Effect of COD:SO4 2− Ratio, HRT and Linoleic Acid Concentration on Mesophilic Sulfate Reduction: Reactor Performance and Microbial Population Dynamics
Abstract
:1. Introduction
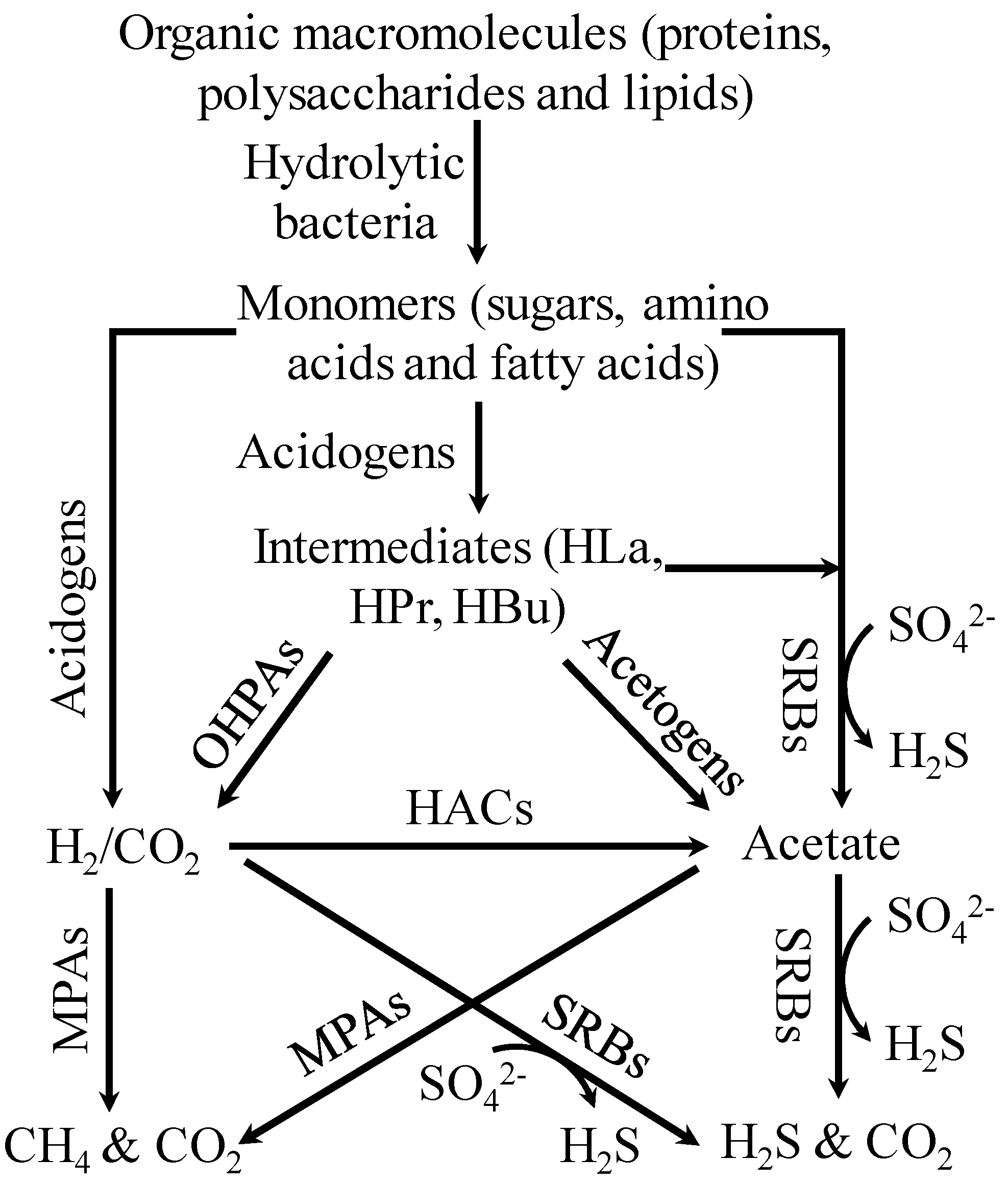
2. Materials and Methods
2.1. Inoculum Source
2.2. Sulfate Reduction Studies
| Experiment #s | LA Concentration (g·L−1) | COD/SO42− Ratio | HRT (h) | Initial SO42− Concentration (g·L−1) |
|---|---|---|---|---|
