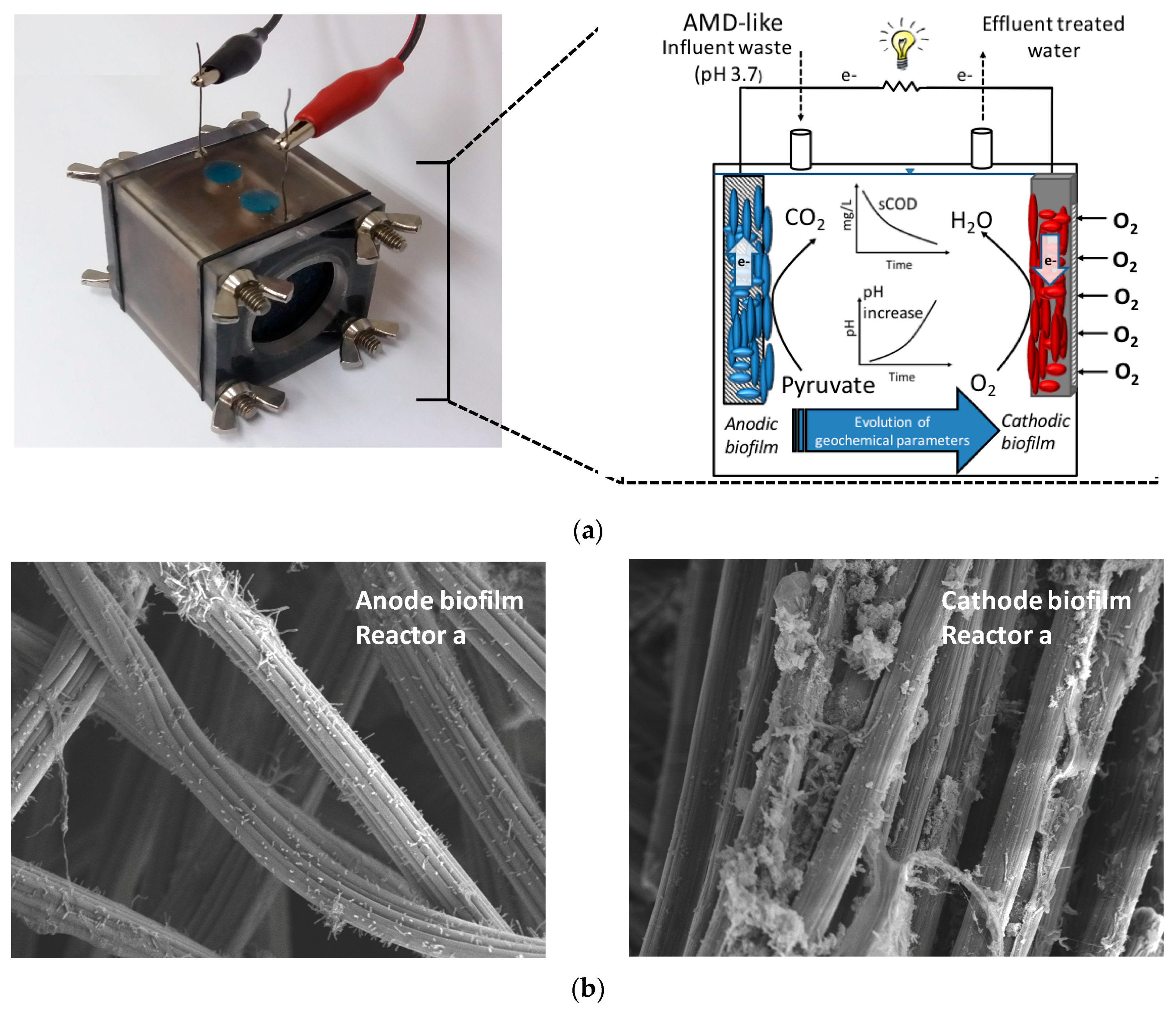
2.1. Single-Chamber MFC Start-Up and Operation
2.2. Continuous-Flow Experiments
2.3. Batch (Non-MFC) Experiments
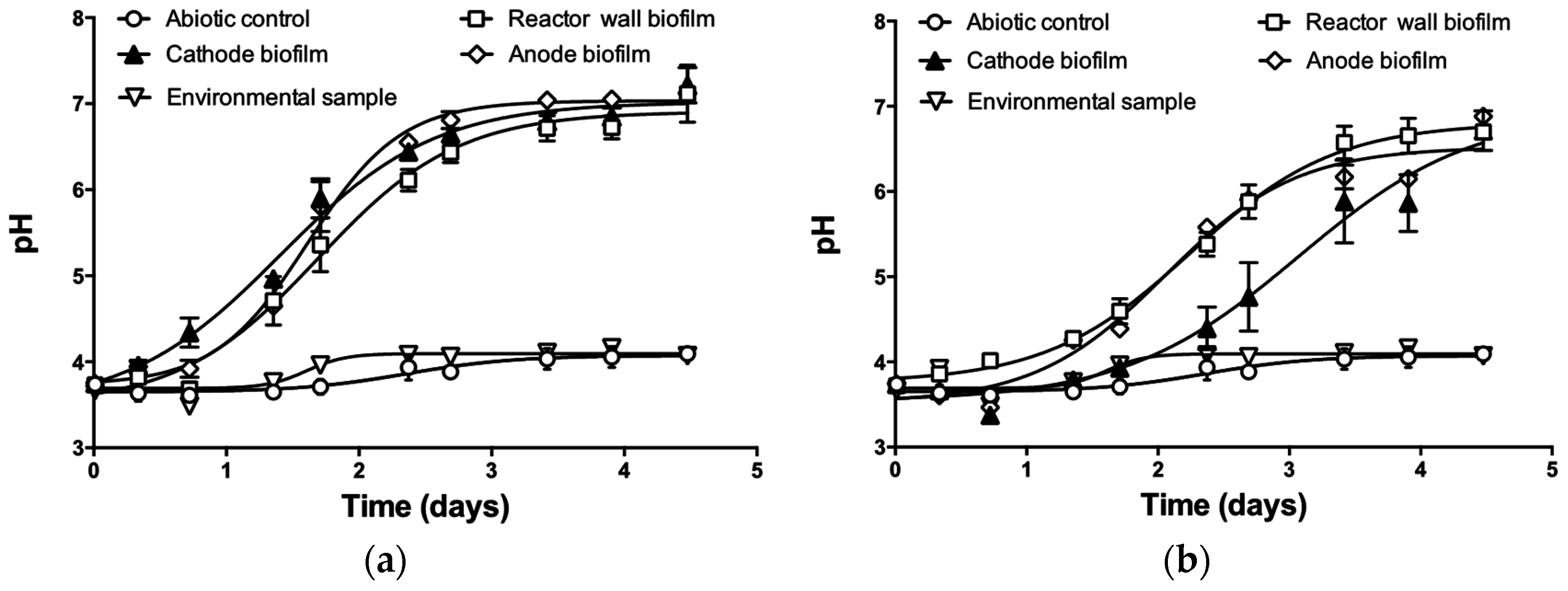
3. Results and Discussion
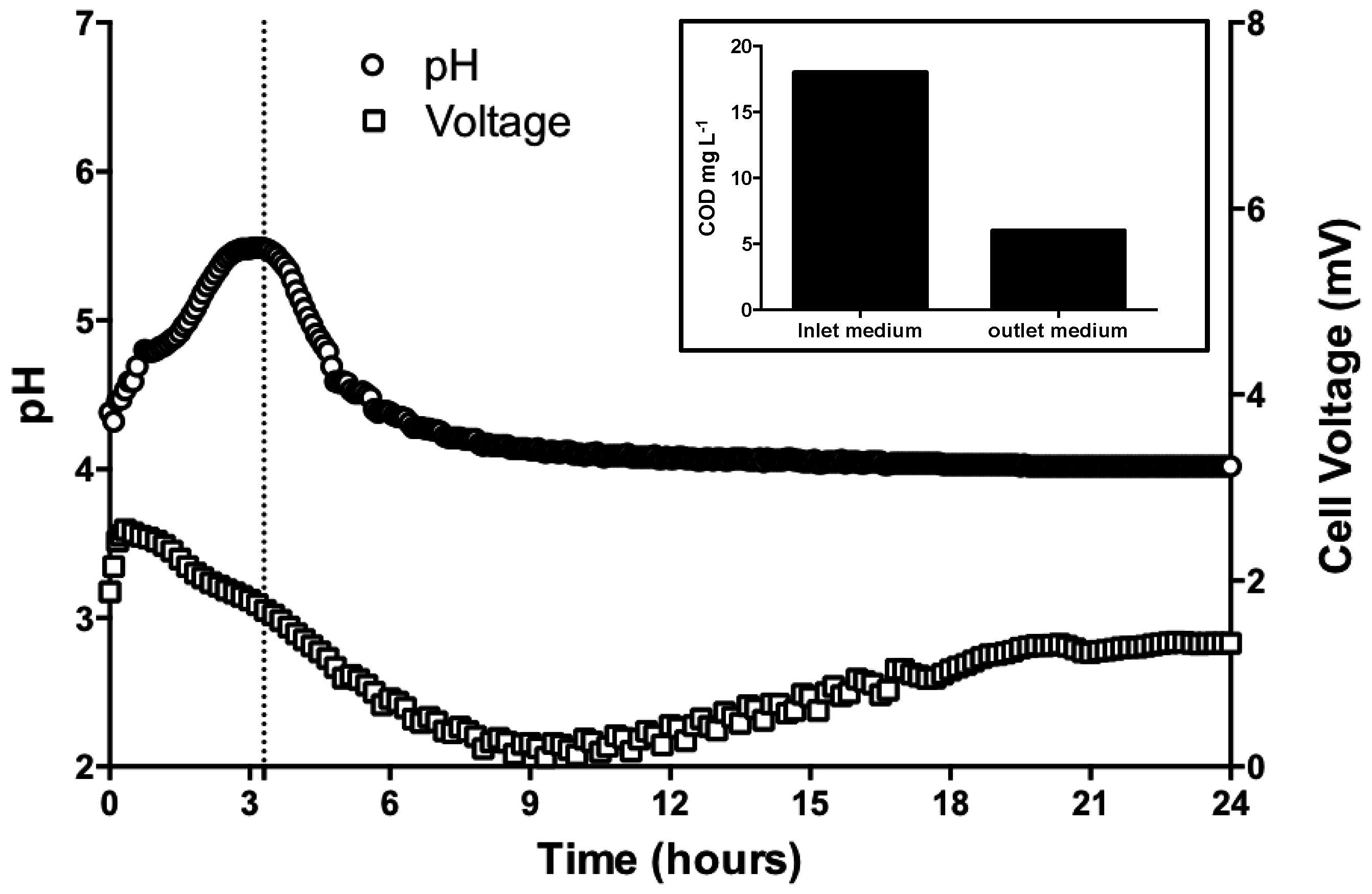
3.1. pH Neutralization by MFC Reactors Operated in Batch-Mode
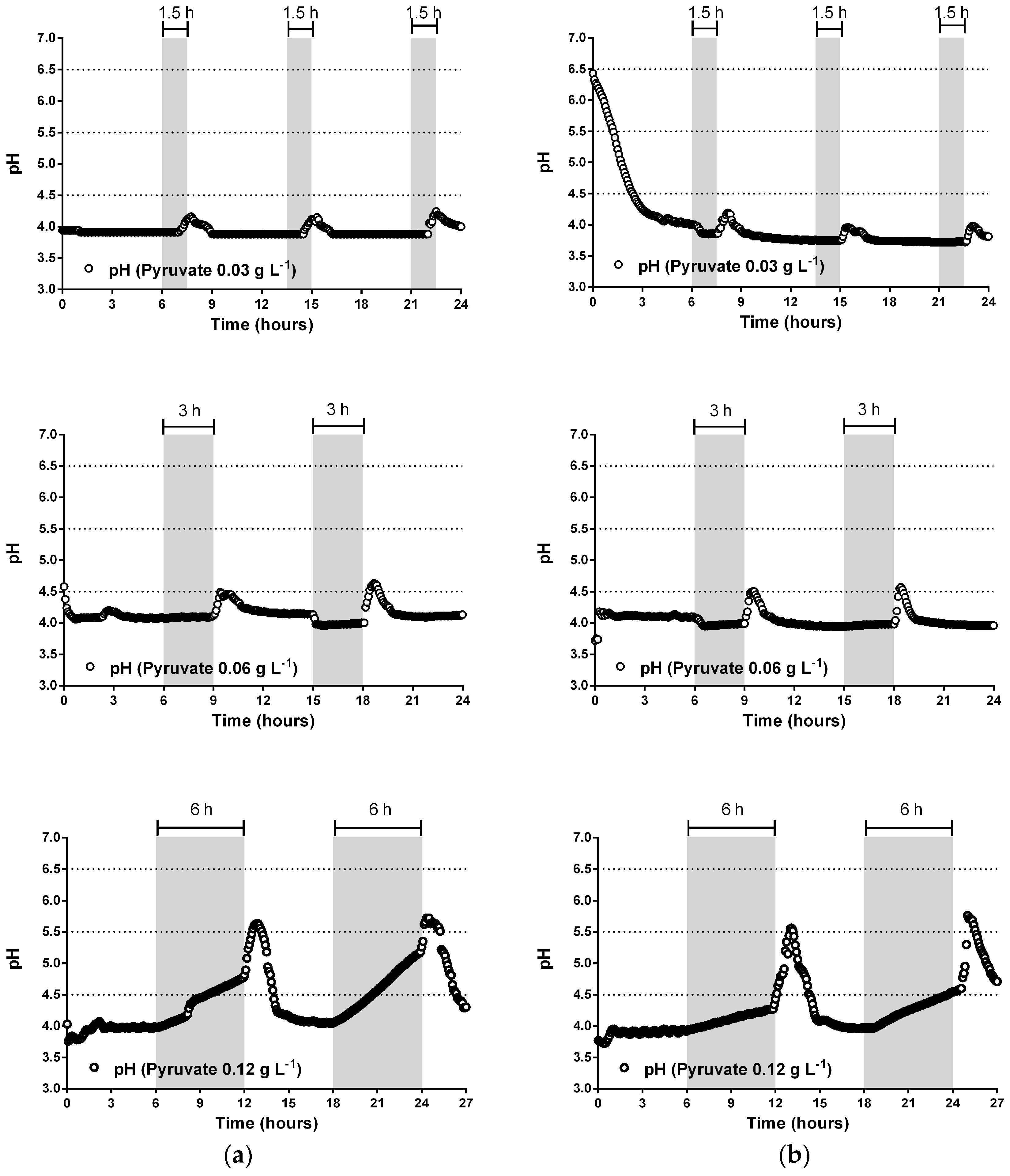
3.2. pH Neutralization and Continuos-Flow Operation Mode
3.3. MFC as an Enrichment Method for Acid-Neutralizing Microorganisms
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
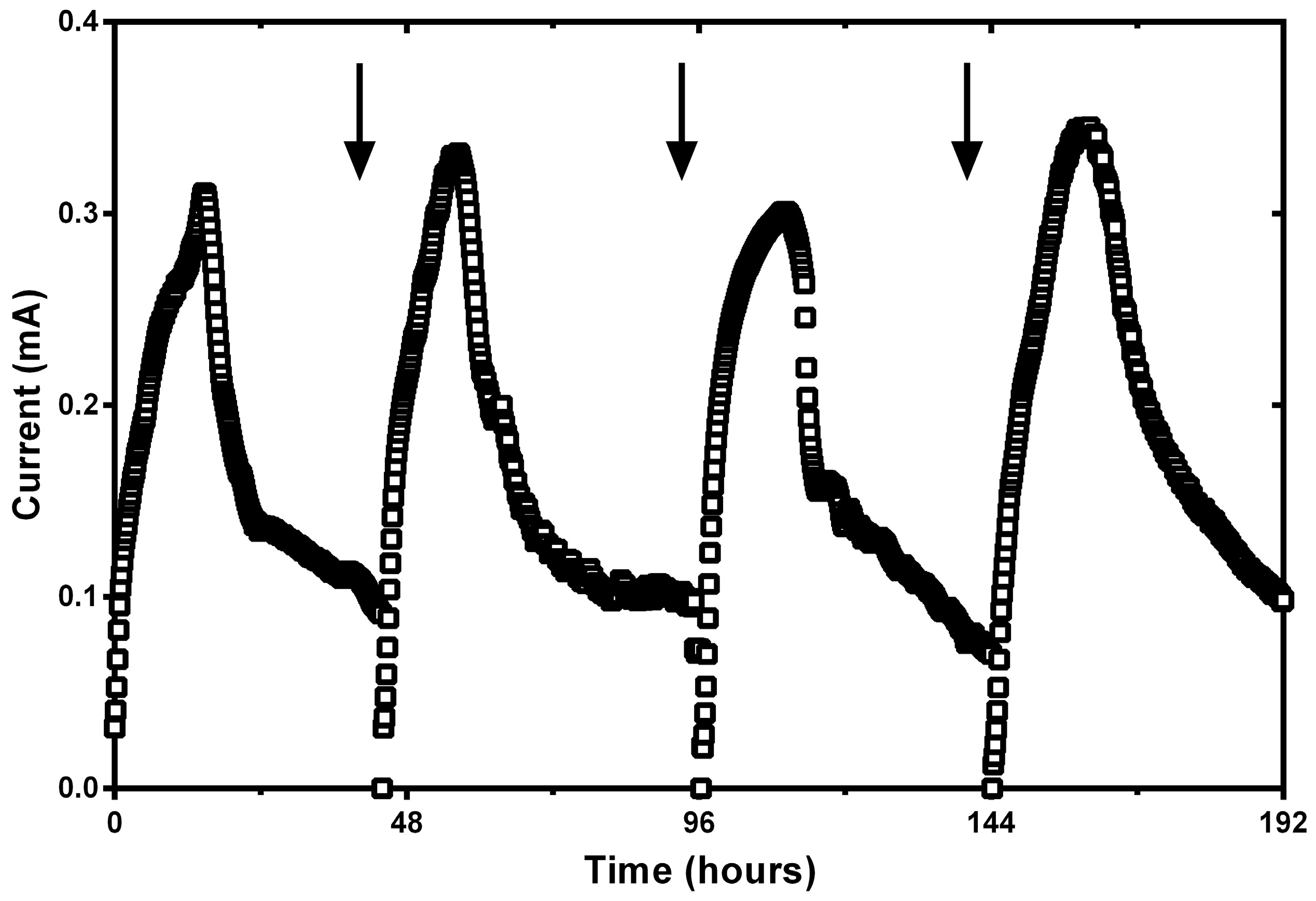
Appendix A. Current Cycles of ~50 h of Duration in SC-MFC Operated under Initial Acidic Conditions (pH = 3.7)
References
- Levings, C.D.; Varela, D.E.; Mehlenbacher, N.M.; Barry, K.L.; Piercey, G.E.; Guo, M.; Harrison, P.J. Effect of an acid mine drainage effluent on phytoplankton biomass and primary production at britannia beach, howe sound, British Columbia. Mar. Pollut. Bull. 2005, 50, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.G.; Blowes, D.W.; Gould, W.D.; Herbert, R.B.; Ptacek, C.J. Geochemistry of a permeable reactive barrier for metals and acid mine drainage. Environ. Sci. Technol. 1999, 33, 2793–2799. [Google Scholar] [CrossRef]
