Benthic Communities of Low-Order Streams Affected by Acid Mine Drainages: A Case Study from Central Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
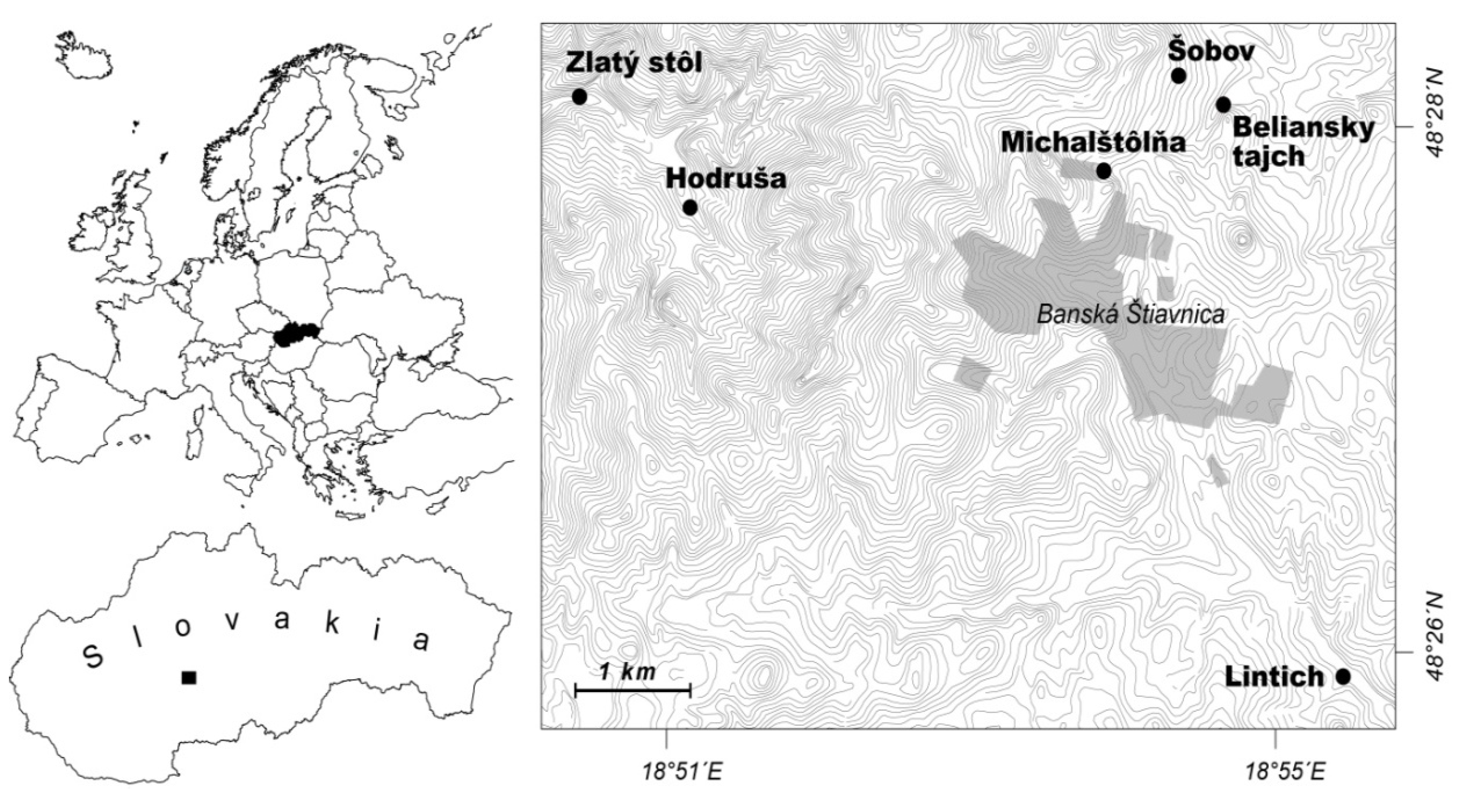
2.2. Water Chemistry and Benthic Invertebrates
2.3. Statistical Analysis
| Variable | Unit | Mean | Minimum | Maximum | PC1.chem Loading |
|---|---|---|---|---|---|
| pH | 5.63 | 2.45 | 7.20 | −0.248 | |
| Conductivity | µS cm−1 | 1971 | 363 | 6,960 | 0.256 |
| SO42− | mg L−1 | 2129.3 | 186.0 | 8330.0 | 0.256 |
| Al | mg L−1 | 72.0 | 0.01 | 430.0 | 0.257 |
| As | µg L−1 | 0.6 | 0.5 | 1.2 | −0.073 |
| Ba | µg L−1 | 41.9 | 6.3 | 135.0 | 0.251 |
| Ca | mg L−1 | 182.9 | 39.0 | 410.0 | −0.019 |
| Cd | µg L−1 | 12.6 | 0.1 | 39.6 | 0.151 |
| Co | µg L−1 | 226.1 | 0.1 | 1082.0 | 0.256 |
| Cr | µg L−1 | 37.4 | 0.5 | 221.0 | 0.257 |
| Cu | µg L−1 | 420.9 | 4.2 | 2240.0 | 0.257 |
| Fe | mg L−1 | 232.2 | 0.01 | 1390.0 | 0.257 |
| K | mg L−1 | 2.9 | 0.5 | 4.1 | −0.234 |
| Mg | mg L−1 | 68.7 | 9.5 | 221.0 | 0.251 |
| Mn | mg L−1 | 6.4 | 0.005 | 30.1 | 0.257 |
| Na | mg L−1 | 8.3 | 4.9 | 14.6 | 0.060 |
| Ni | µg L−1 | 7.9 | 2.4 | 20.6 | 0.077 |
| S | mg L−1 | 537.4 | 40.0 | 2256.0 | 0.257 |
| Si | mg L−1 | 14.5 | 3.1 | 40.2 | 0.243 |
| Sr | µg L−1 | 398.6 | 196.0 | 987.0 | −0.058 |
| Zn | µg L−1 | 780.9 | 42.3 | 4050.0 | 0.255 |
3. Results
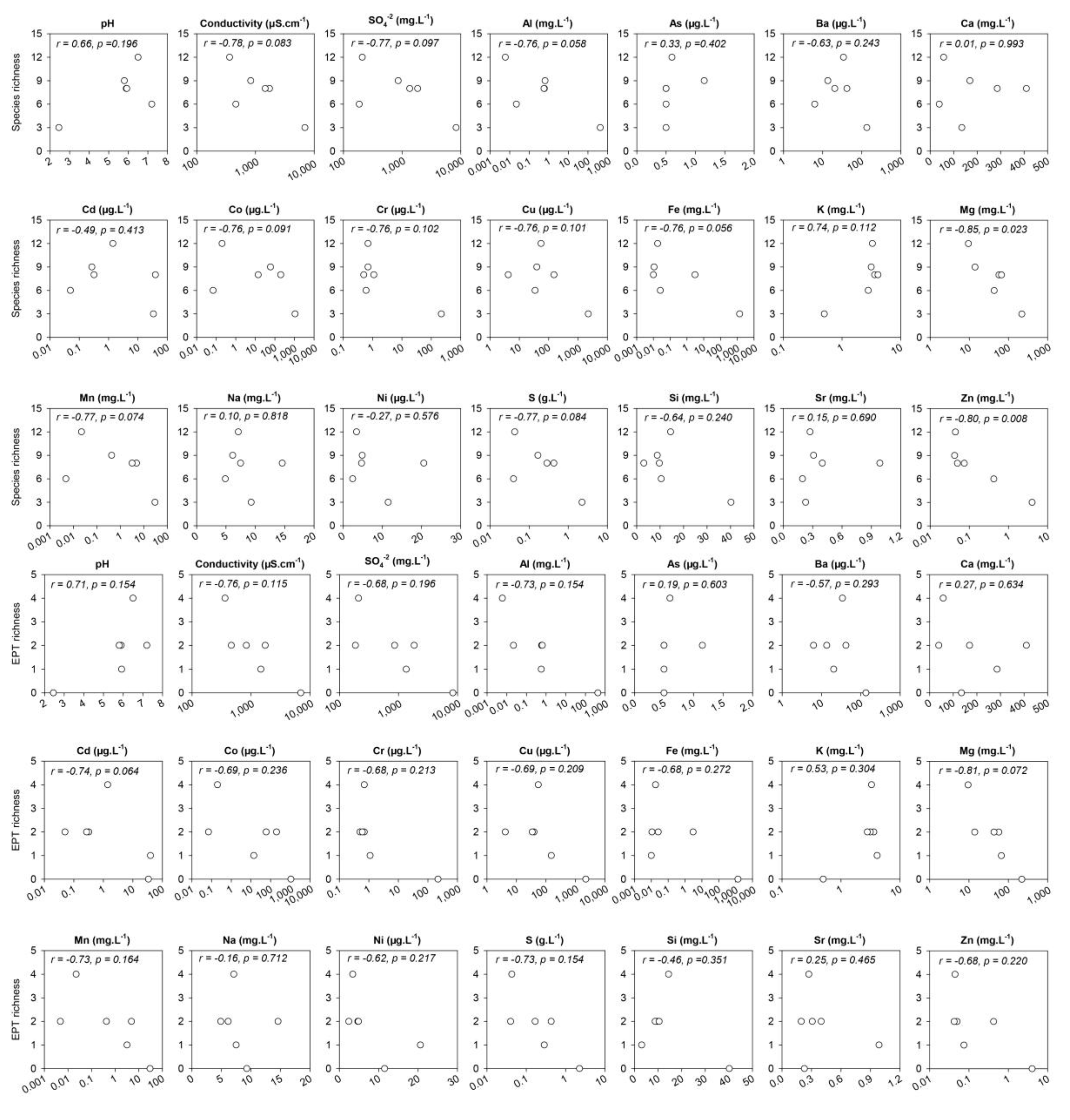
3.1. Water Chemistry
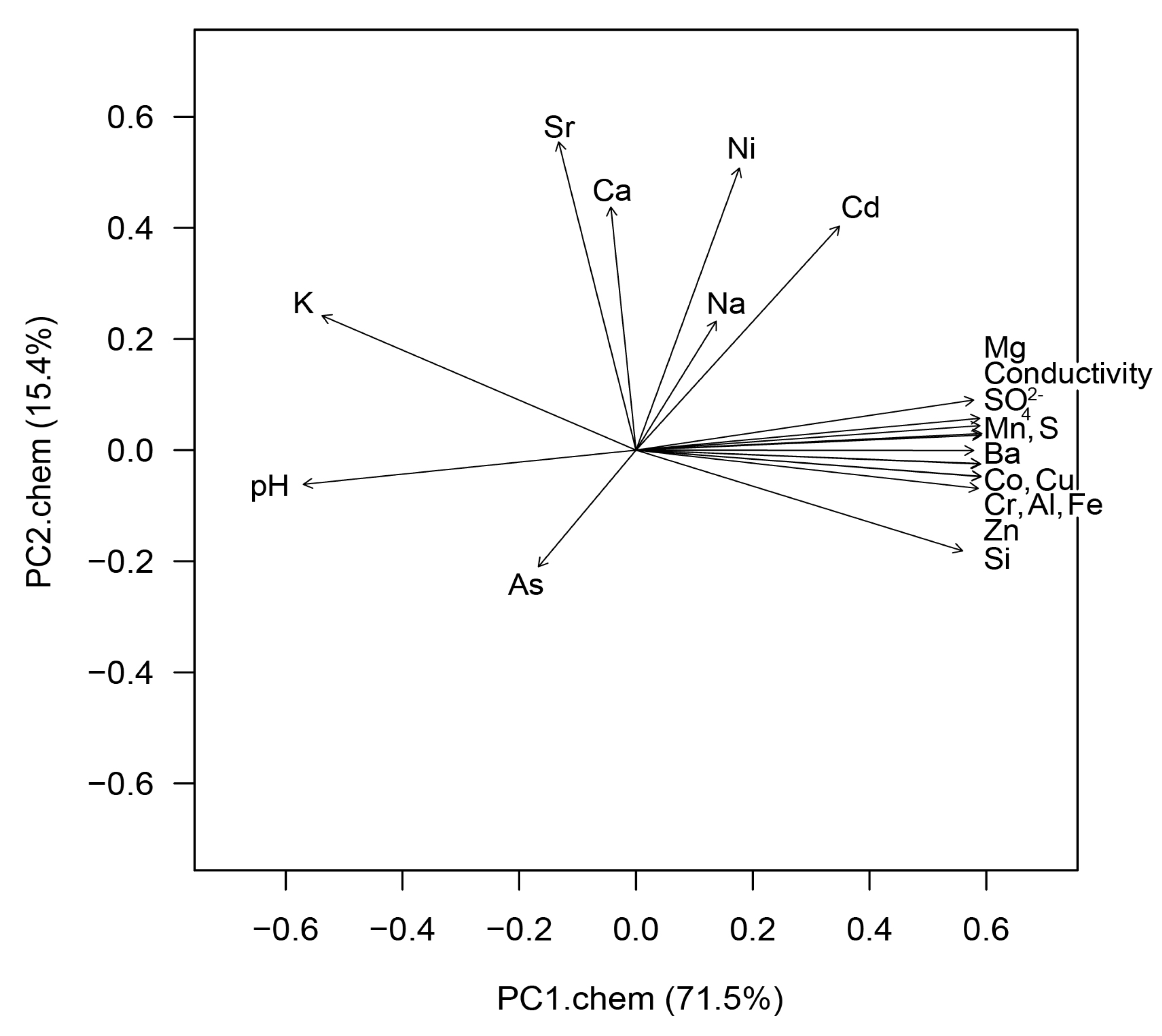
3.2. Diversity
| Taxon | Abbreviation | FFG | Frequency (%) |
|---|---|---|---|
| Turbellaria | |||
| Dugesia polychroa (Schmidt, 1861) | Duge | P | 14 |
| Oligochaeta | |||
| Oligochaeta indet. | Olig | G | 71 |
| Amphipoda | |||
| Gammarus fossarum (Koch, 1836) | Gamm | Sh | 29 |
| Plecoptera | |||
| Nemoura spp. | Nemo | Sh | 29 |
| Nemurella pictetii (Klapálek, 1900) | Nemu | G | 29 |
| Leuctra spp. | Leuc | G/Sc/Sh | 29 |
| Coleoptera | |||
| Hydroporus sp. | Hydr | P | 29 |
| Trichoptera | |||
| Plectrocnemia conspersa (Curtis, 1834) | Plec | P | 57 |
| Micropterna sp. | Micr | Sh | 14 |
| Potamophylax nigricornis (Pictet, 1834) | Pota | Sh | 14 |
| Lepidoptera | |||
| Cataclysta lemnata (Linnaeus, 1758) | Cata | Sh | 14 |
| Diptera | |||
| Tipulini indet. | Tipu | Sh | 14 |
| Molophilus sp. | Molo | G | 29 |
| Rhypholophus haemorrhoidalis (Zetterstedt, 1838) | Rhyp | P | 14 |
| Scleroprocta sp. | Scle | G | 14 |
| Eutonia sp. | Euto | P | 14 |
| Dicranota sp. | Dicr | P | 14 |
| Pedicia sp. | Pedi | P | 29 |
| Macropelopia sp. | Macr | P | 14 |
| Chaetocladius sp. | Chae | G | 29 |
| Heterotrissocladius marcidus (Walker, 1856) | Hete | G | 14 |
| Limnophyes sp. | Limn | G | 57 |
| Ceratopogoninae indet. | Cera | P | 43 |
| Forcipomyiinae indet. | Forc | Sh | 14 |
| Simuliidae indet. | Simu | F | 14 |
| Dolichopodidae indet. | Doli | P | 14 |
3.3. Assemblage Composition

4. Discussion
4.1. Water Chemistry
4.2. Diversity
4.3. Assemblage Composition
5. Conclusions
Acknowledgments
Author Contributions
Appendix
| Source | Richness | Density | Biomass | FFG | Eph | Ple | Tri | Dip | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Fe | MP | pH | Fe | MP | pH | Fe | MP | pH | Fe | MP | pH | Fe | MP | pH | Fe | MP | pH | Fe | MP | pH | Fe | MP | ||
| [11] | + | + | + | + | |||||||||||||||||||||
| [12] | + | + | + | + | + | + | |||||||||||||||||||
| [13] | + | + | + | + | + | + | |||||||||||||||||||
| [14] | + | + | + | ||||||||||||||||||||||
| [17] | + | + | |||||||||||||||||||||||
| [18] | + | + | + | ||||||||||||||||||||||
| [19] | + | + | |||||||||||||||||||||||
| [20] | + | + | + | + | + | + | + | ||||||||||||||||||
| [22] | + | + | |||||||||||||||||||||||
| [23] | + | + | + | + | |||||||||||||||||||||
| [26] | + | + | + | ||||||||||||||||||||||
| [59] | + | + | + | + | + | + | + | + | |||||||||||||||||
| [60] | + | + | + | + | + | ||||||||||||||||||||
| [62] | + | + | + | + | + | + | + | + | |||||||||||||||||
| [70] | + | ||||||||||||||||||||||||
| [84] | + | + | |||||||||||||||||||||||
| [85] | + | + | |||||||||||||||||||||||
| [86] | + | + | + | + | + | ||||||||||||||||||||
| [87] | + | ||||||||||||||||||||||||
| [88] | + | + | |||||||||||||||||||||||
| [89] | + | + | |||||||||||||||||||||||
| [90] | + | + | + | + | |||||||||||||||||||||
| [91] | + | ||||||||||||||||||||||||
| [92] | + | ||||||||||||||||||||||||
| [93] | + | + | |||||||||||||||||||||||
| [94] | + | ||||||||||||||||||||||||
| [95] | + | + | |||||||||||||||||||||||
| [96] | + | + | |||||||||||||||||||||||
| [97] | + | ||||||||||||||||||||||||
| [98] | + | ||||||||||||||||||||||||
| [99] | + | + | + | + | + | ||||||||||||||||||||
| [100] | + | + | + | + | + | + | |||||||||||||||||||