| 1 | 0 | 0.8 | 12 | 2.67 |
| 2 | 1.6 | 36 | 1.34 | |
| 3 | 2.4 | 24 | 0.89 | |
| 4 | 0.5 | 0.8 | 24 | 2.67 |
| 5 | 1.6 | 12 | 1.34 | |
| 6 | 2.4 | 36 | 0.89 | |
| 7 | 1.0 | 0.8 | 36 | 2.67 |
| 8 | 1.6 | 24 | 1.34 | |
| 9 | 2.4 | 12 | 0.89 |
2.3. Analytical Methods
2.4. Microbial Methods
3. Results and Discussion
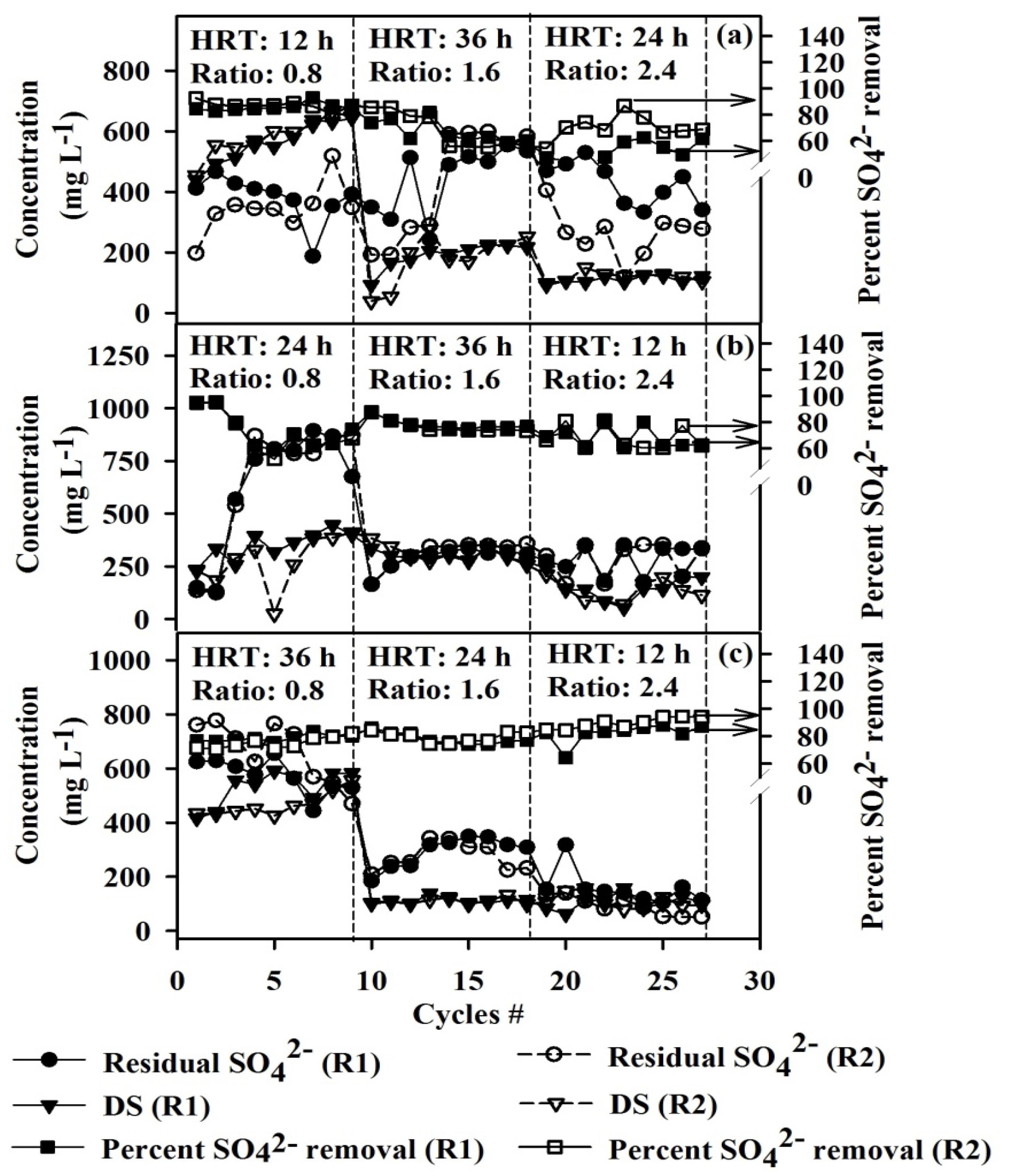
3.1. Comparison of Sulfate Removal and the Degradation Byproducts at Different Operating Conditions
