Role of Plants in a Constructed Wetland: Current and New Perspectives
Abstract
:1. Introduction
2. Role of Plants in CWs
| Role of plants in a constructed wetland | Reference |
|---|---|
| Physical effects of root structure | |
| Filtering effect | [7] |
| Velocity reduction, promotion of sedimentation, decreased resuspension | [7] |
| Prevention of medium clogging | [3] |
| Improved hydraulic conductivity | [2,14] |
| Macrophytes do not increase hydraulic conductivity and even contribute to clogging | [2,4] |
| No effect on removal of suspended solids | [7] |
| Roots as a base for microorganisms | |
| Provision of surface for microbial attachment | [2,7] |
| Root release of gas and exudates | |
| Oxygen leakage—addition of aerobic niches | [1,7,15,16] |
| Oxygen leakage—increased aerobic degradation | [17,18] |
| Oxygen leakage—supports precipitation of heavy metals | [7] |
| Oxygen leakage—increased nitrification Excretion of carbon—increased denitrification | [2,11,19,20,21] |
| Aerobic dynamics are very limited in horizontal flow CWs (HF) | [7] |
| Release of antibiotics, phytometallophores and phytochelatins | [7,22,23,24] |
| Root exudates promote metal chelation to prevent metal toxicity | [7] |
| Plant uptake | |
| Storage and uptake of nutrients | [7,25,26,27,28] |
| Plant nutrient uptake is negligible | [3,5,6,7,29,30,31] |
| Metal phytoremediation | [28,32,33] |
| Salt phytoremediation | [34] |
| Evapotranspiration | |
| Increased water loss | [35,36] |
| Microclimatic conditions | |
| Light attenuation reduces algal growth | [2] |
| Insulation from frost in the winter | [37,38,39] |
| Insulation from radiation in the spring | [3,40] |
| Reduced wind velocity | [7] |
| Stabilization of the sediment surface | [7] |
| Other functions of plants in the CW | |
| Elimination of pathogens | [41] |
| Insect and odor control | [42] |
| Enhanced mosquito reproduction | [43] |
| Wastewater gardens | [44,45] |
| Increased wildlife diversity | [2] |
| Aesthetic appearance of the system | [3,42] |
| Bioindicators | [46] |
| Plant production | |
| Ornamental plant production | [47,48,49] |
| Production of fiber for construction material | [7,50] |
| Bioenergy crops | [7,51] |
| Animal feed | (no reference, suggested in [52]) |
3. New Perspectives
3.1. Salt Phytoremediation in CW
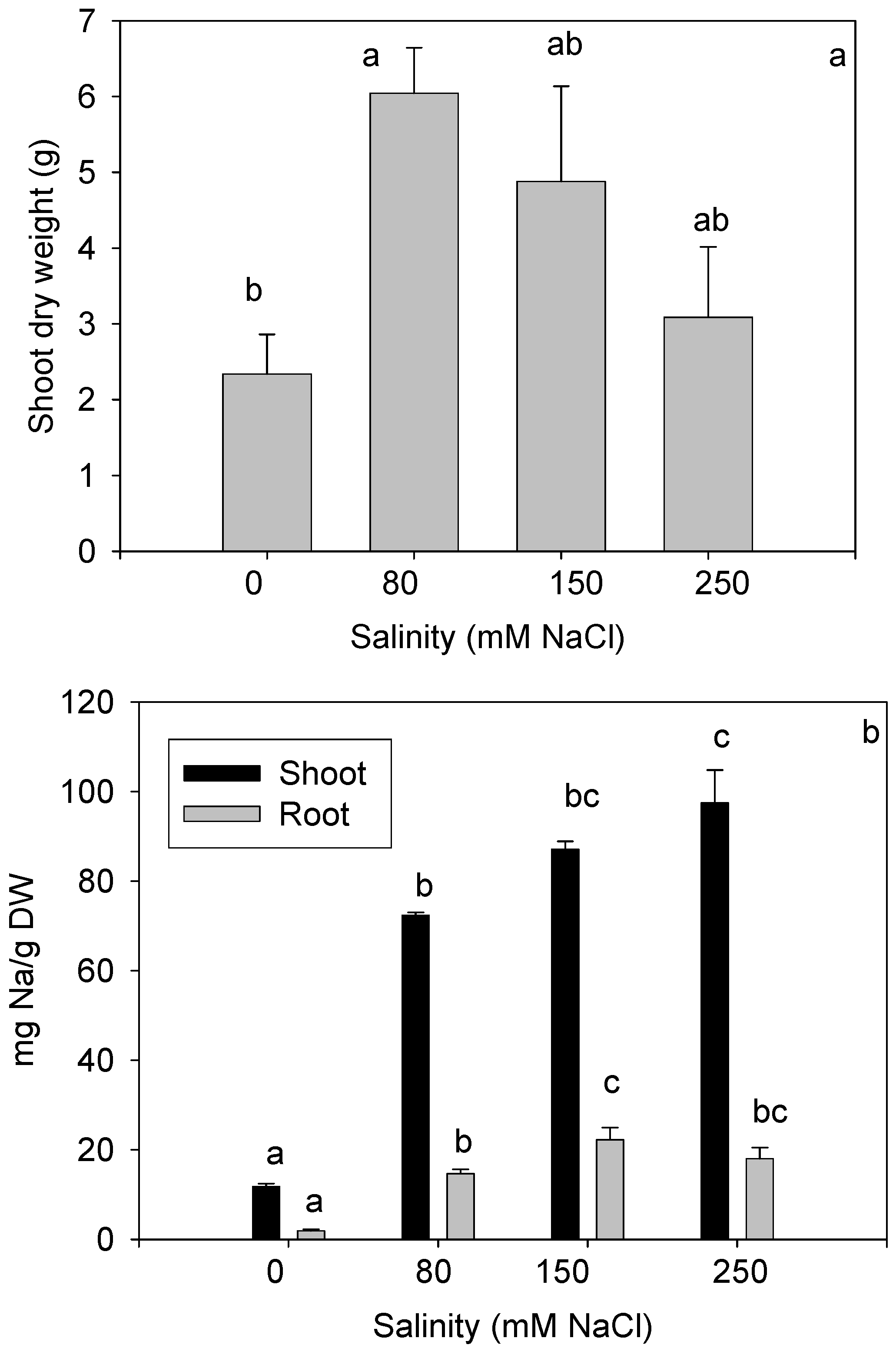
3.2. Plant Physiological Performance for CW Biomonitoring


4. Discussion
5. Conclusions
Acknowledgments
References
- Hammer, D.A.; Bastian, R.K. Wetland ecosystems: Natural water purifiers? In Constructed Wetlands for Wastewater Treatment: Municipal, Industrial and Agricultural; Hammer, D.A., Ed.; Lewis Publishers: Chelsea, MI, USA, 1989; pp. 5–20. [Google Scholar]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar]
- Brix, H. Functions of macrophytes in constructed wetlands. Water Sci. Technol. 1994, 29, 71–78. [Google Scholar]