| [101] | + | + | |||||||||||||||||||||||
| [102] | + | ||||||||||||||||||||||||
| [103] | + | + | + | + | |||||||||||||||||||||
| [104] | + | + | + | + | + | ||||||||||||||||||||
| [105] | + | + | |||||||||||||||||||||||
| [106] | + | ||||||||||||||||||||||||
| [107] | + | + | + | + | + | + | |||||||||||||||||||
| [108] | + | + | |||||||||||||||||||||||
| [109] | + | + | + | + | + | + | |||||||||||||||||||
| [110] | + | + | + | + | + | + | |||||||||||||||||||
| [111] | + | + | |||||||||||||||||||||||
| [112] | + | + | + | + | |||||||||||||||||||||
| [113] | + | + | + | + | + | + | |||||||||||||||||||
| [114] | + | + | + | + | + | ||||||||||||||||||||
| Characteristic | Hodruša | Beliansky tajch | Lintich | Zlatý stôl | Michalštôlňa | Šobov |
|---|---|---|---|---|---|---|
| 48°27'39.10" N 18°51'12.64" E | 48°28'14.23" N 18°54'37.67" E | 48°26'1.18" N 18°55'40.07" E | 48°28'3.18" N 18°50'26.36" E | 48°27'56.17" N 18°53'52.92" E | 48°28'20.23" N 18°54'19.36" E | |
| Altitude (m) | 559 | 582 | 460 | 520 | 660 | 650 |
| Type of mining activity | Abandoned mine adit for polymetallic and silver ores mining | Abandoned drainage mine adit | Abandoned slag heap | Abandoned drainage mine adit | Abandoned spoil dump | Abandoned spoil dump |
| Source of contamination | outflow | outflow | drainage | outflow | drainage | drainage |
| Average stream width (m) | 2.1 | 1.5 | 1.3 | 1.0 | 1.1 | 0.9 |
| Average stream depth (m) | 0.17 | 0.12 | 0.14 | 0.09 | 0.09 | 0.06 |
| Proportion of substrates (% Co/Pe/CG/CPOM) * | 30/15/35/20 | 40/0/20/40 | 30/10/30/30 | 20/10/40/30 | 30/10/30/30 | 50/10/20/20 |
| Presence of metal hydroxides precipitates ** | 3 | 3 | 2 | 1 | 1 + | 2 |
| pH | 5.80 | 5.90 | 6.50 | 7.20 | 5.92 | 2.45 |
| Conductivity (µS cm−1) | 831 | 1740 | 363 | 464 | 1465 | 6960 |
| SO42−(mg L−1) | 860 | 1840 | 210 | 186 | 1350 | 8330 |
| Al (mg L−1) | 0.645 | 0.594 | 0.006 | 0.022 | 0.570 | 430.0 |
| As (µg L−1) | 1.15 | 0.50 | 0.60 | 0.50 | 0.50 | 0.50 |
| Ba (µg L−1) | 13.6 | 41.9 | 34.2 | 6.3 | 20.5 | 135.0 |
| Ca (mg L−1) | 169.5 | 410.0 | 57.7 | 39.0 | 286.0 | 135.0 |
| Cd (µg L−1) | 0.27 | 0.32 | 1.40 | 0.05 | 39.60 | 33.80 |
| Co (µg L−1) | 60.0 | 200.0 | 0.2 | 0.1 | 14.1 | 1082.0 |
| Cr (µg L−1) | 0.7 | 0.5 | 0.7 | 0.6 | 1.1 | 221.0 |
| Cu (µg L−1) | 39.5 | 4.2 | 55.0 | 34.4 | 152.0 | 2240.0 |
| Fe (mg L−1) | 0.01 | 3.02 | 0.02 | 0.03 | 0.01 | 1390.00 |
| K (mg L−1) | 3.2 | 3.6 | 3.3 | 2.8 | 4.1 | 0.5 |
| Mg (mg L−1) | 14.1 | 57.4 | 9.5 | 43.3 | 66.7 | 221.0 |
| Mn (mg L−1) | 0.426 | 4.900 | 0.022 | 0.005 | 3.195 | 30.100 |
| Na (mg L−1) | 6.2 | 14.6 | 7.1 | 4.9 | 7.5 | 9.3 |
| Ni (µg L−1) | 4.9 | 4.7 | 3.4 | 2.4 | 20.6 | 11.5 |
| S (mg L−1) | 168.5 | 430.0 | 43.0 | 40.0 | 287.0 | 2256.0 |
| Si (mg L−1) | 8.8 | 9.7 | 14.5 | 10.5 | 3.1 | 40.2 |
| Sr (µg L−1) | 309.0 | 398.6 | 273.0 | 196.0 | 987.0 | 228.0 |
| Zn (µg L−1) | 42.3 | 50.0 | 44.3 | 424.0 | 74.8 | 4050.0 |
| Variable | pH | Conductivity | SO42− | Al | As | Ba | Ca | Cd | Co | Cr | Cu | Fe | K | Mg | Mn | Na | Ni | S | Si | Sr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | − | −0.97 | −0.98 | −0.95 | 0.09 | −0.95 | −0.08 | −0.61 | −0.96 | −0.95 | −0.95 | −0.95 | 0.81 | −0.92 | −0.97 | −0.30 | −0.38 | −0.97 | −0.87 | 0.11 | −0.92 |
| Conductivity | 0.016 | - | 1.00 | 0.98 | −0.28 | 0.97 | 0.05 | 0.62 | 0.99 | 0.98 | 0.98 | 0.98 | −0.86 | 0.98 | 1.00 | 0.31 | 0.36 | 1.00 | 0.90 | −0.15 | 0.96 |
| SO42− | 0.010 | 0.003 | - | 0.98 | −0.25 | 0.97 | 0.04 | 0.60 | 0.99 | 0.98 | 0.98 | 0.98 | −0.87 | 0.97 | 1.00 | 0.31 | 0.34 | 1.00 | 0.91 | −0.17 | 0.96 |
| Al | 0.010 | 0.013 | 0.013 | - | −0.23 | 0.96 | −0.16 | 0.55 | 0.98 | 1.00 | 1.00 | 1.00 | −0.94 | 0.96 | 0.99 | 0.15 | 0.25 | 0.98 | 0.96 | −0.28 | 1.00 |
| As | 0.832 | 0.472 | 0.505 | 0.598 | - | −0.31 | −0.11 | −0.37 | −0.24 | −0.24 | −0.24 | −0.24 | 0.12 | −0.41 | −0.30 | −0.33 | −0.27 | −0.26 | −0.22 | −0.18 | −0.26 |
| Ba | 0.042 | 0.028 | 0.028 | 0.064 | 0.405 | - | −0.01 | 0.51 | 0.98 | 0.96 | 0.96 | 0.96 | −0.86 | 0.93 | 0.98 | 0.37 | 0.23 | 0.97 | 0.94 | −0.25 | 0.94 |
| Ca | 0.878 | 0.915 | 0.931 | 0.822 | 0.830 | 0.994 | - | 0.18 | −0.02 | −0.16 | −0.16 | −0.16 | 0.40 | 0.03 | 0.00 | 0.81 | 0.36 | 0.01 | −0.30 | 0.54 | −0.21 |
| Cd | 0.205 | 0.154 | 0.224 | 0.257 | 0.598 | 0.295 | 0.743 | - | 0.50 | 0.56 | 0.60 | 0.55 | −0.28 | 0.68 | 0.60 | 0.02 | 0.94 | 0.59 | 0.34 | 0.62 | 0.54 |
| Co | 0.012 | 0.013 | 0.010 | 0.009 | 0.635 | 0.026 | 0.967 | 0.253 | - | 0.98 | 0.98 | 0.98 | −0.91 | 0.96 | 0.99 | 0.31 | 0.22 | 0.99 | 0.94 | −0.29 | 0.97 |
| Cr | 0.052 | 0.072 | 0.073 | 0.080 | 0.634 | 0.095 | 0.765 | 0.177 | 0.143 | - | 1.00 | 1.00 | −0.94 | 0.96 | 0.99 | 0.15 | 0.25 | 0.98 | 0.96 | −0.28 | 1.00 |