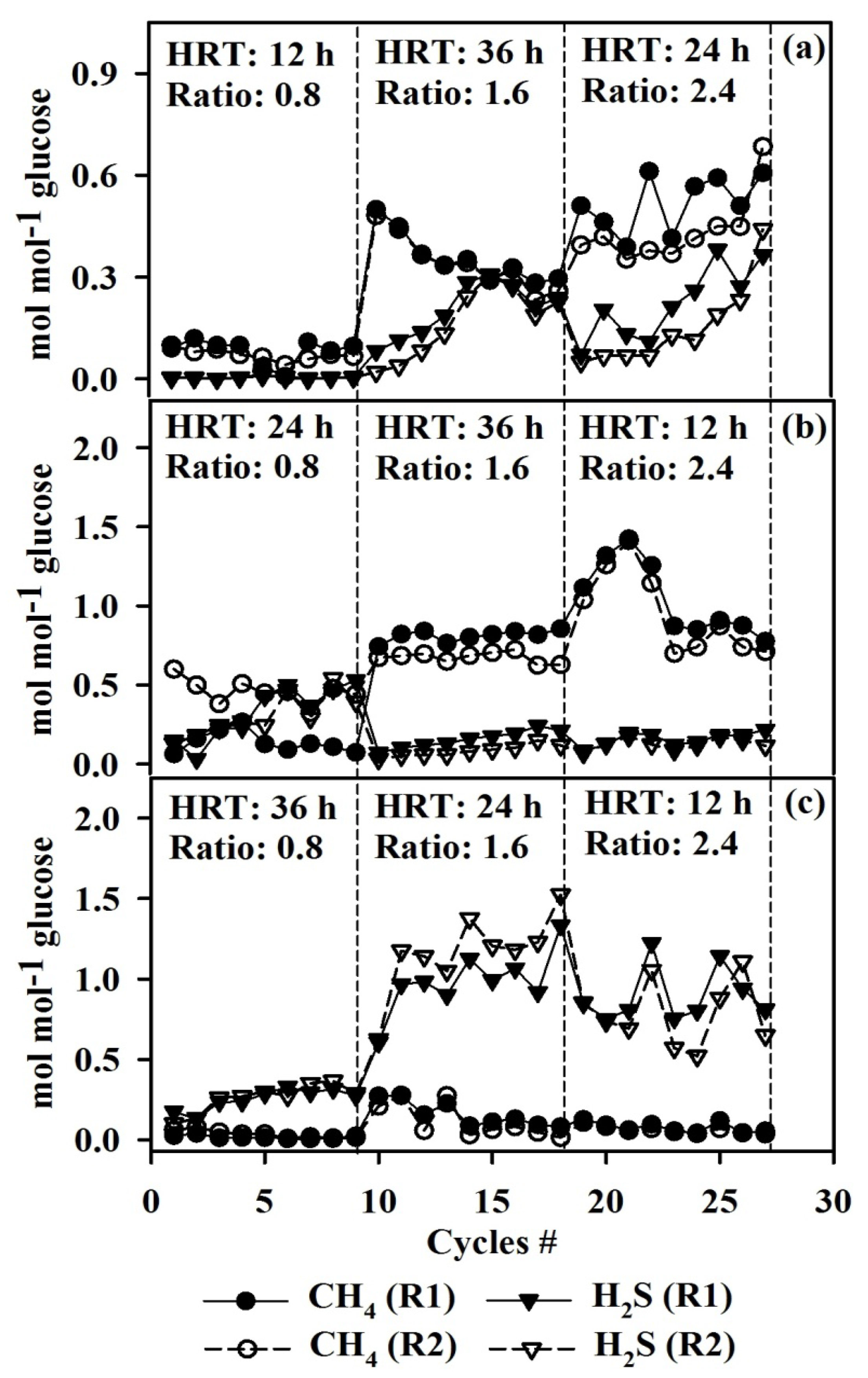
3.2. Effect of COD/SO42− Ratio and HRT on Biogas Production Using LA Treated and Untreated Cultures
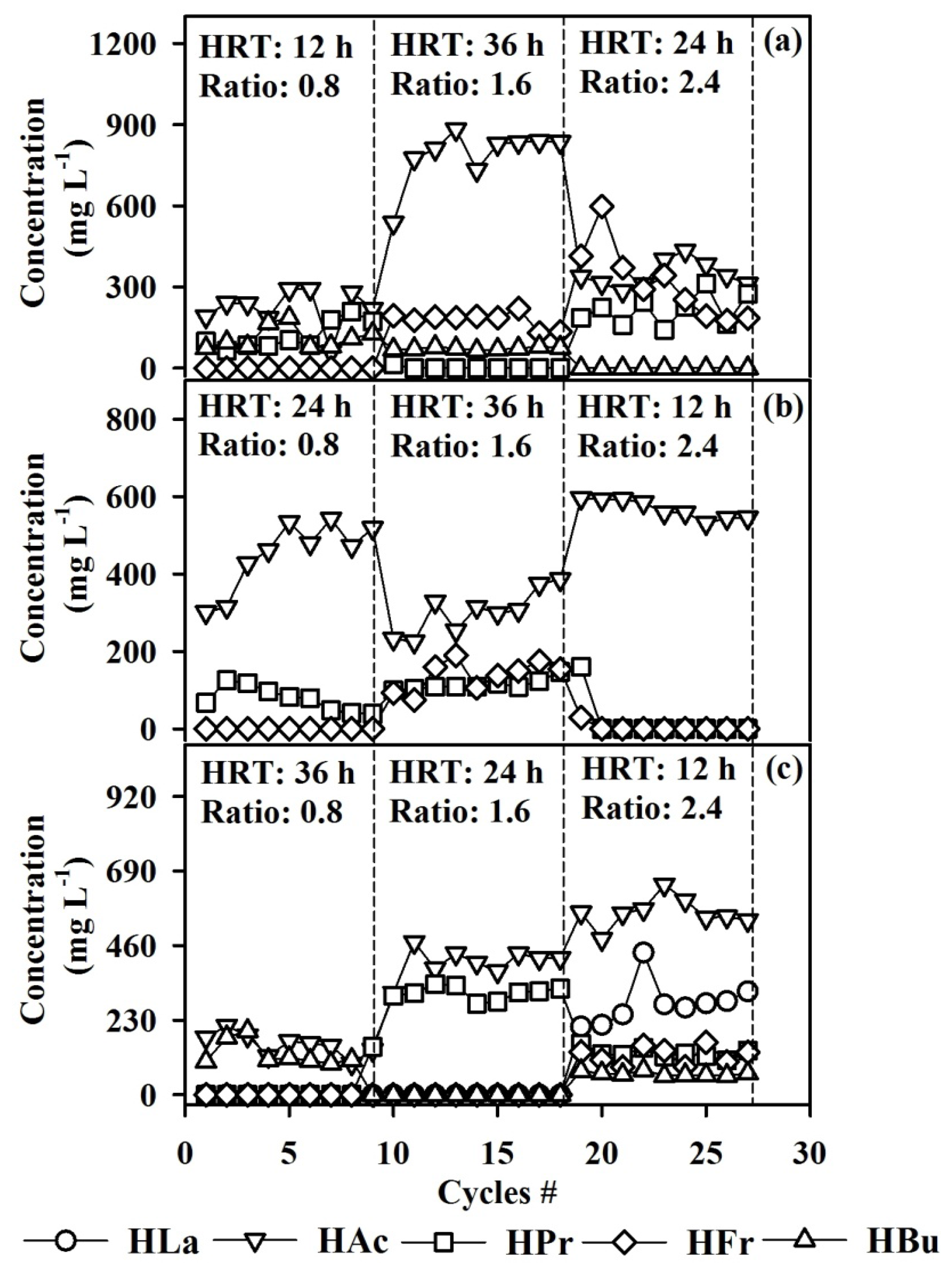
3.3. Volatile Fatty Acid Production
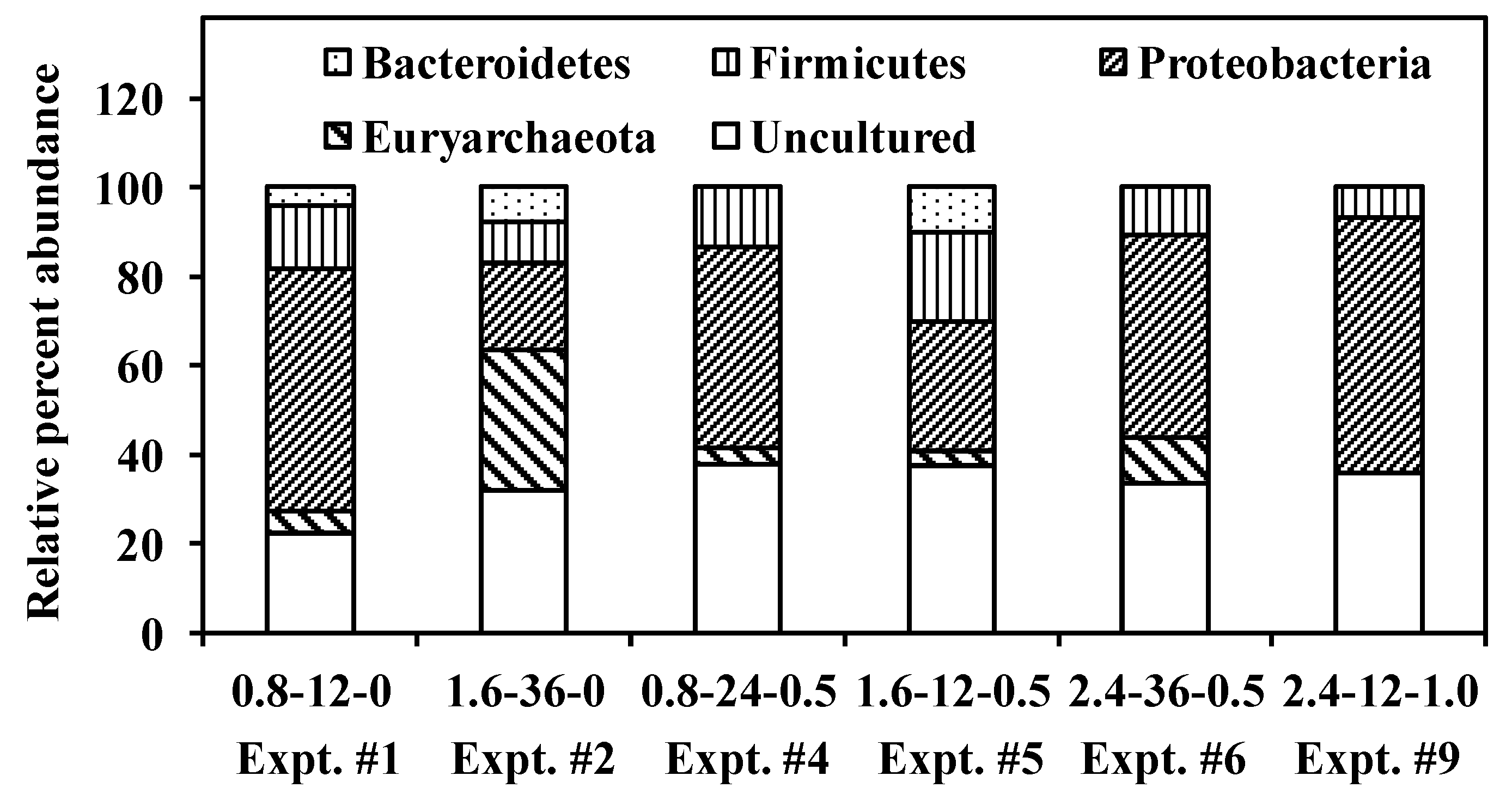
3.4. Competition and Coexistence of Sulfate-Reducing Bacteria, Homoacetogens and Methanogens
