Photocatalytic Degradation of Phenol and Phenol Derivatives Using a Nano-TiO2 Catalyst: Integrating Quantitative and Qualitative Factors Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenols and Cresols
2.2. Titanium Dioxide
| Particle size (nm) | Surface area (m2 g−1) |
|---|---|
| 5 1 | 275 ± 15 2 |
| 10 1 | 131 ± 12 2 |
| 32 1 | 47 ± 2 2 |
2.3. Experimental Design and Statistical Analysis
| Level | Factors | |||||
|---|---|---|---|---|---|---|
| Reactant (R) | (z) | Temperature (°C/K) | (x1) | TiO2 particle size (nm) | (x2) | |
| Type | Coded | Actual | Coded | Actual | Coded | |
| 1 | phenol | –1 | 23/296 | –1 | 5 | –1 |
| 2 | m-cresol | 0 | 30/303 | 0 | 10 | 0 |
| 3 | o-cresol | +1 | 37/313 | +1 | 32 | +1 |
| Expt # | Factors | Response | Residual | |||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | TiO2 diameter (nm) | Reactant | Apparent Degradation rate constant 1 (min−1) | |||||
| Experimental 2 | Predicted 2 | |||||||
| Average | SD | Average | SD | |||||
| 1 | 23 ± 2 | 5 | phenol | 0.0069 | 0.0003 | 0.0073 | 0.0003 | −0.0004 |