| Cu | 0.075 | 0.066 | 0.070 | 0.088 | 0.499 | 0.095 | 0.765 | 0.176 | 0.148 | 0.003 | - | 1.00 | −0.92 | 0.96 | 0.98 | 0.13 | 0.30 | 0.98 | 0.95 | −0.23 | 0.99 |
| Fe | 0.066 | 0.028 | 0.030 | 0.062 | 0.302 | 0.023 | 0.798 | 0.279 | 0.033 | 0.170 | 0.166 | - | −0.94 | 0.96 | 0.99 | 0.15 | 0.25 | 0.98 | 0.96 | −0.28 | 1.00 |
| K | 0.152 | 0.151 | 0.156 | 0.137 | 0.766 | 0.137 | 0.436 | 0.684 | 0.123 | 0.153 | 0.144 | 0.101 | - | −0.84 | −0.88 | 0.02 | 0.05 | −0.88 | −0.97 | 0.57 | −0.95 |
| Mg | 0.081 | 0.014 | 0.026 | 0.034 | 0.226 | 0.073 | 0.968 | 0.128 | 0.057 | 0.056 | 0.051 | 0.051 | 0.143 | - | 0.98 | 0.27 | 0.41 | 0.98 | 0.86 | −0.08 | 0.96 |
| Mn | 0.022 | 0.003 | 0.002 | 0.018 | 0.372 | 0.021 | 1.000 | 0.214 | 0.009 | 0.065 | 0.060 | 0.037 | 0.149 | 0.012 | - | 0.29 | 0.32 | 1.00 | 0.92 | −0.19 | 0.98 |
| Na | 0.465 | 0.429 | 0.425 | 0.699 | 0.400 | 0.352 | 0.052 | 0.942 | 0.434 | 0.791 | 0.791 | 0.696 | 0.959 | 0.536 | 0.435 | - | 0.03 | 0.29 | 0.13 | 0.08 | 0.11 |
| Ni | 0.381 | 0.396 | 0.442 | 0.524 | 0.594 | 0.648 | 0.466 | 0.020 | 0.738 | 0.520 | 0.484 | 0.594 | 0.911 | 0.342 | 0.446 | 0.944 | - | 0.32 | 0.01 | 0.85 | 0.23 |
| S | 0.010 | 0.002 | 0.002 | 0.013 | 0.503 | 0.026 | 0.996 | 0.217 | 0.009 | 0.071 | 0.075 | 0.036 | 0.150 | 0.022 | 0.002 | 0.438 | 0.456 | - | 0.92 | −0.19 | 0.97 |
| Si | 0.140 | 0.142 | 0.137 | 0.152 | 0.534 | 0.062 | 0.585 | 0.529 | 0.101 | 0.143 | 0.138 | 0.077 | 0.018 | 0.158 | 0.132 | 0.730 | 0.979 | 0.133 | - | −0.50 | 0.96 |
| Sr | 0.737 | 0.697 | 0.645 | 0.482 | 0.700 | 0.483 | 0.246 | 0.149 | 0.419 | 0.485 | 0.480 | 0.435 | 0.192 | 0.835 | 0.639 | 0.843 | 0.096 | 0.621 | 0.209 | - | −0.31 |
| Zn | 0.149 | 0.106 | 0.119 | 0.120 | 0.203 | 0.156 | 0.697 | 0.229 | 0.139 | 0.109 | 0.080 | 0.071 | 0.032 | 0.073 | 0.116 | 0.877 | 0.818 | 0.125 | 0.069 | 0.328 | - |
| Taxon | Hodruša | Beliansky tajch | Lintich | Zlatý stôl | Michalštôlňa | Šobov |
|---|---|---|---|---|---|---|
| Turbellaria | ||||||
| Dugesia polychroa (Schmidt, 1861) | + | |||||
| Oligochaeta | ||||||
| Oligochaeta indet | + | + | + | + | + | |
| Amphipoda | ||||||
| Gammarus fossarum (Koch, 1836) | + | + | ||||
| Plecoptera | ||||||
| Nemoura spp. | + | + | ||||
| Nemurella pictetii (Klapálek, 1900) | + | + | ||||
| Leuctra spp. | + | + | ||||
| Coleoptera | ||||||
| Hydroporus sp. | + | + | ||||
| Trichoptera | ||||||
| Plectrocnemia conspersa (Curtis, 1834) | + | + | + | + | ||
| Micropterna sp. | + | |||||
| Potamophylax nigricornis (Pictet, 1834) | + | |||||
| Lepidoptera | ||||||
| Cataclysta lemnata (Linnaeus, 1758) | + | |||||
| Diptera | ||||||
| Tipulini indet | + | |||||
| Molophilus sp. | + | + | ||||
| Rhypholophus haemorrhoidalis (Zetterstedt, 1838) | + | |||||
| Scleroprocta sp. | + | |||||
| Eutonia sp. | + | |||||
| Dicranota sp. | + | |||||
| Pedicia sp. | + | + | ||||
| Macropelopia sp. | + | |||||
| Chaetocladius sp. | + | + | ||||
| Heterotrissocladius marcidus (Walker, 1856) | + | |||||
| Limnophyes sp. | + | + | + | + | ||
| Ceratopogoninae indet. | + | + | + | |||
| Forcipomyiinae indet. | + | |||||
| Simuliidae indet. | + | |||||
| Dolichopodidae indet. | + |
Conflicts of Interest
References
- Kelly, M. Mining and the Freshwater Environment; Elsevier Applied Science: London, UK, 2008; p. 228. [Google Scholar]
- Boult, S.; Collins, D.N.; White, K.N.; Curtis, C.D. Metal transport in a stream polluted by acid mine drainage—The Afon Goch, Anglesey, UK. Environ. Pollut. 1994, 84, 279–284. [Google Scholar] [CrossRef]
- Alpers, C.N.; Blowes, D.W. Environmental Geochemistry of Sulfide Oxidation; American Chemical Society: Washington, DC, USA, 1994; p. 681. [Google Scholar]
- Cherry, D.S.; Currie, R.J.; Soucek, D.J.; Latimer, H.A.; Trent, G.C. An integrative assessment of a watershed impacted by abandoned mine land discharges. Environ. Pollut. 2001, 111, 377–388. [Google Scholar] [CrossRef]
- Niyogi, D.K.; Lewis, W.M., Jr.; McKnight, D.M. Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 2002, 5, 554–567. [Google Scholar]
- Bray, J.P.; Broady, P.A.; Niyogi, D.K.; Harding, J.S. Periphyton communities in New Zealand streams impacted by acid mine drainage. Marine Freshw. Res. 2008, 59, 1084–1091. [Google Scholar] [CrossRef]
- De La Peña, S.; Barreiro, R. Biomonitoring acidic drainage impact in a complex setting using periphyton. Environ. Monit. Assess. 2009, 150, 351–363. [Google Scholar] [CrossRef]
- Hynes, H.B. The Biology of Polluted Waters; Liverpool University Press: Liverpool, UK, 1960; p. 202. [Google Scholar]