- Stottmeister, U.; Wiessner, A.; Kuschk, P.; Kappelmeyer, U.; Kastner, M.; Bederski, O.; Muller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar] [CrossRef]
- Langergraber, G. The role of plant uptake on the removal of organic matter and nutrients in subsurface flow constructed wetlands: A simulation study. Water Sci. Technol. 2005, 51, 213–223. [Google Scholar]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Kalff, J. Limnology: Inland Water Ecosystems; Prentice-Hall: New Jersey, NJ, USA, 2002; p. 592. [Google Scholar]
- Scholz, M. Wetland Systems to Control Urban Runoff; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Sklarz, M.Y.; Gross, A.; Yakirevich, A.; Soares, M.I.M. A recirculating vertical flow constructed wetland for the treatment of domestic wastewater. Desalination 2009, 246, 617–624. [Google Scholar] [CrossRef]
- Fraser, L.H.; Carty, S.M.; Steer, D. A test of four plant species to reduce total nitrogen and total phosphorus from soil leachate in subsurface wetland microcosms. Bioresour. Technol. 2004, 94, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Karathanasis, A.D.; Potter, C.L.; Coyne, M.S. Vegetation effects on fecal bacteria, bod, and suspended solid removal in constructed wetlands treating domestic wastewater. Ecol. Eng. 2003, 20, 157–169. [Google Scholar] [CrossRef]
- Naylor, S.; Brisson, J.; Labelle, M.A.; Drizo, A.; Comeau, Y. Treatment of freshwater fish farm effluent using constructed wetlands: The role of plants and substrate. Water Sci. Technol. 2003, 48, 215–222. [Google Scholar]
- Petticrew, E.L.; Kalff, J. Water-flow and clay retention in submerged macrophyte beds. Can. J. Fish. Aquat. Sci. 1992, 49, 2483–2489. [Google Scholar] [CrossRef]
- Luederitz, V.; Eckert, E.; Lange-Weber, M.; Lange, A.; Gersberg, R.M. Nutrient removal efficiency and resource economics of vertical flow and horizontal flow constructed wetlands. Ecol. Eng. 2001, 18, 157–171. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (cav) trin ex steud. New Phytol. 1990, 114, 121–128. [Google Scholar] [CrossRef]
- Barko, J.W.; Gunnison, D.; Carpenter, S.R. Sediment interactions with submersed macrophyte growth and community dynamics. Aquat. Bot. 1991, 41, 41–65. [Google Scholar] [CrossRef]
- Sorrell, B.K.; Boon, P.I. Biogeochemistry of billabong sediments. II. Seasonal-variations in methane production. Freshw. Biol. 1992, 27, 435–445. [Google Scholar] [CrossRef]
- Yang, L.; Chang, H.T.; Huang, M.N.L. Nutrient removal in gravel- and soil-based wetland microcosms with and without vegetation. Ecol. Eng. 2001, 18, 91–105. [Google Scholar] [CrossRef]
- Munch, C.; Kuschk, P.; Roske, I. Root stimulated nitrogen removal: Only a local effect or important for water treatment? Water Sci. Technol. 2005, 51, 185–192. [Google Scholar]
