1. Introduction
The eutrophication problems caused by wastewater discharge have been in focus during the late 20th century, and in Sweden, wastewater treatment with phosphorus precipitation was introduced at a large scale during the 1970s. Since then, some recipients (lakes, rivers and Baltic Sea costal bays) have recovered significantly and, in some cases, there is even a discussion of whether the wastewater treatment has been too efficient. This is the case for one of Sweden’s largest lakes, Vättern, where a fish population of charr is now decreasing, and one possible reason for this is a drop in nutrient levels. Some even argue that the nutrient levels today of approximately 3–4 µg P/L (total phosphorus) is lower than the original concentrations, estimated to have been 6 µg P/L [
1].
The recovery of subarctic lakes, however, has to only a small extent been described in scientific journals, and, if at all, rather in the grey zone area of reports (e.g., [
2,
3]). Instead, the scientific literature from the subarctic region has focused on heavy metal pollution (e.g., [
4,
5]) or marine systems (e.g., [
6,
7]).
This paper investigates a case in the Swedish mountains, where introduction of advanced wastewater treatment has led to a considerable recovery of two small lakes since 1975. During the pollution and recovery period, a downstream economically interesting Arctic charr population developed a migration pattern that utilized the nutrient resources in the lakes.
1.1. Objectives
The objectives of this paper are:
To present unpublished data from 1985 and 2003 complementing the three previously published investigations of the status in the Åsvalltjärnarna lakes from a phosphorus and microalgae perspective, and to some extent also from a fish perspective;
To review trends in the recovery of the lakes during the last three decades;
To compare optional recovery management strategies for the lakes with regard to the assumed pristine state and preservation of the productive ecotone that has developed between the wastewater treatment and a downstream pristine-like recipient.
The structure of the paper is as follows. In
Section 1.2 the historical and current wastewater treatment and its discharge upstream the lakes are described together with a general description of the surroundings. In
Section 2, the previous unpublished 2003 investigation is presented. In
Section 3, the 2003 results are compared with the previously published data from the 1972–1974, 1978–1980 and 1985–1988 investigations, and some unpublished microalgal data from 1985. In
Section 4, three options for future management of the two lakes are presented and discussed.
1.2. Area Description and History
1.2.1. Study Site
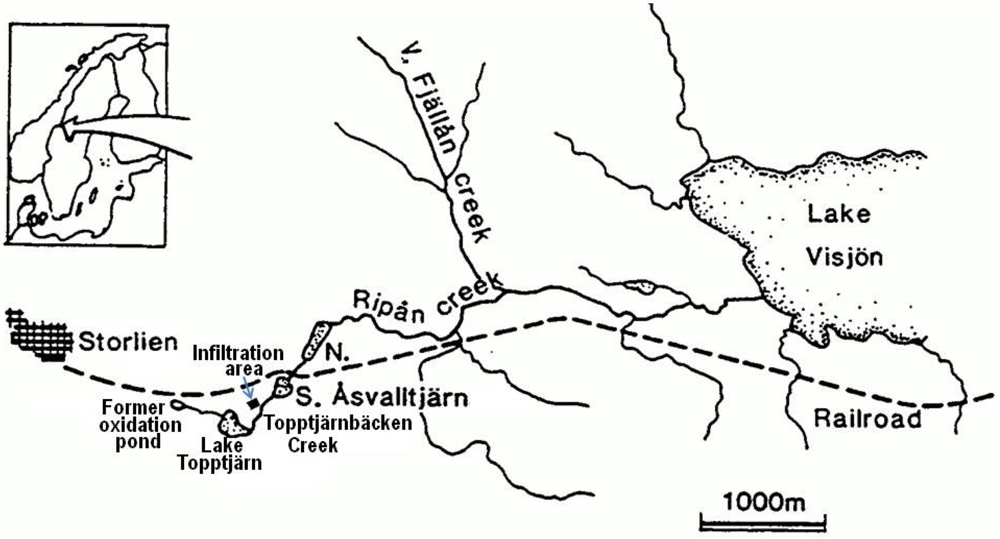
The investigated Åsvalltjärnarna lakes, consisting of the upper Lake South Åsvalltjärn and the lower Lake North Åsvalltjärn, are located in the subarctic mid-Swedish mountain area close to the Norwegian border at latitude 63°18' N. The watershed divider between the Baltic Sea and the Atlantic Ocean is situated only a few hundred meters west of the lakes. The lakes drain to the Baltic side. Lake Topptjärn is the source lake of the drainage area (
Figure 1), and a small stream, the Topptjärnbäcken Creek, connects it with Lake South Åsvalltjärn and further on to Lake North Åsvalltjärn, so that via Ripån Creek is connected with Lake Visjön, about five km downstream. A wetland covers the area between Lake Topptjärn and Lake South Åsvalltjärn, and the Topptjärnbäcken Creek is embedded with dense vegetation of
Salix and
Carex. The area is about 600 m above sea level and just below the tree line surrounded by sparse birch trees (
Betula pubescens). The average precipitation is 1000 mm a year, and the mean average temperature is 0.7 °C over the year, −7.0 °C in January and 11.2 °C in July [
8].