- Thorp, V.J.; Lake, P.S. Pollution of a Tasmanian river by mine efluents. II. Distribution of macroinvertebrates. Int. Rev. Ges. Hydrobiol. 1973, 58, 885–892. [Google Scholar] [CrossRef]
- Rosenberg, D.M.; Resh, V.H. Freshwater Biomonitoring and Benthic Macroinvertebrates; Chapman and Hall: New York, NY, USA, 1993; p. 488. [Google Scholar]
- Kiffney, P.M.; Clements, W.H. Effects of metals on stream macroinvertebrate assemblages from different altitudes. Ecol. Appl. 1996, 6, 472–481. [Google Scholar] [CrossRef]
- Malmqvist, B.; Hoffsten, P.O. Influence of drainage from old mine deposits on benthic macroinvertebrates in central Swedish streams. Water Res. 1999, 33, 2415–2423. [Google Scholar] [CrossRef]
- Merovich, G.T.; Petty, J.T. Continuous response of benthic macroinvertebrate assemblages to a discrete disturbance gradient: Consequences for diagnosing stressors. J. N. Am. Benthol. Soc. 2010, 294, 1241–1257. [Google Scholar] [CrossRef]
- Gray, N.F.; Delaney, E. Comparison of benthic macroinvertebrate indices for the assessment of the impact of acid mine drainage on an Irish river below an abandoned Cu-S mine. Environ. Pollut. 2008, 155, 31–40. [Google Scholar] [CrossRef]
- Vuori, K.M. Direct and indirect effects of iron on river ecosystems. Ann. Zool. Fenn. 1995, 32, 317–329. [Google Scholar]
- Northington, R.M.; Benfield, E.F.; Schoenholtz, S.H.; Timpano, A.J.; Webster, J.R.; Zipper, C. An assessment of structural attributes and ecosystem function in restored Virginia coalfield streams. Hydrobiologia 2011, 671, 51–63. [Google Scholar] [CrossRef]
- Battaglia, M.; Hose, G.C.; Turak, E.; Warden, B. Depauperate macroinvertebrates in a mine affected stream: Clean water may be the key to recovery. Environ. Pollut. 2005, 138, 132–141. [Google Scholar] [CrossRef]
- Fritz, K.M.; Fulton, S.; Johnson, B.R.; Barton, C.D.; Jack, J.D.; Word, D.A.; Burke, R.A. Structural and functional characteristics of natural and constructed channels draining a reclaimed mountaintop removal and valley fill coal mine. J. N. Am. Benthol. Soc. 2010, 29, 673–689. [Google Scholar] [CrossRef]
- Winterbourn, M.J.; McDiffett, W.F.; Eppley, S.J. Aluminium and iron burdens of aquatic biota in New Zealand streams contaminated by acid mine drainage: Effects of trophic level. Sci. Total Environ. 2000, 254, 45–54. [Google Scholar] [CrossRef]
- Ross, R.M.; Long, E.S.; Dropkin, D.S. Response of macroinvertebrate communities to remediation-simulating conditions in Pennsylvania streams influenced by acid mine drainage. Environ. Monit. Assess. 2008, 145, 323–338. [Google Scholar] [CrossRef]
- Hogsden, K.L.; Harding, J.S. Anthropogenic and natural sources of acidity and metals and their influence on the structure of stream food webs. Environ. Pollut. 2012, 162, 466–474. [Google Scholar] [CrossRef]
- Van Damme, P.A.; Hamel, C.; Ayala, A.; Bervoets, L. Macroinvertebrate community response to acid mine drainage in rivers of the High Andes (Bolivia). Environ. Pollut. 2008, 156, 1061–1068. [Google Scholar] [CrossRef]
- Gray, N.F.; Delaney, E. Measuring community response of bentic macroinvertebrates in an erosional river impacted by acid mine drainage by use of a simple model. Ecol. Indic. 2010, 10, 668–675. [Google Scholar] [CrossRef]
- Kimmel, W.G. The impact of acid mine drainage on the stream ecosystem. In Pennsylvania Coal: Resources, Technology and Utilization; Majumdar, S.K., Miller, E.W., Eds.; Pennsylvania Academy of Science: Easton, PA, USA, 1983; pp. 424–437. [Google Scholar]
- Lin, C.; Wu, Y.; Lu, W.; Chen, A.; Liu, Y. Water chemistry and ecotoxicity of an acid mine drainage-affected stream in subtropical China during a major flood event. J. Hazard. Mater. 2007, 142, 199–207. [Google Scholar] [CrossRef]
- Tripole, S.; Gonzales, P.; Vallania, A.; Garbagnati, M.; Mallea, M. Evaluation of the impact of acid mine drainage on the chemistry and the macrobenthos in the Carolina Stream (San Luis–Argentina). Environ. Monit. Assess. 2006, 114, 377–389. [Google Scholar] [CrossRef]
- Allouache, A.; Michalková, E.; Veverka, M.; Veverková, D. Soil moisture variability and acid mine drainage in the spoil dump of pyrytized hydroquarzite in the region of Banská Štiavnica, Slovakia. Carpath. J. Earth Environ. Sci. 2009, 4, 56–64. [Google Scholar]
- Križáni, I.; Andráš, P.; Šlesárová, A. Percolation modelling of the dump and settlig pit sediment at the Banská Štiavnica ore-field (Western Carpathians, Slovakia). Carpath. J. Earth Environ. Sci. 2009, 4, 109–125. [Google Scholar]
- lauková, E.; Michalková, E.; Máša, B.; Welward, L. Chemotrophic. Acidophilic Microflora of Acid Mine Drainage and Their Utilization; Janka Čižmárová—Partner: Poniky, Slovakia, 2011; p. 174. (In Slovak) [Google Scholar]