- Ruiz-Rueda, O.; Hallin, S.; Baneras, L. Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. Fems Microbiol. Ecol. 2009, 67, 308–319. [Google Scholar] [CrossRef]
- Seidel, K. Abbau von bacterium coli durch höhere wasserpflanzen. Naturwiss 1964, 51, 395. [Google Scholar] [CrossRef]
- Drobotko, V.G.; Rashba, E.Y.; Aizenman, B.E.; Zelepukha, S.I.; Novikova, S.I.; Kaganskaya, M.B. Antimicrobial activity of alkaloids obtained from Valeriana officinalis, Chelidonium majus, Nuphar luteum and Asarum europium. Antibiotiki 22. Chem. Abstr. 1958, 53, 12589d. [Google Scholar]
- Seidel, K. Macrophytes and water purification. In Biological Control of Water Pollution; Tourbier, J., Pierson, R.W.J., Eds.; University of Pennsylvania Press: Pennsylvania, PA, USA, 1976; pp. 109–123. [Google Scholar]
- Gersberg, R.M.; Elkins, B.V.; Lyon, S.R.; Goldman, C.R. Role of aquatic plants in waste-water treatment by artificial wetlands. Water Res. 1986, 20, 363–368. [Google Scholar] [CrossRef]
- Wathugala, A.G.; Suzuki, T.; Kurihara, Y. Removal of nitrogen, phosphorus and cod from waste-water using sand filtration system with phragmites-australis. Water Res. 1987, 21, 1217–1224. [Google Scholar] [CrossRef]
- Tanner, C.C.; Clayton, J.S.; Upsdell, M.P. Effect of loading rate and planting on treatment of dairy farm wastewaters in constructed wetlands. 2. Removal of nitrogen and phosphorus. Water Res. 1995, 29, 27–34. [Google Scholar] [CrossRef]
- Lee, B.H.; Scholz, M. What is the role of phragmites australis in experimental constructed wetland filters treating urban runoff? Ecol. Eng. 2007, 29, 87–95. [Google Scholar] [CrossRef]
- Langergraber, G.; Simunek, J. Modeling variably saturated water flow and multicomponent reactive transport in constructed wetlands. Vadose Zone J. 2005, 4, 924–938. [Google Scholar] [CrossRef]
- Lantzke, I.R.; Heritage, A.D.; Pistillo, G.; Mitchell, D.S. Phosphorus removal rates in bucket size planted wetlands with a vertical hydraulic flow. Water Res. 1998, 32, 1280–1286. [Google Scholar] [CrossRef]
- Geller, G. Horizontal subsurface flow systems in the german speaking countries: Summary of long-term scientific and practical experiences; recommendations. Water Sci. Technol. 1997, 35, 157–166. [Google Scholar]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation—A novel strategy for the removal of toxic metals from the environment using plants. Bio-Technology 1995, 13, 468–474. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Shelef, O.; Gross, A.; Rachmilevitch, S. The use of bassia indica for salt phytoremediation in constructed wetlands. Water Res. 2012, 46, 3967–3976. [Google Scholar] [CrossRef]
- Headley, T.R.; Davison, L.; Huett, D.O.; Muller, R. Evapotranspiration from subsurface horizontal flow wetlands planted with phragmites australis in sub-tropical australia. Water Res. 2012, 46, 345–354. [Google Scholar] [CrossRef]