Lake South Åsvalltjärn and Lake North Åsvalltjärn are small lakes (25,000 m
2 each), with a maximum depth of 4.2 m and of 3.1 m respectively. Both are covered in ice from approximately 1 October to 30 May. Lake South Åsvalltjärn, which is the most studied of the two, has a mean depth of 2 m, a volume of 50,000 m
3, and a drainage area of 602,100 m
2 [
8].
Figure 1.
Map of the study area (adapted from [
9]).
Figure 1.
Map of the study area (adapted from [
9]).
1.2.2. Wastewater Treatment History
The Åsvalltjärnarna lakes did not receive any wastewater load until 1964, since before that the sewage from the recreation resort of Storlien was led towards Norway and to the Atlantic. In 1964, an oxidation pond was built and all sewage was diverted to the drainage area of the lakes Åsvalltjärnarna. Storlien had at that time approximately 200 residents and an average of about 300 tourists over the year. No sludge was ever removed from the oxidation pond and all nutrients remained within the drainage area. The oxidation pond discharged to Lake Topptjärn, and the load of total phosphorus was estimated at 450 kg/year [
8]. In 1974, an advanced wastewater treatment plant with chemical precipitation using aluminium sulphate and post-filtration was built and was fully operational by January 1975. The oxidation pond was dredged and the old sludge was deposited beside it. Subsequently, the treated wastewater was discharged into the old oxidation pond, with an estimated load of only 18–20 kg phosphorus per year to the drainage area. However, it was already evident since 1964, that a lot of the phosphorus was undoubtedly retained in sediments in the oxidation pond, in Lake Topptjärn and in Topptjärnbäcken Creek. Based on Arctic Canadian studies [
8,
10] it was estimated that at least 80% would be retained before the wastewater reached Lake South Åsvalltjärn. A true total phosphorus load on Lake South Åsvalltjärn of less than 90 kg P/year before 1974 and 4 kg P/year after 1974 may therefore be a realistic estimate.
In 1991 the chemical precipitation plant was abandoned and a small natural ridge close to Lake South Åsvalltjärn was used as a sand filter in an open infiltration treatment plant (
Figure 2). The discharge to Lake Topptjärn ceased and instead the current discharge is into the groundwater entering the wetland around Topptjärnbäcken Creek and Lake South Åsvalltjärn. Since the 1960s, the population has decreased to approximately 100 inhabitants, but the tourist resort has expanded and the load to the wastewater plant is higher: 1500 person equivalents, p.e. (1 p.e. equals 70 g BOD per person and day), during the winter season and 200 p.e. during the summer [
11].