- Konečný, V. Vysvetlivky. ku Geologickej Mape Štiavnických. Vrchov a Pohronskéhoovca. (Štiavnický. Stratovulkán) I. Diel.; Konečný, V., Ed.; Vydavateľstvo Dionýza Štúra: Bratislava, Slovakia, 1998; p. 248. (In Slovak) [Google Scholar]
- Miklós, L. Landscape Atlas of the Slovak Republic, 1st ed.; Miklós, L., Ed.; Ministry of Environment of the Slovak Republic, Slovak Environmental Agency: Banská Bystrica, Slovakia, 2002. [Google Scholar]
- Horáková, M.; Lischke, P.; Grünwald, A. Chemical and Physical Methods of Water Analyses; Nakladatelství Technické Literatúry: Prague, Czech Republic, 1986; p. 389. (In Czech) [Google Scholar]
- Frost, S.; Huni, A.; Kershaw, W.E. Evaluation of a kicking technique for sampling stream bottom fauna. Can. J. Zool. 1971, 49, 167–173. [Google Scholar] [CrossRef]
- Krno, I. Determinačný. Kľúč pre Hydrobiológov. Časť. II. Pošvatky. (Plecoptera.); Výskumný ústav vodného hospodárstva v Bratislave: Bratislava, Slovakia, 2013; p. 63. (In Slovak) [Google Scholar]
- Nilsson, A.N. Aquatic Insects of North Europe: A Taxonomic Handbook. Odonata—Diptera; Apollo Books: Stenstrup, Denmark, 1997; Volume 2, p. 440. [Google Scholar]
- Rozkošný, R. Klíč. vodních larev hmyzu; Rozkošný, R., Ed.; Academia: Praha, Czech Republic, 1980; p. 523. (In Czech) [Google Scholar]
- Saether, O.A. Nearctic and Palaearctic Heterotrissocladius (Diptera: Chironomidae); Fisheries and Marine Service: Ann Arbor, MI, USA, 1975; Volume 193, p. 67. [Google Scholar]
- Waringer, J.; Graf, W. Atlas der Mitteleuropäischen Köcherfliegenlarven; Erik Mauch Verlag: Dinkelscherben, Germany, 2011; p. 468. [Google Scholar]
- Andersen, T.; Cranston, P.S.; Epler, J.H. Chironomidae of the Holarctic Region. Keys and Diagnoses, Part I. Larvae; Andersen, T., Cranston, P.S., Epler, J.H., Eds.; Entomological Society of Lund: Lund, Sweden, 2013; p. 573. [Google Scholar]
- porka, F. Vodné Bezstavovce (Makroevertebráta) Slovenska, Súpis Druhov a Autekologické Charakteristiky; Šporka, F., Ed.; Slovenský Hydrometeorologický Ústav: Bratislava, Slovakia, 2003; p. 590. (In Slovak) [Google Scholar]
- Jackson, D.A. Stopping rules in principal components analysis: A comparison of heuristical and statistical approaches. Ecology 1993, 74, 2204–2214. [Google Scholar] [CrossRef]
- Gower, J.C. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 1966, 53, 325–338. [Google Scholar] [CrossRef]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application in analysis of the vegetation on Danish commons. Biologiske Skrifter Det Kongelige Danske Videnskabernes Selskab 1948, 5, 1–34. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community ecology package. R package Version 2.0–6. 2013. Available online: http://CRAN.R-project.org/package=vegan (accessed on 20 February 2013).
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall: London, UK, 1989; p. 511. [Google Scholar]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Wood, S.N. Modelling and smoothing parameter estimation with multiple quadratic penalties. J. R. Statist. Soc. B 2000, 62, 413–428. [Google Scholar] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Goslee, S.C.; Urban, D.L. The ecodist package for dissimilarity-based analysis of ecological data. J. Statist. Softw. 2007, 22, 1–19. [Google Scholar]
- Valúchová, M. Hodnotenie. Kvality Povrchovej vody Slovenska. za rok 2010. Slovenský Vodohospodársky Podnik, š.p., Slovenský Hydrometeorologický Ústav; Valúchová, M., Ed.; Výskumný Ústav Vodného Hospodárstva: Bratislava, Slovakia, 2010; p. 128. (In Slovak) [Google Scholar]
- Equeenuddin, S.M.; Tripathy, S.; Sahoo, P.K.; Panigrahi, M.K. Hydrogeochemical characteristics of acid mine drainage and water pollution at Makum Coalfield, India. J. Geochem. Explor. 2010, 105, 75–82. [Google Scholar] [CrossRef]
- Máša, B.; Pulišová, P.; Bezdička, P.; Michalková, E.; Šubrt, J. Ochre precipitates and acid mine drainage in a mine environment. Ceram. Silik. 2012, 56, 9–14. [Google Scholar]
- Sánchez-España, J.S.; Pamo, E.L.; Esther Santofimia, E.; Aduvire, O.; Reyes, J.; Barettino, D. Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): Geochemistry, mineralogy and environmental implications. Appl. Geochem. 2005, 20, 1320–1356. [Google Scholar] [CrossRef]
- Sarmiento, A.M.; Nieto, J.M.; Olías, M.; Cánovas, C.R. Hydrochemical characteristics and seasonal influence on the pollution by acid mine drainage in the Odiel river Basin (SW Spain). Appl. Geochem. 2009, 24, 697–714. [Google Scholar] [CrossRef]
- Armitage, P.D. The effects of mine drainage and organic enrichment in benthos in the river Nent system, northern Pennines. Hydrobiologia 1980, 74, 119–128. [Google Scholar] [CrossRef]