| 2 | 23 ± 2 | 5 | m-cresol | 0.0093 | 0.0003 | 0.0089 | 0.0002 | 0.0004 |
| 3 | 23 ± 2 | 5 | o-cresol | 0.0115 | 0.001 | 0.0106 | 0.0001 | 0.0009 |
| 4 | 30 ± 2 | 5 | phenol | 0.007 | 0.0002 | 0.0081 | 0.0003 | −0.0011 |
| 5 | 30 ± 2 | 5 | m-cresol | 0.0093 | 0.0007 | 0.0098 | 0.0002 | −0.0005 |
| 6 | 30 ± 2 | 5 | o-cresol | 0.0114 | 0.0017 | 0.0115 | 0.0001 | −0.0001 |
| 7 | 37 ± 2 | 5 | phenol | 0.008 | 0.0002 | 0.0089 | 0.0003 | −0.0009 |
| 8 | 37 ± 2 | 5 | m-cresol | 0.0116 | 0.0002 | 0.0106 | 0.0002 | 0.0010 |
| 9 | 37 ± 2 | 5 | o-cresol | 0.013 | 0.0003 | 0.0123 | 0.0001 | 0.0007 |
| 10 | 23 ± 2 | 10 | phenol | 0.008 | 0.0001 | 0.0086 | 0.0002 | −0.0006 |
| 11 | 23 ± 2 | 10 | m-cresol | 0.0098 | 0.0002 | 0.0103 | 0.0001 | −0.0005 |
| 12 | 23 ± 2 | 10 | o-cresol | 0.0119 | 0.0003 | 0.0120 | 0.0000 | −0.0001 |
| 13 | 30 ± 2 | 10 | phenol | 0.0101 | 0.0001 | 0.0094 | 0.0002 | 0.0007 |
| 14 | 30 ± 2 | 10 | m-cresol | 0.0115 | 0.0002 | 0.0111 | 0.0000 | 0.0004 |
| 15 | 30 ± 2 | 10 | o-cresol | 0.0119 | 0.0009 | 0.0128 | −0.0001 | −0.0009 |