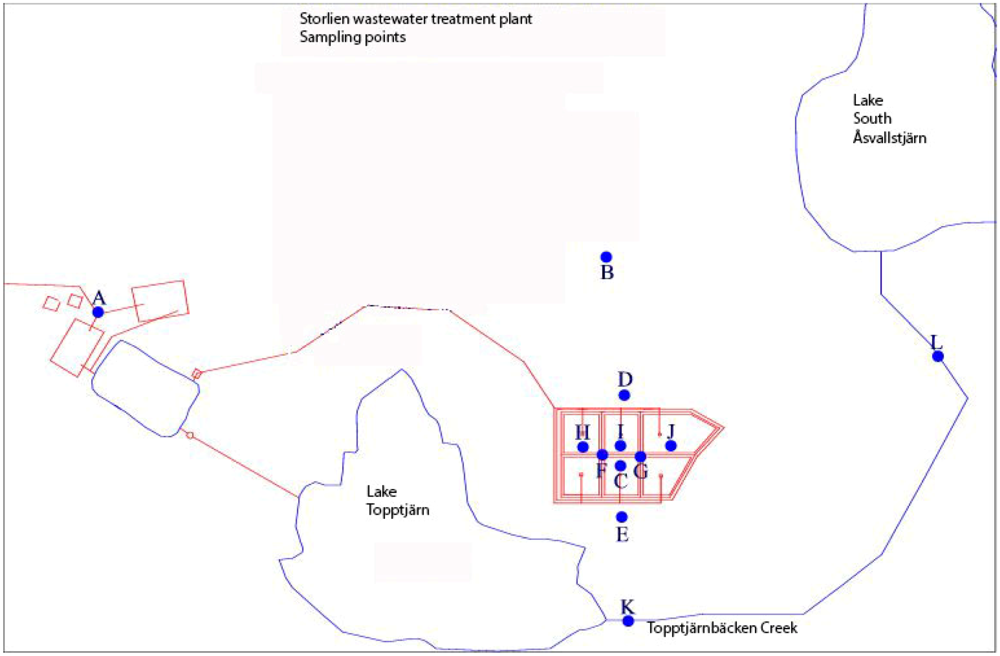
Figure 2.
Sampling points in the Municipality’s wastewater treatment plants control program. The points referred to in
Table 1 are B, C, D, and E, and K and L in
Figure 3. The lake indicated upper right is Lake South Åsvalltjärn (Modified from [
11]).
Figure 2.
Sampling points in the Municipality’s wastewater treatment plants control program. The points referred to in
Table 1 are B, C, D, and E, and K and L in
Figure 3. The lake indicated upper right is Lake South Åsvalltjärn (Modified from [
11]).
1.2.3. Current Phosphorus Load from the Wastewater Treatment
The load to Lake South Åsvalltjärn today comes from three sources: the wastewater plant, from upstream Lake Topptjärn, and diffuse seepage from the surrounding wetlands. The discharge from the wastewater infiltration to the groundwater was in 2002 measured at 78 kg/year [
11]. Occasionally at high wastewater flows (normally in May due to leakage of melting water into the sewage collection system) very diluted but untreated wastewater is discharged to Lake Topptjärn. In 2002, the amount was estimated at 0.67 kg/year [
11]. These sources are monitored four times a year in groundwater sampling pipes around the infiltration system (
Table 1), and once a year (in September) in the Topptjärnbäcken Creek (
Figure 2), upstream and downstream of the infiltration system, close to the outlet of Lake Topptjärn, and near to the outlet in Lake South Åsvalltjärn.
Sampling from the wastewater plant’s control program [
11] shows that there is retention of total phosphorus during the flow through the short Topptjärnbäcken Creek (
Figure 3), even though the infiltration groundwater (downstream southward in
Table 1) is most likely added along the way. Leaking sediments in Lake Topptjärn seems to be the main source of phosphorus reaching Lake South Åsvalltjärn today.
Table 1 also shows that the major part of the infiltrated wastewater phosphorus makes it way south towards the Topptjärnbäcken Creek, and very little goes north until it reaches Lake South Åsvalltjärn via the wetland.
Table 1.
Total phosphorus in groundwater samples upstream and downstream of the current wastewater infiltration. From the the Municipality’s wastewater treatment plants control program [
11]. Sampling points refer to
Figure 2.
Table 1.
Total phosphorus in groundwater samples upstream and downstream of the current wastewater infiltration. From the the Municipality’s wastewater treatment plants control program [11]. Sampling points refer to Figure 2.
| Y-M-D | C, Groundwater at discharge point, µg P/L | E, groundwater downstream south, µg P/L | D, groundwater downstream north, µg P/L | B, groundwater upstream north, µg P/L |
|---|
| 1999-08-17 | 410 | - | - | <10 |
| 1999-09-15 | 150 | - | - | - |
| 2000-03-08 | 80 | - | 30 | - |
| 2000-08-16 | 200 | 280 | <10 | 10 |
| 2001-01-17 | 280 | - | - | - |
| 2001-03-14 | 30 | 230 | <10 | - |
| 2001-05-15 | 40 | - | - | - |
| 2001-08-15 | 180 | 420 | <10 | <10 |
| 2002-03-14 | - | 25 | <5 | <5 |
| 2002-08-12 | - | 34 | <5 | 6 |
| 2003-03-13 | - | 75 | 5 | 5 |
| 2003-08-13 | - | 650 | 5 | 650 |
Figure 3.