- Borin, M.; Milani, M.; Salvato, M.; Toscano, A. Evaluation of Phragmites australis (cav.) trin. Evapotranspiration in northern and southern italy. Ecol. Eng. 2011, 37, 721–728. [Google Scholar] [CrossRef]
- Smith, I.D.; Bis, G.N.; Lemon, E.R.; Rozema, L.R. A thermal analysis of a sub-surface, vertical flow constructed wetland. Water Sci. Technol. 1997, 35, 55–62. [Google Scholar]
- Čížková-Končalová, H.J.; Květ, J.; Lukavská, J. Response of phragmites australis, glyceria maxima, and typha latifolia to additions of piggery sewage in a flooded sand culture. Wetlands Ecol. Manag. 1996, 4, 43–50. [Google Scholar] [CrossRef]
- Brix, H. Denmark. In Constructed Wetlands for Wastewater Treatment in Europe; Vymazal, J., Brix, H., Cooper, P.F., Green, M.B., Haberl, R., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 123–156. [Google Scholar]
- Haslam, S.M. Community regulation in phragmites-communis trin. I. Monodominant stands. J. Ecol. 1971, 59, 65–73. [Google Scholar] [CrossRef]
- Wand, H.; Vacca, G.; Kuschk, P.; Kruger, M.; Kastner, M. Removal of bacteria by filtration in planted and non-planted sand columns. Water Res. 2007, 41, 159–167. [Google Scholar] [CrossRef]
- Wood, A. Constructed wetlands in water pollution control: Fundamentals to their understanding. Water Sci. Technol. 1995, 32, 21–29. [Google Scholar]
- Knight, R.L.; Kadlec, R.H.; Ohlendorf, H.M. The use of treatment wetlands for petroleum industry effluents. Environ. Sci. Technol. 1999, 33, 973–980. [Google Scholar] [CrossRef]
- Nelson, M.; Cattin, F.; Rajendran, M.; Hafouda, L. Value-Adding through Creation of High Diversity Gardens and Ecospaces in Subsurface Flow Constructed Wetlands: Case Studies in Algeria and Australia of Wastewater Gardens Systems. In Proceedings of 11th International Conference on Wetland Systems for Water Pollution Control; Billore, S., Dass, P., Vymazal, J., Eds.; Institute of Environmental Management and Plant Sciences, Vikram University: Ujjain, India, 2008; Volume 1, pp. 344–356. [Google Scholar]
- Tencer, Y.; Idan, G.; Strom, M.; Nusinow, U.; Banet, D.; Cohen, E.; Schroder, P.; Shelef, O.; Rachmilevitch, S.; Soares, I.; et al. Establishment of a constructed wetland in extreme dryland. Environ. Sci. Pollut. Res. 2009, 16, 862–875. [Google Scholar] [CrossRef]
- Shelef, O.; Golan-Goldhirsh, A.; Gendler, T.; Rachmilevitch, S. Physiological parameters of plants as indicators of water quality in a constructed wetland. Environ. Sci. Pollut. Res. 2011, 18, 1234–1242. [Google Scholar] [CrossRef]
- Zurita, F.; de Anda, J.; Belmont, M.A. Treatment of domestic wastewater and production of commercial flowers in vertical and horizontal subsurface-flow constructed wetlands. Ecol. Eng. 2009, 35, 861–869. [Google Scholar] [CrossRef]