- Giberson, D.J.; Mackay, R.J. Life history and distribution of mayflies (Ephemeroptera) in some acid streams in south central Ohio. Can. J. Zool. 1991, 69, 899–910. [Google Scholar] [CrossRef]
- Courtney, L.A.; Clements, W.H. Effects of acidic pH on benthic macroinvertebrate communities in stream microcosms. Hydrobiologia 1998, 379, 135–145. [Google Scholar] [CrossRef]
- Soucek, D.J.; Cherry, D.S.; Currie, R.J.; Latimer, H.A.; Trent, G.C. Laboratory to field validation in an integrative assessment of an acid mine drainage-impacted watershed. Environ. Toxicol. Chem. 2000, 19, 1036–1043. [Google Scholar]
- Gerhardt, A.; Janssens de Bisthoven, L.; Soares, A.M.V.M. Macroinvertebrate response to acid mine drainage: Community metrics and on-line behavioural toxicity bioassay. Environ. Pollut. 2004, 130, 263–274. [Google Scholar] [CrossRef]
- Clements, W.H.; Carlisle, D.M.; Lazorchak, J.M.; Johnson, P.C. Heavy metals structure benthic communities in Colorado mountain streams. Ecol. Appl. 2000, 10, 626–638. [Google Scholar] [CrossRef]
- Winner, R.W.; Boesel, M.W.; Farrell, M.P. Insect community structure as an index of heavy-metal pollution in lotic ecosystems. Can. J. Fish. Aquat. Sci. 1980, 37, 647–655. [Google Scholar] [CrossRef]
- Loayza-Muro, R.A.; Elías-Letts, R.; Marticorena-Ruíz, J.K.; Palomino, E.J.; Duivenvoorden, J.F.; Kraak, M.H.S.; Admiraal, W. Metal-induced shifts in benthic macroinvertebrate community composition in Andean high altitude streams. Environ. Toxicol. Chem. 2010, 29, 1–8. [Google Scholar] [CrossRef]
- Horecký, J.; Rucki, J.; Krám, P.; Křeček, J.; Bitušík, P.; Stuchlík, E. Differences in benthic macroinvertebrate structure of headwater streams with extreme hydrochemistry. Biologia 2013, 68, 303–313. [Google Scholar] [CrossRef]
- García-Criado, F.; Tomé, A.; Vega, F.J.; Antolín, C. Performance of some diversity and biotic indices in rivers affected by coal mining in northwestern Spain. Hydrobiologia 1999, 394, 209–217. [Google Scholar] [CrossRef]
- Krno, I.; Šporka, F.; Galas, J.; Hamerlík, L.; Zaťovičová, Z.; Bitušík, P. Littoral benthic macroinvertebrates of mountain lakes in the Tatra Mountains (Slovakia, Poland). Biologia 2006, 61, 147–166. [Google Scholar] [CrossRef]
- Szczesny, B. Benthic macroinvertebrates in the acidified headstreams of the Vistula river. Stud. Nat. 1998, 44, 145–170. [Google Scholar]
- Horecký, J.; Stuchlík, E.; Chvojka, P.; Hardekopf, D.W.; Mihaljevič, M.; Špaček, J. Macroinvertebrate community and chemistry of the most atmospherically acidified streams in the Czech Republic. Water Air Soil Poll. 2006, 173, 261–272. [Google Scholar] [CrossRef]
- Leland, H.V.; Carter, J.L.; Fend, S.V. Use of detrended correspondence analysis to evaluate factors controlling spatial distribution of benthic insects. Hydrobiologia 1986, 132, 113–123. [Google Scholar] [CrossRef]
- Gower, A.M.; Myers, G.; Kent, M.; Foulkes, M.E. Relationship between macroinvertebrate communities and environmental variables in metal-contaminated streams in south-west England. Freshw. Biol. 1994, 32, 199–221. [Google Scholar] [CrossRef]
- Janssens de Bisthoven, L.; Gerhardt, A.; Soares, A.M.V.M. Chironomidae larvae as bioindicators of an acid mine drainage in Portugal. Hydrobiologia 2005, 532, 181–191. [Google Scholar] [CrossRef]
- Clements, W.H. Benthic invertebrate community responses to heavy metals in the Upper Arkansas River Basin, Colorado. J. N. Am. Benthol. Soc. 1994, 13, 30–44. [Google Scholar] [CrossRef]
- Orendt, C. Chironomids as bioindicators in acidified streams: A contribution to the acidity tolerance of chironomid species with a classification in sensitivity classes. Int. Rev. Ges. Hydrobiol. 1999, 84, 439–449. [Google Scholar]
- Bitušík, P. A Contribution to the Knowledge of the Fauna of Chironomids (Chironomidae), Meniscus Midges (Dixidae) and Mosquitoes (Culicidae) of Some Water Biotopes in the Zvolen Environs; Jančová, G., Sláviková, D., Eds.; Vypra: Zvolen, Slovakia, 1994; pp. 98–106. (In Slovak) [Google Scholar]
- Oboňa, J. Structure and diversity of aquatic invertebrate communities of water filled tree holes. Ph.D. Thesis, Technical University in Zvolen, Zvolen, Slovakia, 2013. [Google Scholar]
- Schlief, J.; Mutz, M. Long-term leaf litter decomposition and associated microbial processes in extremely acidic (pH ˂ 3) mining waters. Arch. Hydrobiol. 2005, 164, 53–68. [Google Scholar]
- Lecerf, A.; Chauvet, E. Diversity and functions of leafdecaying fungi in human-altered streams. Freshw. Biol. 2008, 53, 1658–1672. [Google Scholar]