| 16 | 37 ± 2 | 10 | phenol | 0.0117 | 0.0012 | 0.0103 | 0.0001 | 0.0014 |
| 17 | 37 ± 2 | 10 | m-cresol | 0.0123 | 0.0004 | 0.0119 | 0.0000 | 0.0004 |
| 18 | 37 ± 2 | 10 | o-cresol | 0.0128 | 0.0005 | 0.0136 | −0.0001 | −0.0008 |
| 19 | 23 ± 2 | 32 | phenol | 0.0023 | 0.0002 | 0.0020 | 0.0001 | 0.0004 |
| 20 | 23 ± 2 | 32 | m-cresol | 0.0042 | 0.0003 | 0.0036 | 0.0000 | 0.0006 |
| 21 | 23 ± 2 | 32 | o-cresol | 0.0052 | 0.0001 | 0.0053 | −0.0001 | −0.0001 |
| 22 | 30 ± 2 | 32 | phenol | 0.0028 | 0 | 0.0028 | 0.0001 | 0.0000 |
| 23 | 30 ± 2 | 32 | m-cresol | 0.0046 | 0.0003 | 0.0045 | 0.0000 | 0.0001 |
| 24 | 30 ± 2 | 32 | o-cresol | 0.0063 | 0.0001 | 0.0061 | −0.0001 | 0.0002 |
| 25 | 37 ± 2 | 32 | phenol | 0.0034 | 0.0003 | 0.0036 | 0.0001 | −0.0002 |
| 26 | 37 ± 2 | 32 | m-cresol | 0.005 | 0.0003 | 0.0053 | 0.0000 | −0.0003 |
| 27 | 37 ± 2 | 32 | o-cresol | 0.0063 | 0.00062 | 0.0070 | −0.0001 | −0.0007 |
2.4. Experimental Methods
2.4.1. Degradation Experiments
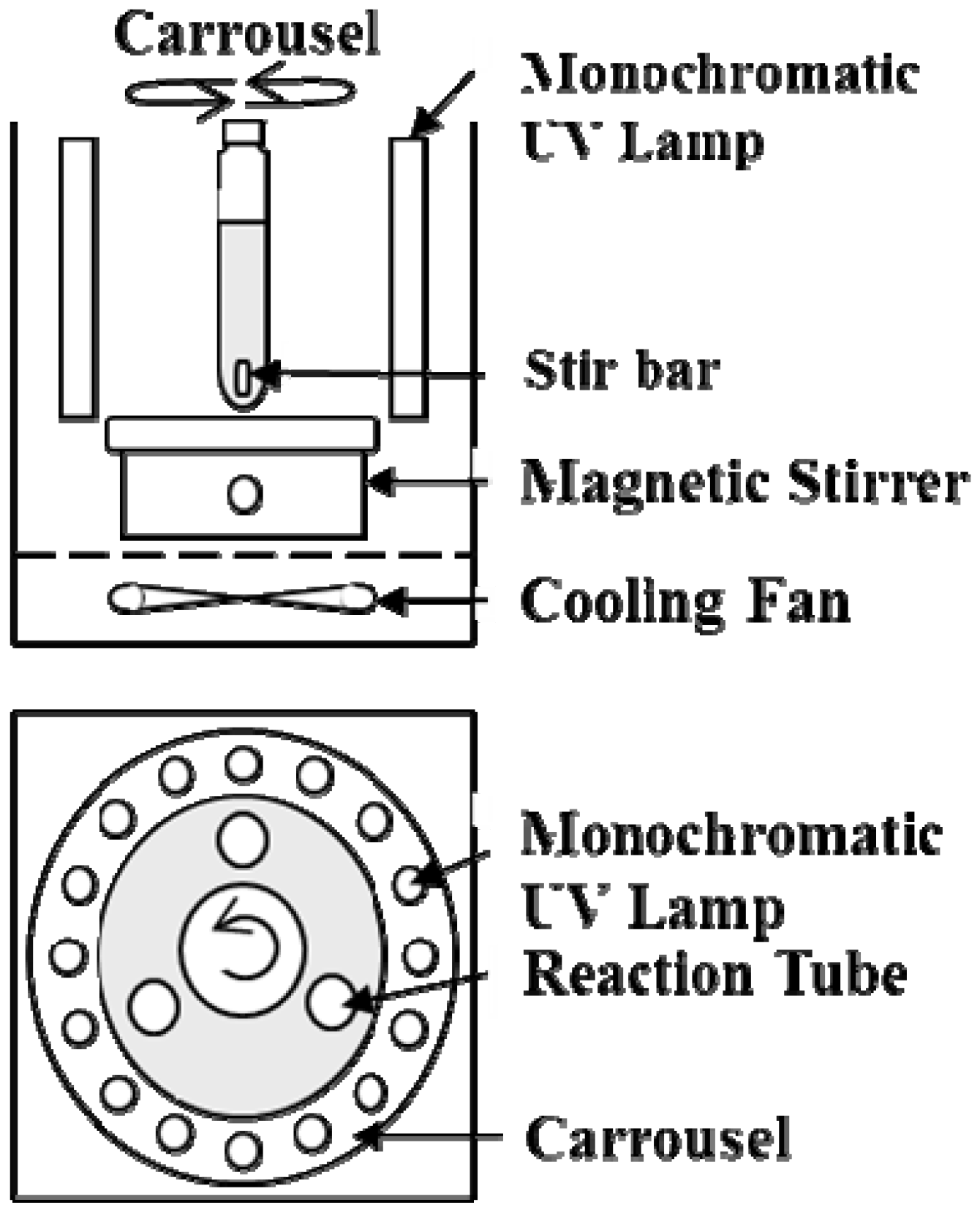
2.4.2. Surface Area Measurements