- Belmont, M.A.; Metcalfe, C.D. Feasibility of using ornamental plants (Zantedeschia aethiopica) in subsurface flow treatment wetlands to remove nitrogen, chemical oxygen demand and nonylphenol ethoxylate surfactants—A laboratory-scale study. Ecol. Eng. 2003, 21, 233–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Zhang, Z.; Zhou, X.; Tu, Y. Chapter 3: Ornamental plants in constructed wetlands. In Ornamental Plants: Type, Cultivation and Nutrition; Aquino, J.C., Ed.; Nova Science Publishers, Inc: New York, NY, USA, 2011; pp. 81–96. [Google Scholar]
- Maddison, M.; Mauring, T.; Remm, K.; Lesta, M.; Mander, U. Dynamics of typha latifolia l. Populations in treatment wetlands in estonia. Ecol. Eng. 2009, 35, 258–264. [Google Scholar] [CrossRef]
- Aronsson, P.; Perttu, K. Willow vegetation filters for wastewater treatment and soil remediation combined with biomass production. For. Chron. 2001, 77, 293–299. [Google Scholar]
- Kivaisi, A.K. The potential for constructed wetlands for wastewater treatment and reuse in developing countries: A review. Ecol. Eng. 2001, 16, 545–560. [Google Scholar] [CrossRef]
- Tanner, C.C. Plants as ecosystem engineers in subsurface-flow treatment wetlands. Water Sci. Technol. 2001, 44, 9–17. [Google Scholar]
- Cooper, P.; Boon, A. The Use of Phragmites for Wastewater Treatment by the Root Zone Method: The UK Approach. In Aquatic Plants for Water Treatment and Resource Recovery; Reddy, S.W., Ed.; Magnolia Publishing: Orlando, FL, USA, 1987; pp. 153–174. [Google Scholar]
- Armstrong, W.; Webb, T.; Darwent, M.; Beckett, P.M. Measuring and interpreting respiratory critical oxygen pressures in roots. Ann. Bot. 2009, 103, 281–293. [Google Scholar]
- Armstrong, W.; Cousins, D.; Armstrong, J.; Turner, D.W.; Beckett, P.M. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: A microelectrode and modelling study with Phragmites australis. Ann. Bot. 2000, 86, 687–703. [Google Scholar] [CrossRef]
- Armstrong, M. Aeration in Higher Plants. In Advances in Botanical Research; Woolhouse, H.W., Ed.; Academic Press: London, UK, 1979; pp. 225–332. [Google Scholar]
- Drew, M.C.; Saglio, P.H.; Pradet, A. Larger adenylate energy-charge and atp/adp ratios in aerenchymatous roots of zea-mays in anaerobic media as a consequence of improved internal oxygen-transport. Planta 1985, 165, 51–58. [Google Scholar] [CrossRef]
- Williams, H.G.; Bialowiec, A.; Slater, F.; Randerson, P.F. Diurnal cycling of dissolved gas concentrations in a willow vegetation filter treating landfill leachate. Ecol. Eng. 2010, 36, 1680–1685. [Google Scholar] [CrossRef]
- Stein, O.R.; Hook, P.B. Temperature, plants, and oxygen: How does season affect constructed wetland performance? Environ. Sci. Heal. A 2005, 40, 1331–1342. [Google Scholar] [CrossRef]
- Bialowiec, A.; Davies, L.; Albuquerque, A.; Randerson, P.F. The influence of plants on nitrogen removal from landfill leachate in discontinuous batch shallow constructed wetland with recirculating subsurface horizontal flow. Ecol. Eng. 2012, 40, 44–52. [Google Scholar] [CrossRef]