| Experiment #s | LA Concentration (g·L−1) | COD/SO42− Ratio | HRT (h) | COD Equivalent (mg·L−1) SO42− Reduced | COD Equivalent (mg·L−1) Methane Produced | Percent Electron Flow to SRBs | Percent Electron Flow to MPAs |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0.8 | 12 | 1281.4 | 28.7 | 60.0 | 1.4 |
| 2 | 1.6 | 36 | 543.6 | 101.7 | 25.4 | 4.8 | |
| 3 | 2.4 | 24 | 351.8 | 194.7 | 16.4 | 9.1 | |
| 4 | 0.5 | 0.8 | 24 | 971.3 | 91.9 | 45.4 | 4.3 |
| 5 | 1.6 | 12 | 663.8 | 265.4 | 31.0 | 12.4 | |
| 6 | 2.4 | 36 | 394.8 | 288.8 | 18.5 | 13.5 | |
| 7 | 1.0 | 0.8 | 36 | 1187.1 | 5.4 | 55.5 | 0.3 |
| 8 | 1.6 | 24 | 681.1 | 26.6 | 31.8 | 1.2 | |
| 9 | 2.4 | 12 | 562.4 | 21.8 | 26.3 | 1.0 |
4. Conclusions
- Operating the ASBRs was suitable for SO42− reduction with SO42− removal efficiencies greater than 60%, under the conditions examined in this study.