- Cowie, R.; Williams, M.; Wireman, M.; Runkel, R. Use of natural and applied tracers to guide targeted remediation efforts in an acid mine drainage system, Colorado Rockies, USA. Water 2014, 6, 745–777. [Google Scholar] [CrossRef]
- Kalin, M.; Fyson, A.; Wheeler, W.N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Sci. Total Environ. 2006, 366, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Ziemkiewicz, P.F.; Skousen, J.G.; Brant, D.L.; Sterner, P.L.; Lovett, R.J. Acid mine drainage treatment with armored limestone in open limestone channels. J. Environ. Qual. 1997, 26, 1017–1024. [Google Scholar] [CrossRef]
- Burgess, J.E.; Stuetz, R.M. Activated sludge for the treatment of sulphur-rich wastewaters. Miner. Eng. 2002, 15, 839–846. [Google Scholar] [CrossRef]
- Bejan, D.; Bunce, N.J. Acid mine drainage: Electrochemical approaches to prevention and remediation of acidity and toxic metals. J. Appl. Electrochem. 2015, 45, 1239–1254. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, D.; Ma, J.; Huang, G.; Cai, L.; Zhang, L. Behavior of metal ions in bioelectrochemical systems: A review. J. Power Sources 2015, 275, 243–260. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Shantaram, A.; Beyenal, H.; Veluchamy, R.R.A.; Lewandowski, Z. Wireless sensors powered by microbial fuel cells. Environ. Sci. Technol. 2005, 39, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Rahunen, N.; Varcoe, J.R.; Roberts, A.J.; Avignone-Rossa, C.; Thumser, A.E.; Slade, R.C.T. Factors affecting the performance of microbial fuel cells for sulfur pollutants removal. Biosens. Bioelectron. 2009, 24, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-Y.; Zhou, S.-G.; Chen, Q.; Zhao, B.; Yuan, Y.; Zhuang, L. Enhanced anaerobic degradation of organic pollutants in a soil microbial fuel cell. Chem. Eng. J. 2011, 172, 647–653. [Google Scholar] [CrossRef]
- Butler, C.S.; Clauwaert, P.; Green, S.J.; Verstraete, W.; Nerenberg, R. Bioelectrochemical perchlorate reduction in a microbial fuel cell. Environ. Sci. Technol. 2010, 44, 4685–4691. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Dempsey, B.A.; Logan, B.E. Electricity generation from synthetic acid-mine drainage (AMD) water using fuel cell technologies. Environ. Sci. Technol. 2007, 41, 8149–8153. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.-C.; Gao, Z.-Y.; Ding, H.; Xu, N.; Wu, W.-M. Recovery of silver from silver(I)-containing solutions in bioelectrochemical reactors. Bioresour. Technol. 2012, 111, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Hu, N. The modeling of gold recovery from tetrachloroaurate wastewater using a microbial fuel cell. Bioresour. Technol. 2013, 133, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, T.; Liu, C.; Quan, X.; Chen, L.; Wang, A.; Chen, G. Synergetic interactions improve cobalt leaching from lithium cobalt oxide in microbial fuel cells. Bioresour. Technol. 2013, 128, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Heijne, A.T.; Liu, F.; Weijden, R.V.D.; Weijma, J.; Buisman, C.J.N.; Hamelers, H.V.M. Copper recovery combined with electricity production in a microbial fuel cell. Environ. Sci. Technol. 2010, 44, 4376–4381. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.; Pu, W.-C.; Cai, C.-F.; Xu, J.-P.; He, W.-J. Remediation of acid mine drainage based on a novel coupled membrane-free microbial fuel cell with permeable reactive barrier system. Pol. J. Environ. Stud. 2016, 25, 107–112. [Google Scholar] [CrossRef]
- Lefebvre, O.; Neculita, C.M.; Yue, X.; Ng, H.Y. Bioelectrochemical treatment of acid mine drainage dominated with iron. J. Hazard. Mater. 2012, 241, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Leiva, E.D.; Rámila, C.d.P.; Vargas, I.T.; Escauriaza, C.R.; Bonilla, C.A.; Pizarro, G.E.; Regan, J.M.; Pasten, P.A. Natural attenuation process via microbial oxidation of arsenic in a high Andean watershed. Sci. Total Environ. 2014, 466–467, 490–502. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, Y.; Manohar, A.K.; Mansfeld, F. Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochemistry 2008, 74, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Biffinger, J.C.; Byrd, J.N.; Dudley, B.L.; Ringeisen, B.R. Oxygen exposure promotes fuel diversity for shewanella oneidensis microbial fuel cells. Biosens. Bioelectron. 2008, 23, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Erable, B.; Etcheverry, L.; Bergel, A. Increased power from a two-chamber microbial fuel cell with a low-ph air-cathode compartment. Electrochem. Commun. 2009, 11, 619–622. [Google Scholar] [CrossRef]