- Ueckert, J.; Hurek, T.; Fendrik, I.; Niemann, E.G. Radial gas-diffusion from roots of rice (oryza-sativa-l) and kallar grass (leptochloa-fusca l kunth), and effects of inoculation with azospirillum-brasilense cd. Plant Soil 1990, 122, 59–65. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology; Saunders: Philadelphia, PA, USA, 1975. [Google Scholar]
- El Hamouri, B.; Nazih, J.; Lahjouj, J. Sub surface-horizontal flow constructed wetland for sewage treatment under moroccan climate conditions. Desalination 2007, 215, 153–158. [Google Scholar] [CrossRef]
- Gregersen, P.; Brix, H. Zero-discharge of nutrients and water in a willow dominated constructed wetland. Water Sci. Technol. 2001, 44, 407–412. [Google Scholar]
- Green, M.; Shaul, N.; Beliavski, M.; Sabbah, I.; Ghattas, B.; Tarre, S. Minimizing land requirement and evaporation in small wastewater treatment systems. Ecol. Eng. 2006, 26, 266–271. [Google Scholar] [CrossRef]
- Masi, F.; Martinuzzi, N. Constructed wetlands for the mediterranean countries: Hybrid systems for water reuse and sustainable sanitation. Desalination 2007, 215, 44–55. [Google Scholar] [CrossRef]
- Thomas, K.L.; Benstead, J.; Davies, K.L.; Lloyd, D. Role of wetland plants in the diurnal control of CH4 and CO2 fluxes in peat. Soil Biol. Biochem. 1996, 28, 17–23. [Google Scholar] [CrossRef]
- Reddy, K.R.; Patrick, W.H.; Lindau, C.W. Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol. Oceanogr. 1989, 34, 1004–1013. [Google Scholar] [CrossRef]
- El Shaer, H.M. Halophytes and salt-tolerant plants as potential forage for ruminants in the near east region. Small Ruminant. Res. 2010, 91, 3–12. [Google Scholar] [CrossRef]
- Youssef, K.M.; Fahmy, A.A.; El Essawy, A.M.; El Shaer, H.M. Nutritional studies on Pennisetum americanum and Kochia indica fed to sheep under saline conditions of sinai, Egypt. Am. Eur. J. Agric. Environ. Sci. 2009, 5, 63–68. [Google Scholar]
- Brown, J.J.; Glenn, E.P.; Fitzsimmons, K.M.; Smith, S.E. Halophytes for the treatment of saline aquaculture effluent. Aquaculture 1999, 175, 255–268. [Google Scholar] [CrossRef]
- Zhao, F.; Mcgrath, S.P.; Crosland, A.R. Comparison of 3 wet digestion methods for the determination of plant sulfur by inductively-coupled plasma-atomic emission-spectroscopy (icpaes). Commun. Soil Sci. Plant Anal. 1994, 25, 407–418. [Google Scholar]
- Welz, B.; Sperling, M. Frontmatte. In Atomic Absorption Spectrometry, 3rd ed; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007. [Google Scholar]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen peroxide and plant stress: A challenging relationship. Plant Stress 2007, 1, 4–15. [Google Scholar]
- Wolff, S.P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Oxyg. Radic. Biol. Syst. C 1994, 233, 182–189. [Google Scholar] [CrossRef]