- ASBRs operating under low COD/SO42− ratio (0.8) with low HRT (12 h) is preferred for SO42− removal efficiencies greater than 75%.
- When compared to the control cultures, in cultures fed 1 g·L−1 LA and at COD/SO42− ratios 1.6 and 2.4, the SO42− removal efficiencies improved by approximately 20% and 28%, respectively.
- Dissolved sulfide and butyrate were associated with cultures fed with low COD/SO42− ratios, while H2S, acetate and propionate were linked with high COD/SO42− ratios.
- Methanogens belonging to Methanobacteriales and Methanosarcinales were abundant in control cultures operating at high HRTs while the addition of LA suppressed Methanobacteriales alone; however, with a reduction in HRT, washout of Methanosarcinales was observed.
- In controls and cultures fed 0.5 g·L−1 LA, HACs belonging the Bacteroidetes phylum was abundant.
- Desulfovibrio sp. and Desulfatibacillum sp., the major SRBs responsible for SO42− reduction, were able to out-compete MPAs and HACs at low HRTs and low COD/SO42− ratios.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lens, P.N.L.; Visser, A.; Janssen, A.J.H.; Pol, L.W.H.; Lettinga, G. Biotechnological treatment of sulfate-rich wastewaters. Crit. Rev. Environ. Sci. Technol. 1998, 28, 41–88. [Google Scholar]
- Zitomer, D.H.; Shrout, J.D. High-sulfate, high-chemical oxygen demand wastewater treatment using aerated methanogenic fluidized beds. Water Environ. Res. 2000, 72, 90–97. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, J.; Ghavipanjeh, F.; Mirjafari, P. The effect of influent COD and upward flow velocity on the behaviour of sulphate-reducing bacteria. Process Biochem. 2005, 40, 2305–2310. [Google Scholar] [CrossRef]
- White, C.; Gadd, G.M. Mixed sulphate-reducing bacterial cultures for bioprecipitation of toxic metals: Factorial and response-surface analysis of the effects of dilution rate, sulphate and substrate concentration. Microbiology 1996, 142, 2197–2205. [Google Scholar] [CrossRef]
- Omil, F.; Lens, P.; Visser, A.; Pol, L.W.H.; Lettinga, G. Long-term competition between sulfate reducing and methanogenic bacteria in UASB reactors treating volatile fatty acids. Biotechnol. Bioeng. 1998, 57, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Colleran, E.; Finnegan, S.; Lens, P. Anaerobic treatment of sulfate-containing waste streams. Antonie Van Leeuwenhoek 1995, 67, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [PubMed]
- Schonheit, P.; Kristjansson, J.K.; Thauer, R.K. Kinetic mechanism for the ability of sulfate reducers to out-compete methanogens for acetate. Arch. Microbiol. 1982, 132, 285–288. [Google Scholar] [CrossRef]
- Isa, Z.; Grusenmeyer, S.; Verstraete, W. Sulfate reduction relative to methane production in high-rate anaerobic-digestion—Microbiological aspects. Appl. Environ. Microbiol. 1986, 51, 580–587. [Google Scholar] [PubMed]
- Findlay, S.E.G.; Sinsabaugh, R.L.; Sobczak, W.V.; Hoostal, M. Metabolic and structural response of hyporheic microbial communities to variations in supply of dissolved organic matter. Limnol. Oceanogr. 2003, 48, 1608–1617. [Google Scholar] [CrossRef]
- Dar, S.A.; Yao, L.; van Dongen, U.; Kuenen, J.G.; Muyzer, G. Analysis of diversity and activity of sulfate-reducing bacterial communities in sulfidogenic bioreactors using 16S rRNA and dsrB genes as molecular markers. Appl. Environ. Microbiol. 2007, 73, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Raskin, L.; Rittmann, B.E.; Stahl, D.A. Competition and coexistence of sulfate-reducing and methanogenic populations in anaerobic biofilms. Appl. Environ. Microbiol. 1996, 62, 3847–3857. [Google Scholar] [PubMed]
- Sipma, J.; Lettinga, G.; Stams, A.J.M.; Lens, P.N.L. Hydrogenogenic CO conversion in a moderately thermophilic (55 °C) sulfate-fed gas lift reactor: Competition for CO-derived H2. Biotechnol. Progr. 2006, 22, 1327–1334. [Google Scholar] [CrossRef]