2.5. Analytical Methods
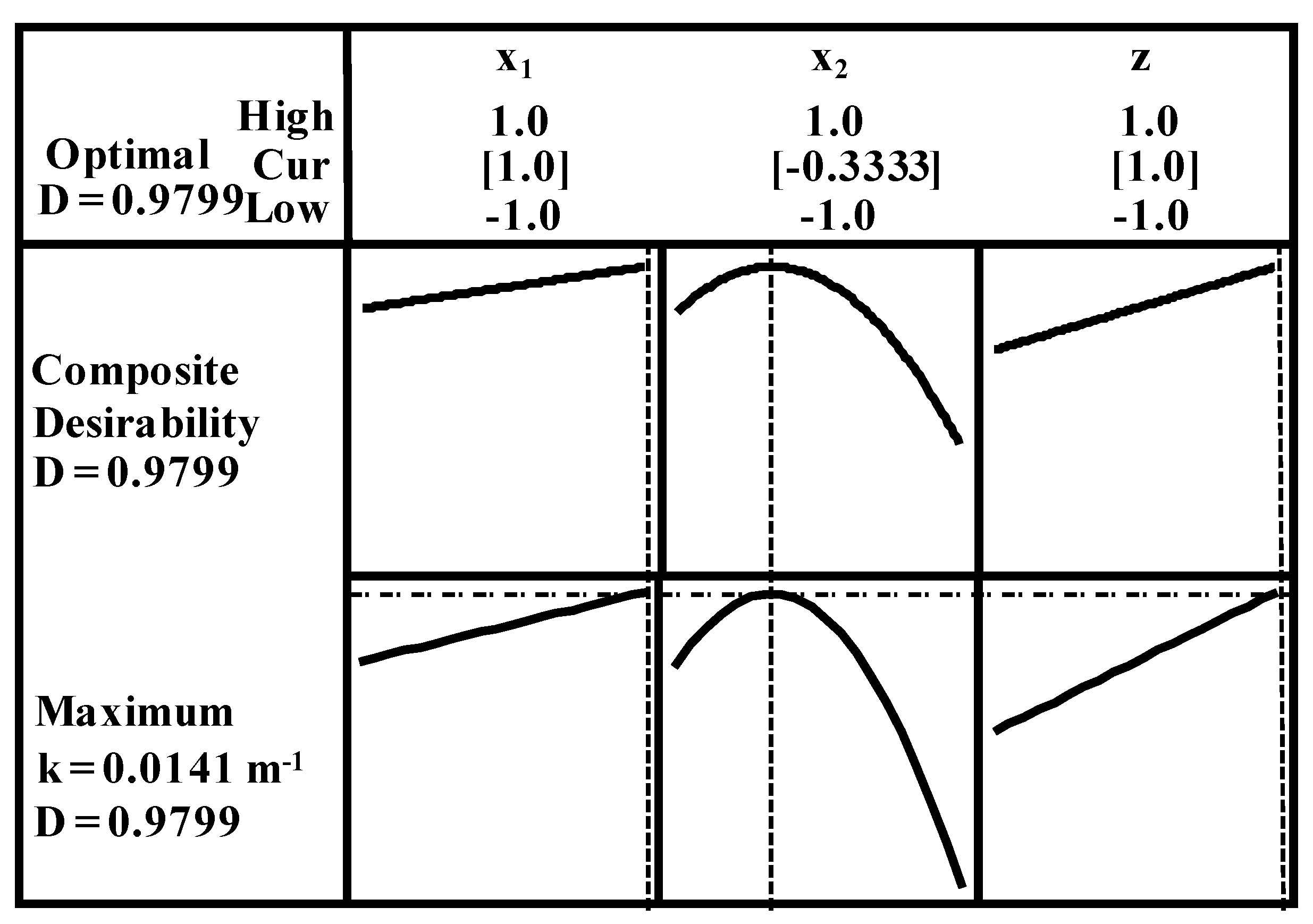
2.6. Optimization Study
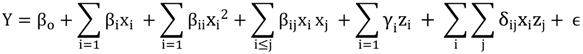
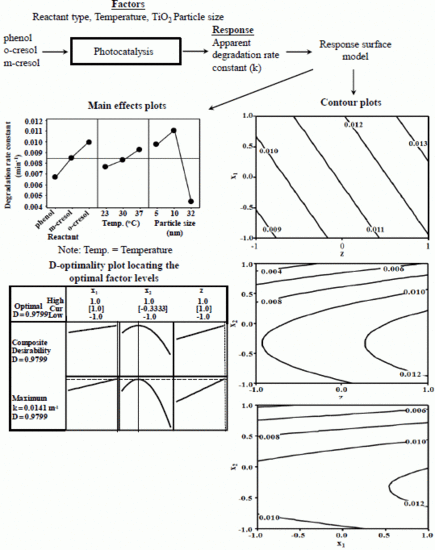
3. Results and Discussion
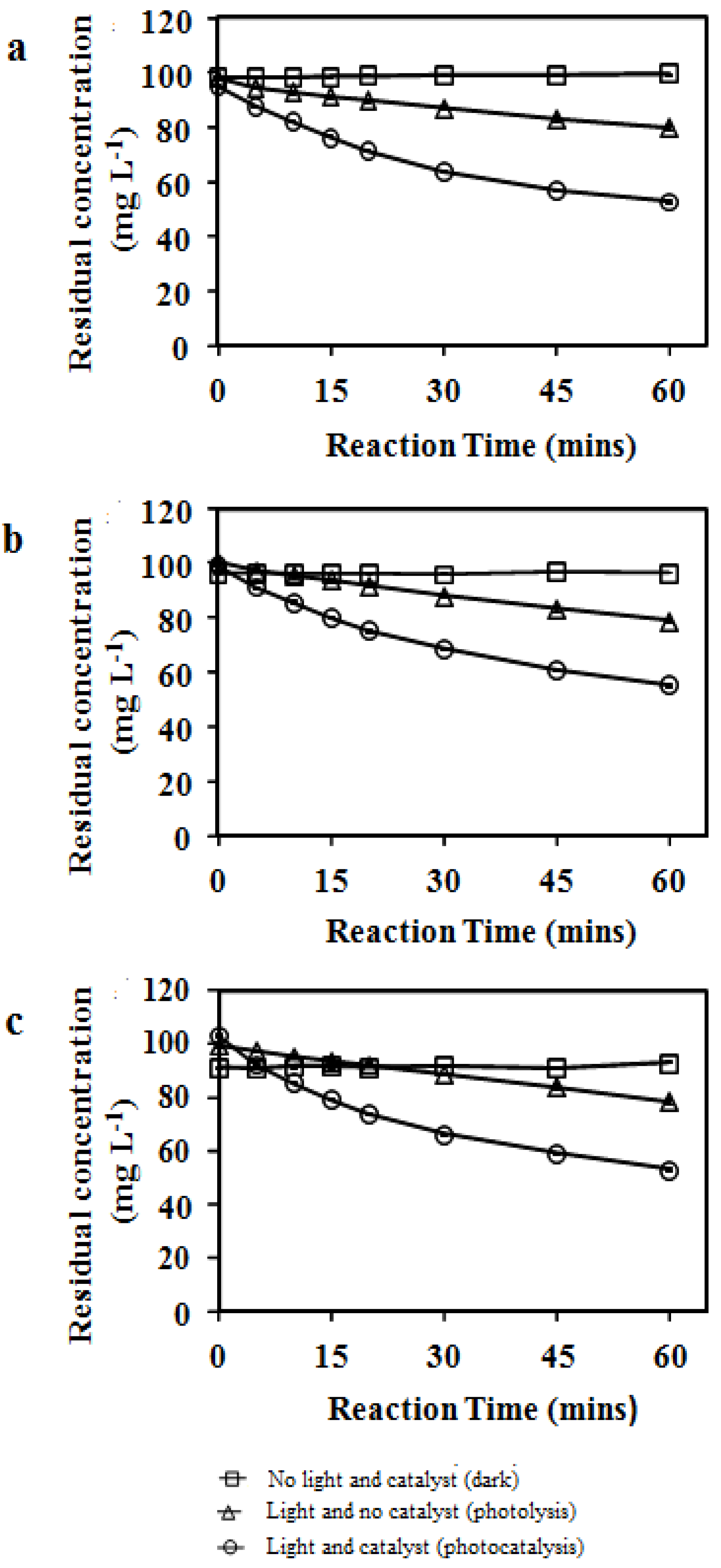
3.1. Photolytic and Photocatalytic Degradation
| Reactant | Temperature (°C) | Apparent degradation rate constant (min−1) | |
|---|---|---|---|
| Photolysis 1 | |||
| Average | SD2 | ||
| Phenol | 23 | 0.0029 | 0.0001 |
| Phenol | 30 | 0.0037 | 0.0003 |
| Phenol | 37 | 0.0037 | 0.0003 |
| m-Cresol | 23 | 0.0039 | 0.0002 |
| m-Cresol | 30 | 0.0043 | 0.0002 |
| m-Cresol | 37 | 0.0056 | 0.0004 |
| o-Cresol | 23 | 0.0034 | 0.0001 |
| o-Cresol | 30 | 0.0037 | 0.0001 |
| o-Cresol | 37 | 0.0050 | 0.0006 |
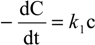
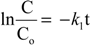
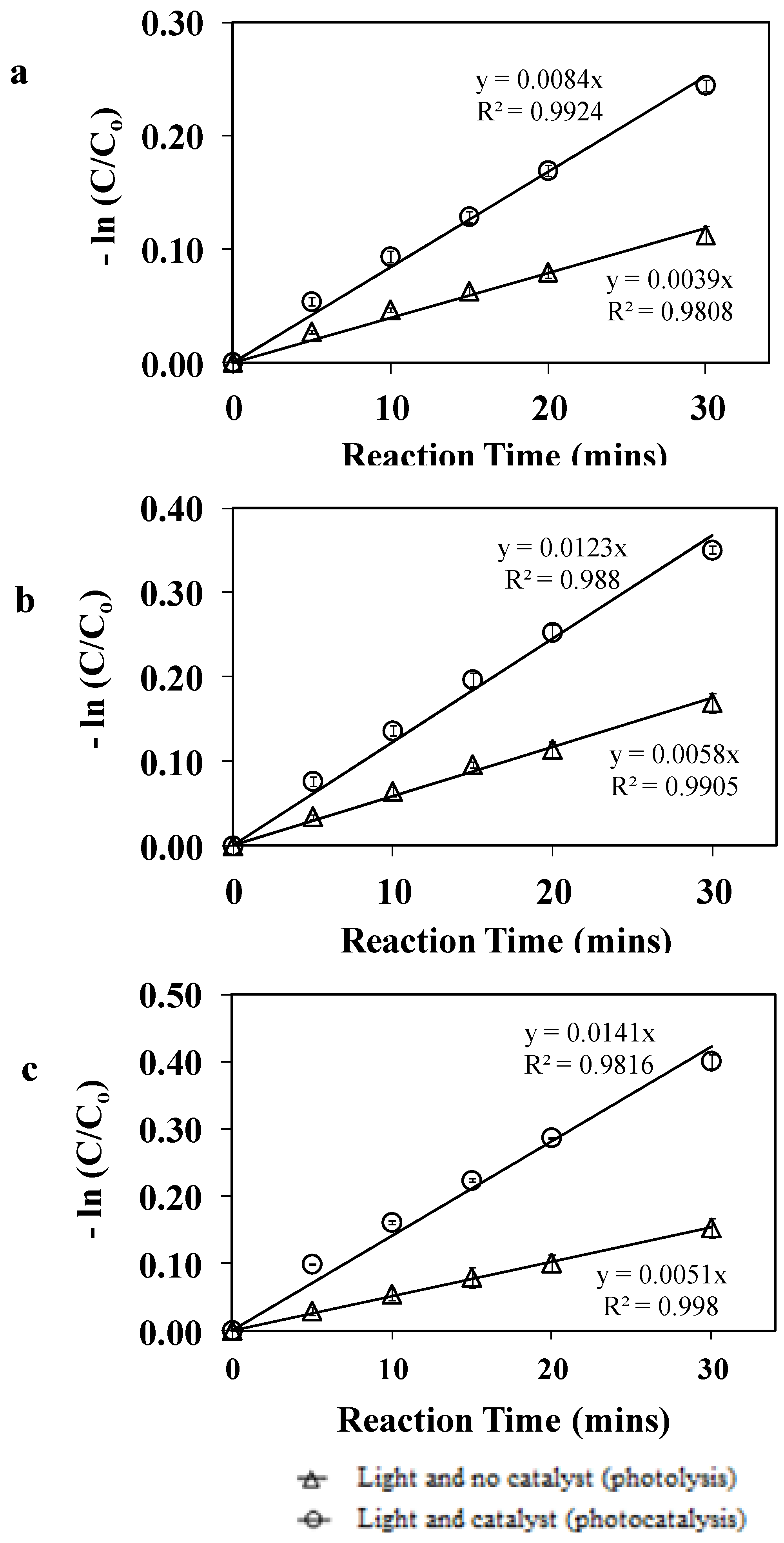
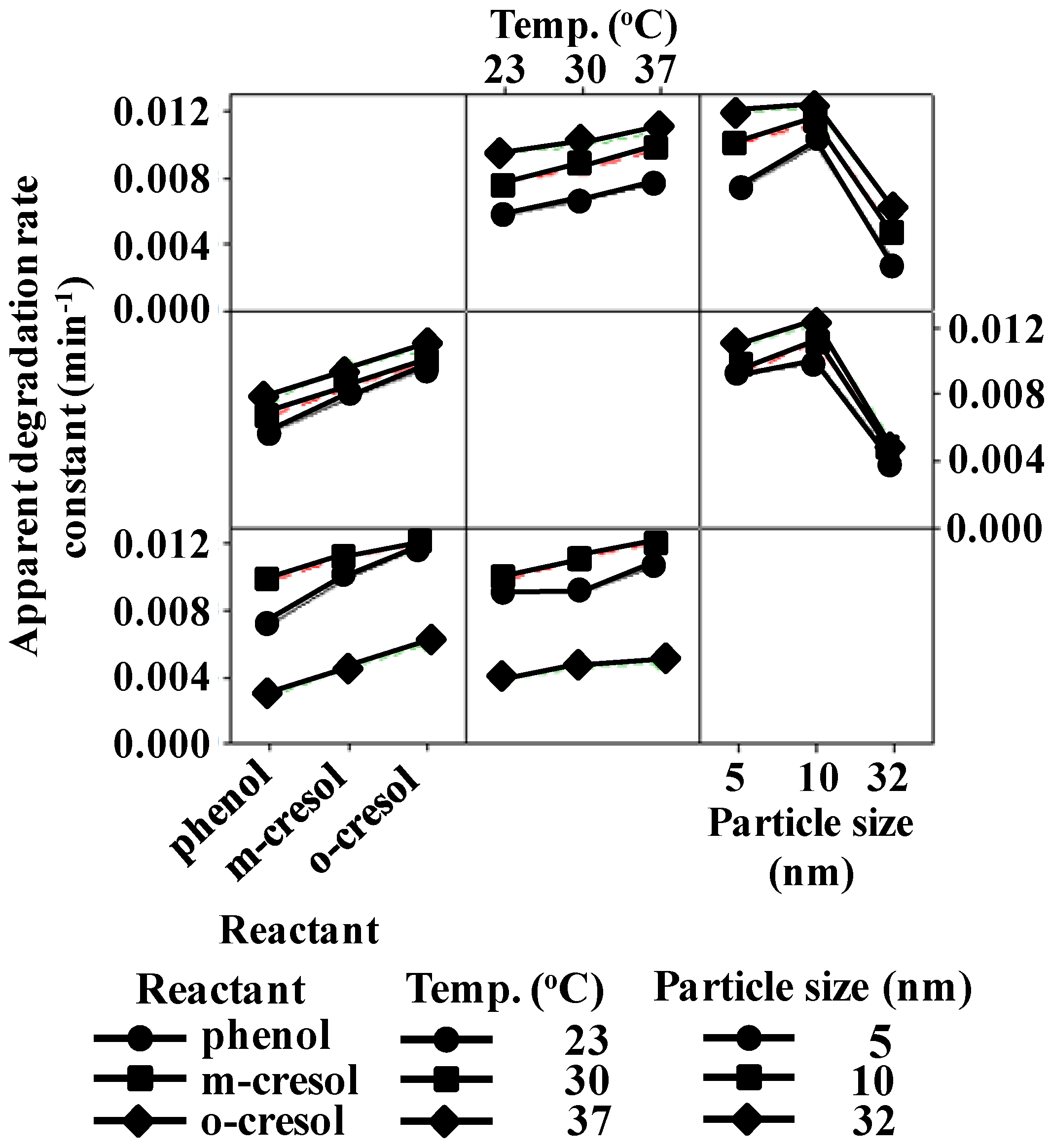
3.2. Effect of the Variables on the apparent degradation constant
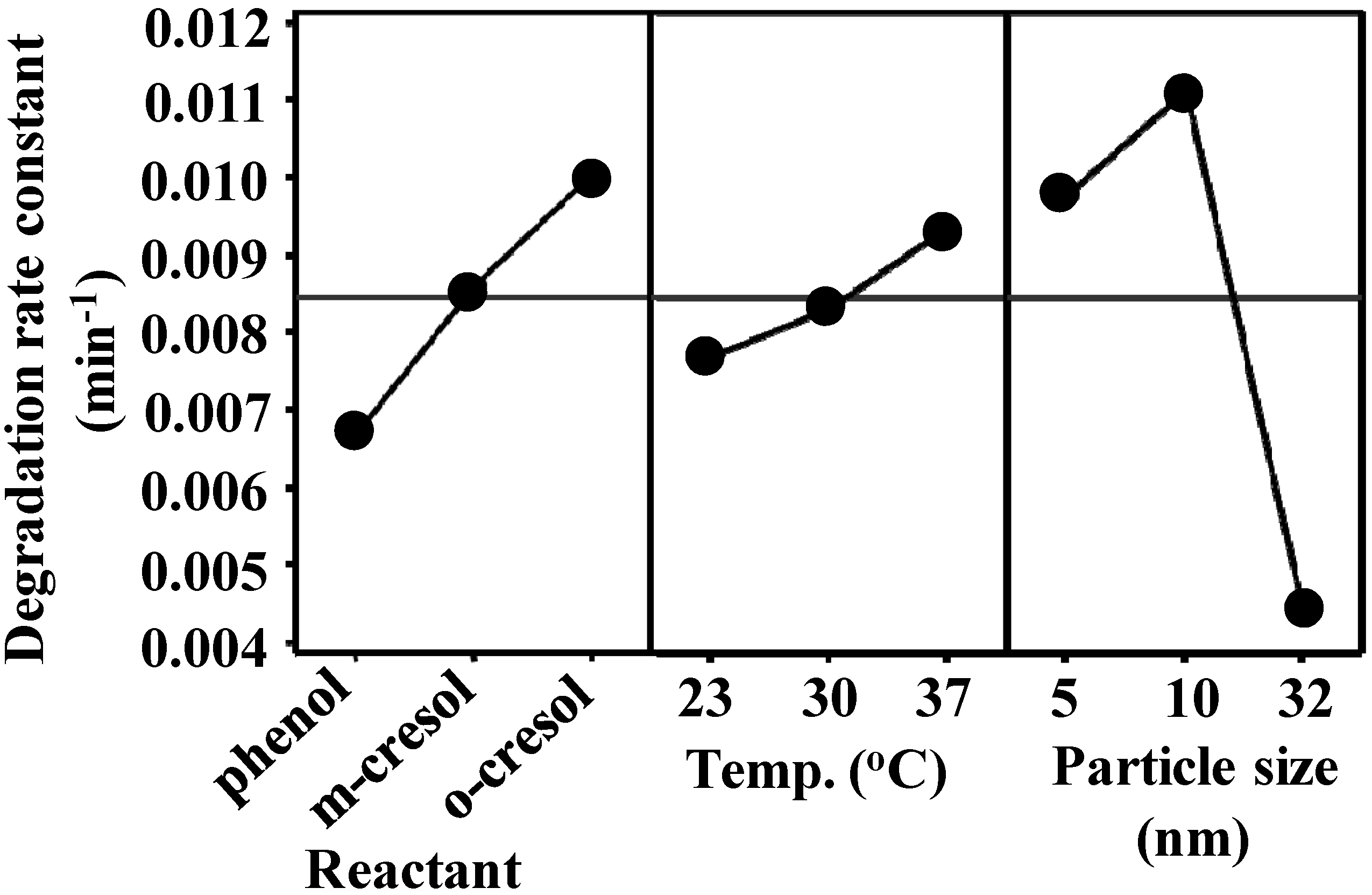
3.3. Combined Effects of the Experimental Variables