- Shelef, O.; Lazarovitch, N.; Rewald, B.; Golan-Goldhirsh, A.; Rachmilevitch, S. Root halotropism: Salinity effects on bassia indica root. Plant Biosyst. 2010, 144, 471–478. [Google Scholar] [CrossRef]
- Ma, C.J.; Naidu, R.; Liu, F.G.; Lin, C.H.; Ming, H. Influence of hybrid giant napier grass on salt and nutrient distributions with depth in a saline soil. Biodegradation 2012, 23, 907–916. [Google Scholar] [CrossRef]
- Young, M.A.; Rancier, D.G.; Roy, J.L.; Lunn, S.R.; Armstrong, S.A.; Headley, J.V. Seeding conditions of the halophyte Atriplex patula for optimal growth on a salt impacted site. Int. J. Phytoremediat. 2011, 13, 674–680. [Google Scholar] [CrossRef]
- Keiffer, C.H.; Ungar, I.A. Germination and establishment of halophytes on brine-affected soils. J. Appl. Ecol. 2002, 39, 402–415. [Google Scholar] [CrossRef]
- Carty, D.T.; Swetish, S.M.; Crawley, W.W.; Priebe, W.F. In Major Variables Influencing Technology Solution for Remediation of Salt Affected Soils. In Proceedings of the Rocky Mountain Symposium of Environmental Issues in Oil and Gas Operations, Olorado School of Mines: Golden, CO, USA, 14–15 July 1997; pp. 145–152.
- Hbirkou, C.; Martius, C.; Khamzina, A.; Lamers, J.P.A.; Welp, G.; Amelung, W. Reducing topsoil salinity and raising carbon stocks through afforestation in khorezm, uzbekistan. J. Arid Environ. 2011, 75, 146–155. [Google Scholar] [CrossRef]
- Rabhi, M.; Hafsi, C.; Lakhdar, A.; Hajji, S.; Barhoumi, Z.; Hamrouni, M.H.; Abdelly, C.; Smaoui, A. Evaluation of the capacity of three halophytes to desalinize their rhizosphere as grown on saline soils under nonleaching conditions. Afr. J. Ecol. 2009, 47, 463–468. [Google Scholar] [CrossRef]
- Ravindran, K.C.; Venkatesan, K.; Balakrishnan, V.; Chellappan, K.P.; Balasubramanian, T. Restoration of saline land by halophytes for indian soils. Soil Biol. Biochem. 2007, 39, 2661–2664. [Google Scholar] [CrossRef]
- Zhao, K.F. Desalinization of saline soils by suaeda-salsa. Plant Soil 1991, 135, 303–305. [Google Scholar] [CrossRef]
- Kimball, K.D.; Levin, S.A. Limitations of laboratory bioassays—The need for ecosystem-level testing. Bioscience 1985, 35, 165–171. [Google Scholar] [CrossRef]
- Doust, J.L.; Schmidt, M.; Doust, L.L. Biological assessment of aquatic pollution—A review, with emphasis on plants as biomonitors. Biol. Rev. Camb. Philos. Soc. 1994, 69, 147–186. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shelef, O.; Gross, A.; Rachmilevitch, S. Role of Plants in a Constructed Wetland: Current and New Perspectives. Water 2013, 5, 405-419. https://doi.org/10.3390/w5020405
Shelef O, Gross A, Rachmilevitch S. Role of Plants in a Constructed Wetland: Current and New Perspectives. Water. 2013; 5(2):405-419. https://doi.org/10.3390/w5020405
Chicago/Turabian StyleShelef, Oren, Amit Gross, and Shimon Rachmilevitch. 2013. "Role of Plants in a Constructed Wetland: Current and New Perspectives" Water 5, no. 2: 405-419. https://doi.org/10.3390/w5020405