- Liamleam, W.; Annachhatre, A.P. Electron donors for biological sulfate reduction. Biotechnol. Adv. 2007, 25, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Kleerebezem, R.; Stams, A.J.M.; Kuenen, J.G.; Muyzer, G. Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl. Microbiol. Biotechnol. 2008, 78, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Chaiprapat, S.; Preechalertmit, P.; Boonsawang, P.; Karnchanawong, S. Sulfidogenesis in pretreatment of high-sulfate acidic wastewater using anaerobic sequencing batch reactor and upflow anaerobic sludge blanket reactor. Environ. Eng. Sci. 2011, 28, 597–604. [Google Scholar] [CrossRef]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T.; Allison, C.; Segal, I.; Vorster, H.H.; Walker, A.R.P. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut 1990, 31, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Singh, R.; Chaganti, S.R.; Lalman, J.A. Modeling sulfate removal by inhibited mesophilic mixed anaerobic communities using a statistical approach. Water Res. 2013, 47, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.Z.; Alves, J.I.; Alves, M.M.; Smidt, H.; Stams, A.J.M. Effect of sulfate on methanogenic communities that degrade unsaturated and saturated long-chain fatty acids (LCFA). Environ. Microbiol. 2009, 11, 68–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheoran, A.S.; Sheoran, V.; Choudhary, R.P. Bioremediation of acid-rock drainage by sulphate-reducing prokaryotes: A review. Miner. Eng. 2010, 23, 1073–1100. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Plumb, J.J.; Franzmann, P.D.; Puhakka, J.A. Simple organic electron donors support diverse sulfate-reducing communities in fluidized-bed reactors treating acidic metal- and sulfate-containing wastewater. FEMS Microbiol. Ecol. 2004, 47, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Chowdhury, N.; Lalman, J.A.; Seth, R.; Biswas, N. Impact of initial pH and linoleic acid (C18:2) on hydrogen production by a mesophilic anaerobic mixed culture. J. Environ. Eng. ASCE 2008, 134, 110–117. [Google Scholar] [CrossRef]
- Wiegant, W.M.; Lettinga, G. Thermophilic anaerobic-digestion of sugars in upflow anaerobic sludge blanket reactors. Biotechnol. Bioeng. 1985, 27, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Moon, C.; Veeravalli, S.S.; Shanmugam, S.R.; Chaganti, S.R.; Lalman, J.A. Using a statistical model to examine the effect of COD: SO42− Ratio, HRT and LA concentration on sulfate reduction in an anaerobic sequencing batch reactor. Water 2014, 6, 3478–3494. [Google Scholar] [CrossRef]
- Speece, R.E. Gas Flow Totalizer. US Patent 4064750 A, 27 December 1977. [Google Scholar]
- Chowdhury, N.; Lalman, J.A.; Seth, R.; Ndegwa, P. Biohydrogen production by mesophilic anaerobic fermentation of glucose in the presence of linoleic acid. J. Environ. Eng. ASCE 2007, 133, 1145–1152. [Google Scholar] [CrossRef]
- American Publishers Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1999. [Google Scholar]
- Chaganti, S.R.; Lalman, J.A.; Heath, D.D. 16S rRNA gene based analysis of the microbial diversity and hydrogen production in three mixed anaerobic cultures. Int. J. Hydrogen Energy 2012, 37, 9002–9017. [Google Scholar] [CrossRef]
- Alvarez, M.T.; Pozzo, T.; Mattiasson, B. Enhancement of sulphide production in anaerobic packed bed bench-scale biofilm reactors by sulphate reducing bacteria. Biotechnol. Lett. 2006, 28, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sarti, A.; Zaiat, M. Anaerobic treatment of sulfate-rich wastewater in an anaerobic sequential batch reactor (AnSBR) using butanol as the carbon source. J. Environ. Manag. 2011, 92, 1537–1541. [Google Scholar] [CrossRef]