| Source | DF | Seq SS | Adj SS | Adj MS | F | p | |
|---|---|---|---|---|---|---|---|
| Regression | 8 | 0.000289 | 0.000289 | 0.000036 | 82.10 | 0.000 | S |
| Linear | |||||||
| x1 | 1 | 0.000012 | 0.000012 | 0.000012 | 28.42 | 0.000 | S |
| x2 | 1 | 0.000127 | 0.000127 | 0.000127 | 289.81 | 0.000 | S |
| z | 1 | 0.000050 | 0.000050 | 0.000050 | 114.44 | 0.000 | S |
| Square | |||||||
| x1 × x1 | 1 | 0.000000 | 0.000000 | 0.000000 | 0.49 | 0.494 | NS |
| x2 × x2 | 1 | 0.000096 | 0.000096 | 0.000096 | 217.66 | 0.000 | S |
| Interaction | |||||||
| x1 × x2 | 1 | 0.000000 | 0.000000 | 0.000000 | 0.68 | 0.419 | NS |
| x1× z | 1 | 0.000000 | 0.000000 | 0.000000 | 1.09 | 0.310 | NS |
| x2 × z | 1 | 0.000002 | 0.000002 | 0.000002 | 4.19 | 0.056 | NS |
| Residual Error | 18 | 0.000008 | 0.000008 | 0.000000 | |||
| Total | 26 | 0.000297 |
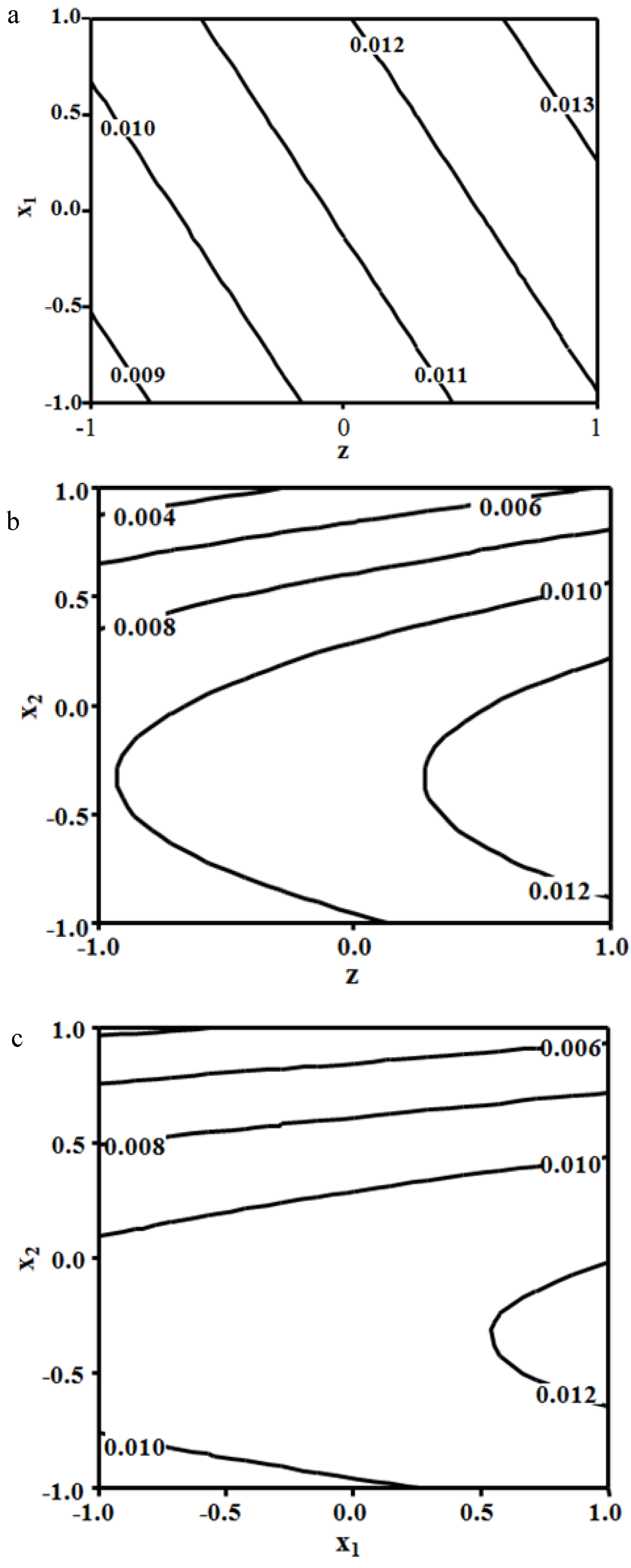
3.4. Model Development
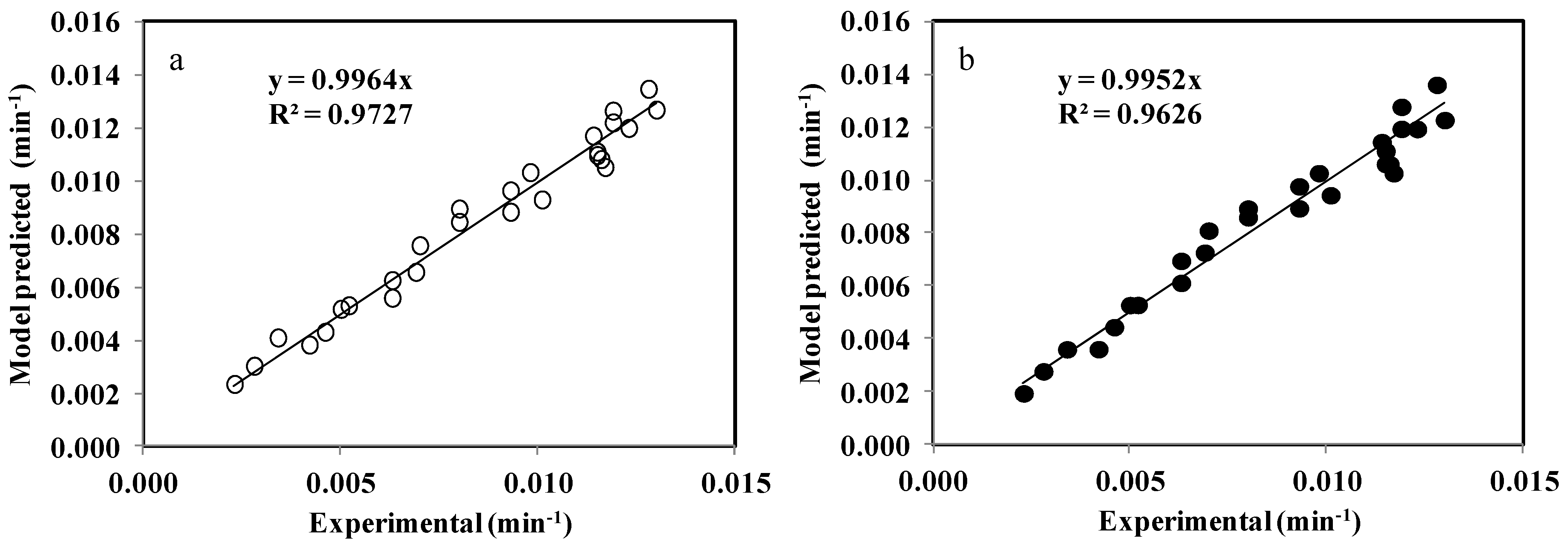
3.5. Assessment of the Model
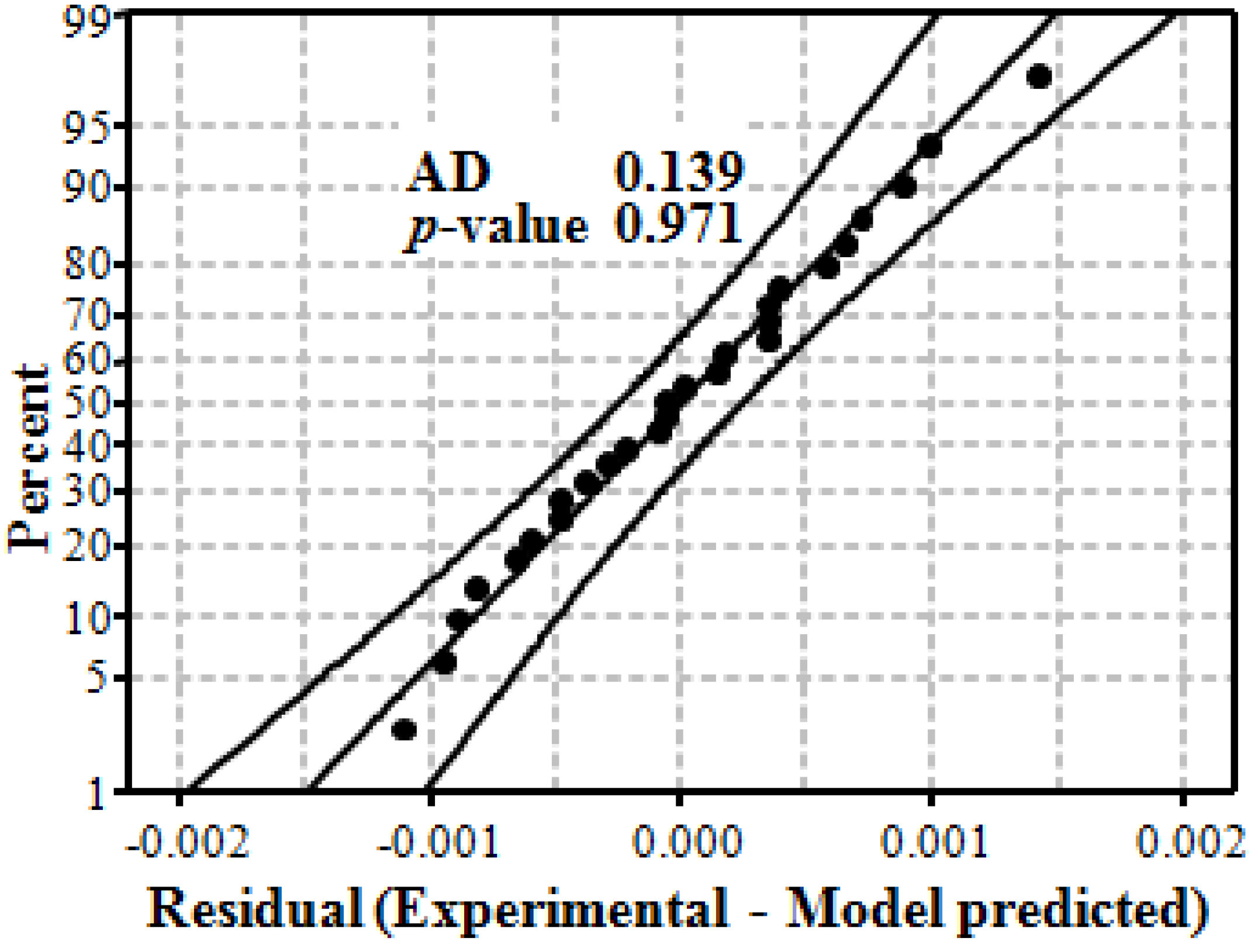
3.6. Activation Energy