- Schlief, J.; Mutz, M. Palatability of leaves conditioned in streams affected by mine drainage: a feeding experiment with Gammarus pulex (L.). Hydrobiologia 2006, 563, 445–452. [Google Scholar] [CrossRef]
- Hogsden, K.L.; Harding, J.S. Consequences of acid mine drainage for the structure and function of benthic stream communities: A review. Freshw. Sci. 2012, 31, 108–120. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Goodyear, K.L.; McNeill, S. Bioaccumulation of heavy metals by aquatic macro-invertebrates of different feeding guilds: A review. Sci. Total Environ. 1999, 229, 1–19. [Google Scholar] [CrossRef]
- Cardwell, R.D.; DeForest, D.K.; Brix, K.V.; Adams, W.J. Do Cd, Cu, Ni, Pb, and Zn biomagnify in aquatic ecosystems? Environ. Contam. Toxicol. 2013, 226, 101–122. [Google Scholar]
- Benke, A.C. Concepts and patterns of invertebrate production in running waters. Verh. Int. Ver. Limnol. 1993, 25, 15–38. [Google Scholar]
- Menezes, S.; Baird, D.J.; Soares, A.M. Beyond taxonomy: A review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. J. Appl. Ecol. 2010, 47, 711–719. [Google Scholar] [CrossRef]
- Arnakleiv, J.V.; Størset, L. Downstream effects of mine drainage on benthos and fish in a Norwegian river: a comparison of the situation before and after river rehabilitation. J. Geochem. Explor. 1995, 52, 35–43. [Google Scholar] [CrossRef]
- Bradley, H.A. Sediment chemistry and benthic macroinvertebrate communities within an acid mine drainage impacted stream. Earth and Environment 2008, 3, 1–31. [Google Scholar]
- Bruns, D.A. Macroinvertebrate response to land cover, habitat, and water chemistry in a mining-impacted river ecosystem: A GIS watershed analysis. Aquat. Sci. 2005, 67, 403–423. [Google Scholar] [CrossRef]
- Cain, D.J.; Carter, J.L.; Fend, S.V.; Luoma, S.N.; Alpers, Ch.N.; Taylor, H.E. Metal exposure in a benthic macroinvertebrate, Hydropsyche californica, related to mine drainage in the Sacramento River. Can. J. Fish. Aquat. Sci. 2000, 57, 380–390. [Google Scholar] [CrossRef]
- Canton, S.P.; Ward, J.V. The aquatic insects, with emphasis on Trichoptera, of a Colorado Stream affected by coal strip-mine drainage. Southwest. Nat. 1981, 25, 453–460. [Google Scholar] [CrossRef]
- Clements, W.H. Metal tolerance and predator-prey interactions in benthic macroinvertebrate stream communities. Ecol. Appl. 1999, 9, 1073–1084. [Google Scholar]
- Clements, W.H. Small-scale experiments support causal relationships between metal contamination and macroinvertebrate community responses. Ecol. Appl. 2004, 14, 954–967. [Google Scholar] [CrossRef]
- Cole, M.B.; Arnold, D.E.; Watten, B.J. Physiological and behavioural response of stonefly nymphs to enhanced limestone treatment of acid mine drainage. Water Res. 2001, 35, 625–632. [Google Scholar] [CrossRef]
- David, C.P. Establishing the impact of acid mine drainage through metal bioaccumulation and taxa richness of benthic insects in a tropical Asian stream (The Philippines). Environ. Toxicol. Chem. 2003, 22, 2952–2959. [Google Scholar] [CrossRef]
- DeNicola, D.M.; Stapleton, M.G. Impact of acid mine drainage on benthic communities in streams: the relative roles of substratum vs. aqueous effects. Environ. Pollut. 2002, 119, 303–315. [Google Scholar] [CrossRef]
- Dsa, J.V.; Johnson, K.S.; Lopez, D.; Kanuckel, C.; Tumlinson, J. Residual toxicity of acid mine drainage-contaminated sediment to stream macroinvertebrates: Relative contribution of acidity vs. metals. Water Air Soil Poll. 2008, 194, 185–197. [Google Scholar] [CrossRef]
- Freund, J.G.; Petty, J.T. Response of fish and macroinvertebrate bioassessment indices to water chemistry in a mined appalachian watershed. Environ. Manage. 2007, 39, 707–720. [Google Scholar] [CrossRef]
- Gerhardt, A. Short term toxicity of iron (Fe) and lead (Pb) to the mayfly Leptophlebia marginata (L.) (Insecta) in relation to freshwater acidification. Hydrobiologia 1994, 284, 157–168. [Google Scholar] [CrossRef]
- Gerhardt, A.; Westermann, F. Effects of precipitations of iron hydroxides on Leptophlebia marginata (L.) (Insecta: Ephemeroptera) in the field. Archiv. Hydrobiol. 1995, 133, 81–93. [Google Scholar]
- Harding, J.S. Impacts of metals and mining on stream communities. In Metal Contaminants in New Zealand; Moore, T.A., Black, A., Centeno, J.A., Harding, J.S., Trumm, D.A., Eds.; Resolutionz Press: Christchurch, New Zealand, 2005; pp. 343–357. [Google Scholar]
- Heatherly, T.II.; Whiles, M.R.; Knuth, D.; James, E.; Garvey, J.E. Diversity and community structure of littoral zone macroinvertebrates in Southern Illinois reclaimed surface mine lakes. Am. Midl. Nat. 2005, 154, 67–77. [Google Scholar] [CrossRef]