- Wei, C.H.; Wang, W.X.; Deng, Z.Y.; Wu, C.F. Characteristics of high-sulfate wastewater treatment by two-phase anaerobic digestion process with jet-loop anaerobic fluidized bed. J. Environ. Sci. China 2007, 19, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.; Gao, Y.; Lettinga, G. Effects of short-term temperature increases on the mesophilic anaerobic breakdown of sulfate containing synthetic wastewater. Water Res. 1993, 27, 541–550. [Google Scholar] [CrossRef]
- McCartney, D.M.; Oleszkiewicz, J.A. Competition between methanogens and sulfate reducers: Effect of COD/Sulfate ratio and acclimation. Water Environ. Res. 1993, 65, 655–664. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Musat, N.; Musat, F.; Mussmann, M. Structure of microbial communities and hydrocarbon-dependent sulfate reduction in the anoxic layer of a polluted microbial mat. Mar. Pollut. Bull. 2011, 62, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.L.; Patel, B.K.C.; Ollivier, B. Taxonomic phylogenetic and ecological diversity of methanogenic Archaea. Anaerobe 2000, 6, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Zinder, S.H. Physiological ecology of methanogens. In Methanogens: Ecology, Physiology, Biochemistryt and Genetics; Ferry, J.G., Ed.; Chapman and Hall: London, UK, 1993; pp. 128–206. [Google Scholar]
- Tan, B.; Dong, X.L.; Sensen, C.W.; Foght, J. Metagenomic analysis of an anaerobic alkane-degrading microbial culture: Potential hydrocarbon-activating pathways and inferred roles of community members. Genome 2013, 56, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, L.P.; Hiibel, S.R.; Pruden, A.; Reardon, K.F. Comparison of microbial community composition and activity in sulfate-reducing batch systems remediating mine drainage. Biotechnol. Bioeng. 2008, 101, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Zhao, Y.; Wang, A.; Gao, C.; Shang, H.; Liu, Y.; Wan, C. The effect of decreasing alkalinity on microbial community dynamics in a sulfate-reducing bioreactor as analyzed by PCR-SSCP. Sci. China Ser. C 2006, 49, 370–378. [Google Scholar] [CrossRef]
- Bijmans, M.F.M.; Dopson, M.; Ennin, F.; Lens, P.N.L.; Buisman, C.J.N. Effect of sulfide removal on sulfate reduction at pH 5 in a hydrogen fed gas-lift bioreactor. J. Microbiol. Biotechnol. 2008, 18, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Weijma, J.; Gubbels, F.; Pol, L.W.H.; Stams, A.J.M.; Lens, P.; Lettinga, G. Competition for H2 between sulfate reducers, methanogens and homoacetogens in a gas-lift reactor. Water Sci. Technol. 2002, 45, 75–80. [Google Scholar] [PubMed]
- Jackson, B.E.; Bhupathiraju, V.K.; Tanner, R.S.; Woese, C.R.; McInerney, M.J. Syntrophus aciditrophicus sp. nov., a new anaerobic bacterium that degrades fatty acids and benzoate in syntrophic association with hydrogen-using microorganisms. Arch. Microbiol. 1999, 171, 107–114. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, C.; Singh, R.; Veeravalli, S.S.; Shanmugam, S.R.; Chaganti, S.R.; Lalman, J.A.; Heath, D.D. Effect of COD:SO4 2− Ratio, HRT and Linoleic Acid Concentration on Mesophilic Sulfate Reduction: Reactor Performance and Microbial Population Dynamics. Water 2015, 7, 2275-2292. https://doi.org/10.3390/w7052275
Moon C, Singh R, Veeravalli SS, Shanmugam SR, Chaganti SR, Lalman JA, Heath DD. Effect of COD:SO4 2− Ratio, HRT and Linoleic Acid Concentration on Mesophilic Sulfate Reduction: Reactor Performance and Microbial Population Dynamics. Water. 2015; 7(5):2275-2292. https://doi.org/10.3390/w7052275
Chicago/Turabian StyleMoon, Chungman, Rajesh Singh, Sathyanarayan S. Veeravalli, Saravanan R. Shanmugam, Subba Rao Chaganti, Jerald A. Lalman, and Daniel D. Heath. 2015. "Effect of COD:SO4 2− Ratio, HRT and Linoleic Acid Concentration on Mesophilic Sulfate Reduction: Reactor Performance and Microbial Population Dynamics" Water 7, no. 5: 2275-2292. https://doi.org/10.3390/w7052275