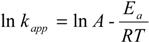
| Particle size (nm) | o-cresol | m-cresol | phenol |
|---|---|---|---|
| Activation energy (kJ mol−1) | |||
| 5 | 6.97 ± 5.88 a,c | 17.12 ± 1.18 a,d | 10.62 ± 2.01 a,d |
| 10 | 3.13 ± 1.61 a,c | 13.02 ± 1.18 b,d | 12.52 ± 2.76 a,d |
| 32 | 10.45 ± 4.81 a,c | 11.63 ± 1.57 b,c | 15.78 ± 1.56 a,c |
| TiO2 catalyst | Reactant | Temperature (K) | Activation Energy (kJ mol−1) | Reference |
|---|---|---|---|---|
| Degussa P25 | Phenol | 303–329 | 16.2 | Kartal et al. [55] |
| Degussa P25 | Naphthalene | 283–313 | 22.0 | Lair et al. [56] |
| Degussa P25 | Imazaquin | 293–313 | 24.8 | Garcia et al. [57] |
| Degussa P25 | Phenol | 290–303 | 13.6 | Ray et al. [27] |
| Degussa P25 | Phenol | 296–310 | 10.6–15.8 | This study |
| Degussa P25 | o-Cresol | 296–310 | 7.0–10.5 | This study |
| Degussa P25 | m-Cresol | 296–310 | 11.6–17.1 | This study |
4. Conclusions
- Ten nanometer diameter TiO2 particles combined with an operating temperature of 37 °C were the optimum conditions to effectively degrade the reactants.