- Howse, R. Chironomids abound in the acid mine drainage of the Dee River, Mt Morgan. Austr. J. Ecotoxicol. 2007, 13, 3–8. [Google Scholar]
- De Bisthoven, L.J.; Gerhardt, A.; Soares, A.M.V.M. Effects of acid mine drainage on Chironomus (Diptera, Chironomidae) measured with the multispecies freshwater biomonitor. Environ. Toxicol. Chem. 2004, 23, 1123–1128. [Google Scholar] [CrossRef]
- Kaye, A. The effects of mine drainage water from Carrock Mine on the water quality and benthic macroinvertebrate communities of Grainsgill Beck: A preliminary study. Earth Environ. 2005, 1, 120–154. [Google Scholar]
- MacCausland, A.; McTammany, M.E. The impact of episodic coal mine drainage pollution on benthic macroinvertebrates in streams in the Anthracite region of Pennsylvania. Environ. Pollut. 2007, 149, 216–226. [Google Scholar] [CrossRef]
- Merovich, G.T.; Petty, J.T. Interactive effects of multiple stressors and restoration priorities in a mined Appalachian watershed. Hydrobiologia 2007, 575, 13–31. [Google Scholar] [CrossRef]
- Merricks, T.C.H.; Cherry, D.S.; Zipper, C.E.; Currie, R.J.; Valenti, T.W. Coal-Mine Hollow Fill and Settling Pond Influences on Headwater Streams in Southern West Virginia, USA. Environ. Monit. Assess. 2007, 129, 359–378. [Google Scholar] [CrossRef]
- Nelson, S.M.; Roline, R.A. Selection of the Mayfly Rithrogena hageni as an indicator of metal pollution in the Upper Arkansas River. J. Freshw. Ecol. 1993, 8, 111–119. [Google Scholar] [CrossRef]
- Nelson, S.M.; Roline, R.A. Recovery of a stream macroinvertebrate community from mine drainage disturbance. Hydrobiologia 1996, 339, 73–84. [Google Scholar] [CrossRef]
- O’Halloran, K.; Cavanagh, J.A.; Harding, J.S. Response of a New Zealand mayfly (Deleatidium spp.) to acid mine drainage: Implications for mine remediation. Environ. Toxicol. Chem. 2008, 27, 1135–1140. [Google Scholar] [CrossRef]
- Poulton, B.C.; Monda, D.P.; Woodward, D.F.; Wildhaber, M.L.; Brumbaugh, W.G. Relation between Benthic Community Structure and Metals Concentrations in Aquatic Macroinvertebrates: Clark Fork River, Montana. J. Freshwater Ecol. 1995, 10, 277–293. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Soucek, D.J.; Cherry, D.S. Integrative assessment of benthic macroinvertebrate community impairment from metal-contaminated waters in tributaries of the Upper Powell River, Virginia, USA. Environ. Toxicol. Chem. 2002, 21, 2233–2241. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lock, R.A.C.; Van der Velde, G.; Medina Hoyos, R.I.; Roelofs, J.G.M. Effects of Mining Activities on Heavy Metal Concentrations in Water, Sediment, and Macroinvertebrates in Different Reaches of the Pilcomayo River, South America. Arch. Environ. Contam. Toxicol. 2003, 44, 314–323. [Google Scholar] [CrossRef]
- Sola, C.; Burgos, M.; Plazuelo, Á.; Toja, J.; Plans, M.; Prat, N. Heavy metal bioaccumulation and macroinvertebrate community changes in a Mediterranean stream affected by acid mine drainage and an accidental spill (Guadiamar River, SW Spain). Sci. Total Environ. 2004, 333, 109–126. [Google Scholar] [CrossRef]
- Stoertz, M.W.; Bourne, H.; Knotts, Ch.; White, M.M. The effects of isolation and acid mine drainage on fish and macroinvertebrate communities of Monday Creek, Ohio, USA. Mine Water Environ. 2002, 21, 60–72. [Google Scholar] [CrossRef]
- Watanabe, N.C.; Harada, S.; Komai, Y. Long-term recovery from mine drainage disturbance of a macroinvertebrate community in the Ichikawa River, Japan. Hydrobiologia 2000, 429, 171–180. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Svitok, M.; Novikmec, M.; Bitušík, P.; Máša, B.; Oboňa, J.; Očadlík, M.; Michalková, E. Benthic Communities of Low-Order Streams Affected by Acid Mine Drainages: A Case Study from Central Europe. Water 2014, 6, 1312-1338. https://doi.org/10.3390/w6051312
Svitok M, Novikmec M, Bitušík P, Máša B, Oboňa J, Očadlík M, Michalková E. Benthic Communities of Low-Order Streams Affected by Acid Mine Drainages: A Case Study from Central Europe. Water. 2014; 6(5):1312-1338. https://doi.org/10.3390/w6051312
Chicago/Turabian StyleSvitok, Marek, Milan Novikmec, Peter Bitušík, Branislav Máša, Jozef Oboňa, Miroslav Očadlík, and Eva Michalková. 2014. "Benthic Communities of Low-Order Streams Affected by Acid Mine Drainages: A Case Study from Central Europe" Water 6, no. 5: 1312-1338. https://doi.org/10.3390/w6051312
APA StyleSvitok, M., Novikmec, M., Bitušík, P., Máša, B., Oboňa, J., Očadlík, M., & Michalková, E. (2014). Benthic Communities of Low-Order Streams Affected by Acid Mine Drainages: A Case Study from Central Europe. Water, 6(5), 1312-1338. https://doi.org/10.3390/w6051312