- Gregory, K.; Bond, D.; Lovley, D. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 2004, 6, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, M.P.; Ter Heijne, A.; van der Weijden, R.; Saakes, M.; Buisman, C.J.N.; Sleutels, T.H.J.A. High rate copper and energy recovery in microbial fuel cells. Front. Microbiol. 2015, 6, 527. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva, E.; Leiva-Aravena, E.; Vargas, I. Acid Water Neutralization Using Microbial Fuel Cells: An Alternative for Acid Mine Drainage Treatment. Water 2016, 8, 536. https://doi.org/10.3390/w8110536
Leiva E, Leiva-Aravena E, Vargas I. Acid Water Neutralization Using Microbial Fuel Cells: An Alternative for Acid Mine Drainage Treatment. Water. 2016; 8(11):536. https://doi.org/10.3390/w8110536
Chicago/Turabian StyleLeiva, Eduardo, Enzo Leiva-Aravena, and Ignacio Vargas. 2016. "Acid Water Neutralization Using Microbial Fuel Cells: An Alternative for Acid Mine Drainage Treatment" Water 8, no. 11: 536. https://doi.org/10.3390/w8110536
APA StyleLeiva, E., Leiva-Aravena, E., & Vargas, I. (2016). Acid Water Neutralization Using Microbial Fuel Cells: An Alternative for Acid Mine Drainage Treatment. Water, 8(11), 536. https://doi.org/10.3390/w8110536