- The apparent degradation rate constant trend for the reactants was as follows: o-cresol > m-cresol > phenol.
- No interaction effects were observed between the experimental factors (particle size, temperature and reactant). The interaction was observed only for a paired combination of particle sizes.
- The modified response surface regression model was adequate for relating the apparent degradation rate constant to the experimental factors within the range of conditions under consideration.
- The apparent degradation rate constant followed an Arrhenius temperature dependence with an increasing linear trend for the three reactants.
- The activation energy was lowest for the degradation of o-cresol using 10-nm TiO2 particles.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Phenol: Environmental Health Criteria 161; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- World Health Organization. Cresols: Environmental Health Criteria 168; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- European Union. The list of priority substances in the field of water policy and amending directive, Council directive 2455/2001/ECC. Official Journal of the European Communities L331. 20 November 2001; 1–5. [Google Scholar]
- Environment Canada. The Second Priority Substances List (PSL2) of the Canadian Environmental Protection Act (CEPA); Environment Canada: Gatineau, Canada, 1995. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Sampling and Analysis Procedure for Screening of Industrial Effluents for Priority Pollutants; Environment Monitoring and Support Laboratory: Cincinnati, OH, USA, 1977. [Google Scholar]
- Pfeffer, F.M. The 1977 screening survey for measurement of organic priority pollutants in petroleum refinery wastewaters. ASTM Specif. Tech. Publ. 1979, 686, 181–190. [Google Scholar]
- Keith, L.H. Identification of organic compounds in unbleached treated kraft paper mill wastewaters. Environ. Sci. Technol. 1976, 10, 555–564. [Google Scholar] [CrossRef]
- Parkhurst, B.R.; Bradshaw, A.S.; Forte, J.L. An evaluation of the acute toxicity to aquatic biota of a coal conversion effluent and it major components. Bull. Environ. Contam. Toxicol. 1979, 23, 349–356. [Google Scholar] [CrossRef]
- Jungclaus, G.; Avila, V.; Hites, R. Organic compounds in an industrial wastewater: A case study of their environmental impact. Environ. Sci. Technol. 1978, 12, 88–96. [Google Scholar] [CrossRef]
- Kamenev, I.; Munter, R.; Pikkov, L.; Kekisheva, L. Wastewater treatment in oil shale chemical industry. Oil Shale 2003, 20, 443–457. [Google Scholar]
- Guo, Z.; Ma, R.; Li, G. Degradation of phenol by nanomaterial TiO2 in wastewater. Chem. Eng. J. 2006, 119, 55–59. [Google Scholar] [CrossRef]
- Auriol, M.; Filali-Meknassi, Y.; Tyagi, R.D.; Adams, C.D.; Surampalli, R.Y. Endocrine disrupting compounds removal from wastewater, a new challenge. Process Biochem. 2006, 41, 525–539. [Google Scholar] [CrossRef]
- Cooper, V.A.; Nicell, J.A. Removal of phenols from a foundry wastewater using horseradish peroxidise. Water Res. 1996, 30, 954–964. [Google Scholar] [CrossRef]
- Veeresh, G.S.; Kumar, P.; Mehrotra, I. Treatment of phenol and cresols in upflow anaerobic sludge blanket (UASB) process: A review. Water Res. 2005, 39, 154–170. [Google Scholar] [CrossRef]
- Hobson, M.J.; Millis, N.F. Chemostat studies of a mixed culture growing on phenolics. Res. J. Water Poll. Control Fed. 1990, 62, 684–691. [Google Scholar]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Wang, K.H.; Hsieh, Y.H.; Chen, L.J. The heterogeneous photocatalytic degradation, intermediates and mineralization for the aqueous solution of cresols and nitrophenols. J. Hazard. Mater. 1998, 59, 251–260. [Google Scholar] [CrossRef]
- Matilainena, A.; Sillanpaa, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. Chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2001, 77, 102–116. [Google Scholar]
- Dalrymple, O.K.; Yeh, D.H.; Trotz, M.A. Review: Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatlysis. J. Chem. Technol. Biotechnol. 2007, 82, 121–134. [Google Scholar] [CrossRef]
- Bouzouba, A.; Markovits, A.; Calatayud, M.; Minot, C. Comparison of the reduction of metal oxide surfaces: TiO2-anatase, TiO2-rutile and SnO2-rutile. Surf. Sci. 2005, 583, 107–117. [Google Scholar] [CrossRef]
- Hengerer, R.; Bolliger, B.; Erbudak, M.; Grätzel, M. Structure and stability of the anatase TiO2 (101) and (001) surfaces. Surf. Sci. 2000, 460, 162–169. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: A review. Water Air Soil Pollut. 2011, 215, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, K.; Yablonsky, G.S.; Ray, A.K. Kinetic studies of photocatalytic degradation in a TiO2 slurry system: Distinguishing working regimes and determining rate dependences. Ind. Eng. Chem. Res. 2003, 42, 2273–2281. [Google Scholar] [CrossRef]
- Mehrotra, K.; Yablonsky, G.S.; Ray, A.K. Macro kinetic studies for photocatalytic degradation of benzoic acid in immobilized systems. Chemosphere 2005, 60, 1427–1436. [Google Scholar] [CrossRef]
- Ray, S.; Lalman, J.A.; Biswas, N. Using the Box-Benkhen technique to statistically model phenol photocatalytic degradation by titanium dioxide nanoparticles. Chem. Eng. J. 2009, 150, 15–24. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Optimization of fermentative hydrogen production process using genetic algorithm based on neural network and response surface methodology. Int. J. Hydrog. Energy 2009, 34, 255–261. [Google Scholar] [CrossRef]
- Yetilmezsoya, K.; Demirel, S.; Vanderbei, R.J. Response surface modeling of Pb (II) removal from aqueous solution by Pistacia vera L.: Box-Behnken experimental design. J. Hazard. Mater. 2009, 171, 551–562. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 3rd ed.; Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Kowalski, S.; Cornell, J.A.; Vining, G.G. A new model and class of designs for mixture experiments with process variables. Commun. Stat. Theory Methods 2000, 29, 2255–2280. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Multiple and complex regression. In Experimental Design and Data Analysis for Biologists; Cambridge University Press: London, UK, 2002; pp. 130–139. [Google Scholar]
- Daly, L.; Bourke, G.J. Multivariate analysis and the control of cofounding. In Interpretation and Uses of Medical Statistics; Blackwell Sciences Ltd.: London, UK, 2000; pp. 340–348. [Google Scholar]
- Draper, N.R.; John, J.A. Response-surface designs for quantitative and qualitative factors. Technometrics 1988, 30, 423–428. [Google Scholar] [CrossRef]
- Aggarwal, M.L.; Chowdhury, S.R.; Bansa, A.; Mita, N. Efficient response surface designs with quantitative and qualitative factors. J. Ind. Soc. Agric. Stat. 2006, 60, 90–99. [Google Scholar]
- Allen, T.T.; Tseng, S.-H. Variance plus bias optimal response surface designs with qualitative factors applied to stem choice modeling. Qual. Reliab. Eng. Int. 2011, 27, 1199–1210. [Google Scholar] [CrossRef]
- Ray, S.; Lalman, J.A.; Biswas, N. Heterogeneous photocatalytic degradation of phenol by titanium dioxide nanoparticles: An optimization approach. In Proceedings of the AIChE Annual Meeting, Salt Lake City, UT, USA, 4–7 November 2007.
- Aliabadi, M.; Ghahremani, H.; Izadkhah, F.; Sagharigar, T. Photocatalytic degradation of N-methyl-2-pyrrolidone in aqueous solutions using light sources of UVA, UVC and UVLED. Fresenius Environ. Bull. 2012, 21, 2120–2125. [Google Scholar]
- Stephens, M.A. Tests based on EDF statistics. In Goodness-of-fit Techniques; D’Agostino, R.B., Stephens, M.A., Eds.; CRC Press: New York, NY, USA, 1986; pp. 97–194. [Google Scholar]
- Labbe, M. Photocatalytic degradation of select drinking water pollutants using nano-titanium dioxide catalyst. M.A.Sc Thesis, University of Windsor, Windsor, Ontario, Canada, 2008. [Google Scholar]
- Zhu, S.; Yang, X.; Yang, W.; Zhang, L.; Wang, J.; Huo, M. Application of porous nickel-coated TiO2 for the photocatalytic degradation of aqueous quinoline in an internal airlift loop reactor. Int. J. Environ. Res. Public Health 2012, 9, 548–563. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, C.Y. Degradation of Di-(2-ethylhexyl) phthalate (DEHP) by TiO2 photocatalysis. Water Air Soil Pollut. 2009, 200, 191–198. [Google Scholar] [CrossRef]
- Kaneco, S.; Katsumata, K.; Suzuki, T.; Ohta, K. Titanium dioxide mediated photocatalytic degradation of dibutyl phthalate in aqueous solution: Kinetics, mineralization and reaction mechanism. Chem. Eng. J. 2006, 125, 59–66. [Google Scholar] [CrossRef]
- Almquist, C.B.; Biswas, P. Role of synthesis method and particle size of nanostructured TiO2 on its photoactivity. J. Catal. 2002, 212, 145–156. [Google Scholar]
- Zhang, Z.; Wang, C.C.; Zakaria, R.; Ying, J.Y. Role of particle size in nanocrystalline TiO2 based photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B 2006, 68, 1–11. [Google Scholar]
- Albarran, G.; Bentley, J.; Schuler, R.H. Substituent effects in the reaction of OH radicals with aromatics: Toluene. J. Phys. Chem. A 2003, 107, 7770–7774. [Google Scholar] [CrossRef]
- Stephens, M.A. EDF statistics for goodness of fit and some comparisons. J. Am. Stat. Assoc. 1974, 69, 730–737. [Google Scholar] [CrossRef]
- Pande, S.; Jana, S.; Basu, S.; Sinha, A.K.; Datta, A.; Pal, T. Nanoparticle-catalyzed clock reaction. J. Phys. Chem. C 2008, 112, 3619–3626. [Google Scholar] [CrossRef]
- Musselwhite, N.E.; Wagner, S.B.; Manbeck, K.A.; Carl, L.M.; Gross, K.M.; Marsh, A.L. Activity and selectivity of colloidal platinum nanocatalysts for aqueous phase cyclohexenone hydrogenation. Appl. Catal. A 2011, 402, 104–109. [Google Scholar] [CrossRef]
- Shah, S.I.; Huang, C.P.; Chen, J.G.; Doren, D.; Barteau, M. Semiconductor metal oxide nanoparticles for visible light photocatalysis. In Proceedings of the NSF Nanoscale Science and Engineering Grantees Conference, Arlington, VA, USA, 16–18 December 2003.
- Patil, S.R.; Akpan, U.G.; Hameed, B.H.; Samdarshi, S.K. A comparative study of the photocatalytic efficiency of Degussa P25, Qualigens, and Hombikat UV-100 in the degradation kinetic of congo red dye. Desalin. Water Treat. 2012, 46, 188–195. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Kartal, Ö.E.; Erol, M.; Oǧuz, H. Photocatalytic destruction of phenol by TiO2 powders. Chem. Eng. Technol. 2001, 24, 645–649. [Google Scholar]
- Lair, A.; Ferronato, C.; Chovelon, J.M.; Herrmann, J.M. Naphthalene degradation in water by heterogeneous photocatalysis: An investigation of the influence of inorganic anions. J. Photochem. Photobiol. A 2008, 193, 193–203. [Google Scholar] [CrossRef]
- Garcia, J.C.; Takashima, K. Photocatalytic degradation of imazaquin in an aqueous suspension of titanium dioxide. J. Photochem. Photobiol. A 2003, 155, 215–222. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Choquette-Labbé, M.; Shewa, W.A.; Lalman, J.A.; Shanmugam, S.R. Photocatalytic Degradation of Phenol and Phenol Derivatives Using a Nano-TiO2 Catalyst: Integrating Quantitative and Qualitative Factors Using Response Surface Methodology. Water 2014, 6, 1785-1806. https://doi.org/10.3390/w6061785
Choquette-Labbé M, Shewa WA, Lalman JA, Shanmugam SR. Photocatalytic Degradation of Phenol and Phenol Derivatives Using a Nano-TiO2 Catalyst: Integrating Quantitative and Qualitative Factors Using Response Surface Methodology. Water. 2014; 6(6):1785-1806. https://doi.org/10.3390/w6061785
Chicago/Turabian StyleChoquette-Labbé, Marissa, Wudneh A. Shewa, Jerald A. Lalman, and Saravanan R. Shanmugam. 2014. "Photocatalytic Degradation of Phenol and Phenol Derivatives Using a Nano-TiO2 Catalyst: Integrating Quantitative and Qualitative Factors Using Response Surface Methodology" Water 6, no. 6: 1785-1806. https://doi.org/10.3390/w6061785