Total phosphorus at the inlet (K in
Figure 2) and outlet (L in
Figure 2) of the Topptjärnbäcken Creek, which connects Lake Topptjärn with Lake South Åsvalltjärn. The measurements are considered being upstream and downstream of the current wastewater infiltration discharge into the groundwater. From the the municipality’s wastewater treatment plants control program. [
11].
Figure 3.
Total phosphorus at the inlet (K in
Figure 2) and outlet (L in
Figure 2) of the Topptjärnbäcken Creek, which connects Lake Topptjärn with Lake South Åsvalltjärn. The measurements are considered being upstream and downstream of the current wastewater infiltration discharge into the groundwater. From the the municipality’s wastewater treatment plants control program. [
11].
2. The 2003 Investigation
2.1. Materials and Methods
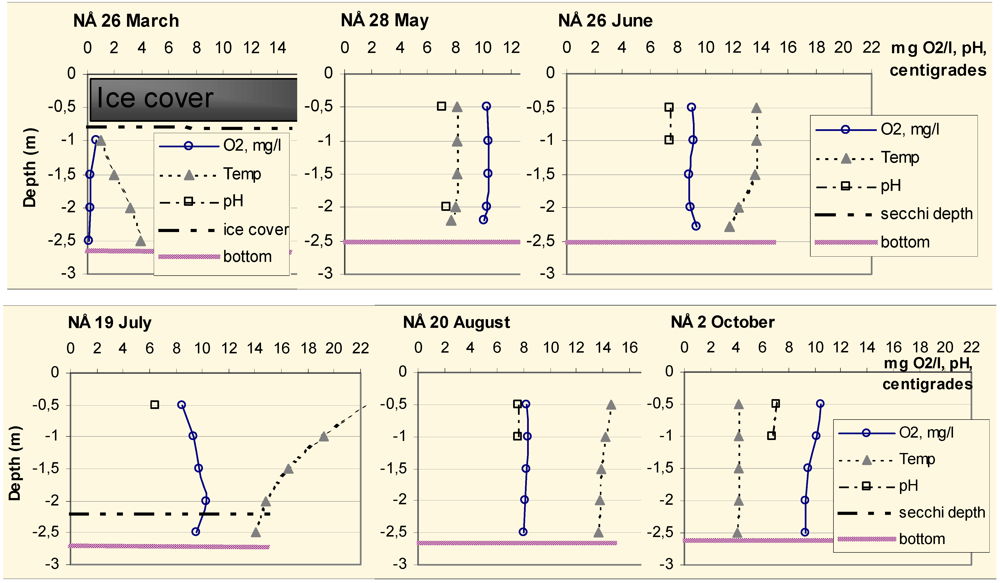
Field sampling was performed upon six occasions during 2003: 26 March, 28 May, 26 June, 18–19 July, 20 August, and 2 October. Samples were taken in the middle of both lakes at the deepest parts. The temperature and oxygen were measured every 0.5 m of the water column with a field oxygen measuring device (WTW Oxi 338, Wissenschaftlich-Technische Werkstätten GmbH, Weilheim, Germany). pH was measured in the surface water with a portable pH-meter (WTW pH 340i, WTW, Weilheim, Germany), and in the May sample, additionally in the bottom water (see
Figure 5,
Figure 6 and
Figure 7 for depth details). All chemical and microalgae samples were taken with a two-liter Ruttner sampler. Phosphate and total phosphorous were preserved in the field with sulphuric acid (H
2SO
4), and then analyzed by an accredited laboratory (AnalyCen, Gothenburg, Sweden) with the colorimetric TRAACS method. Algae samples were collected at 0.5 to 1 m depth in both lakes, conserved with Lugol’s solution in field, and then after sedimentation in a 5 ml sedimentation chamber identified to group and counted. Volume formulas were used from Tikkanen and Willén [
12] and Olrik
et al. [
13]. Fish biomass estimates were obtained from gillnetting using nets with multiple mesh sizes (nets described in [
14]). Two nets in each lake were used for one night on 18–19 July 2003 in the deepest part of the lakes. To estimate total fish biomass and Arctic charr biomass, the gillnet catch (kg × net
−1 × night
−1) was multiplied by the factor 8. This factor was derived from a previous study of these lakes, in which both gillnet catches and fish trapping of emigrating fish was performed [
9]. Thus an estimate of gillnetting efficiency could be obtained, and hence also a biomass estimate.
2.2. Results
2.2.1. Phosphorus
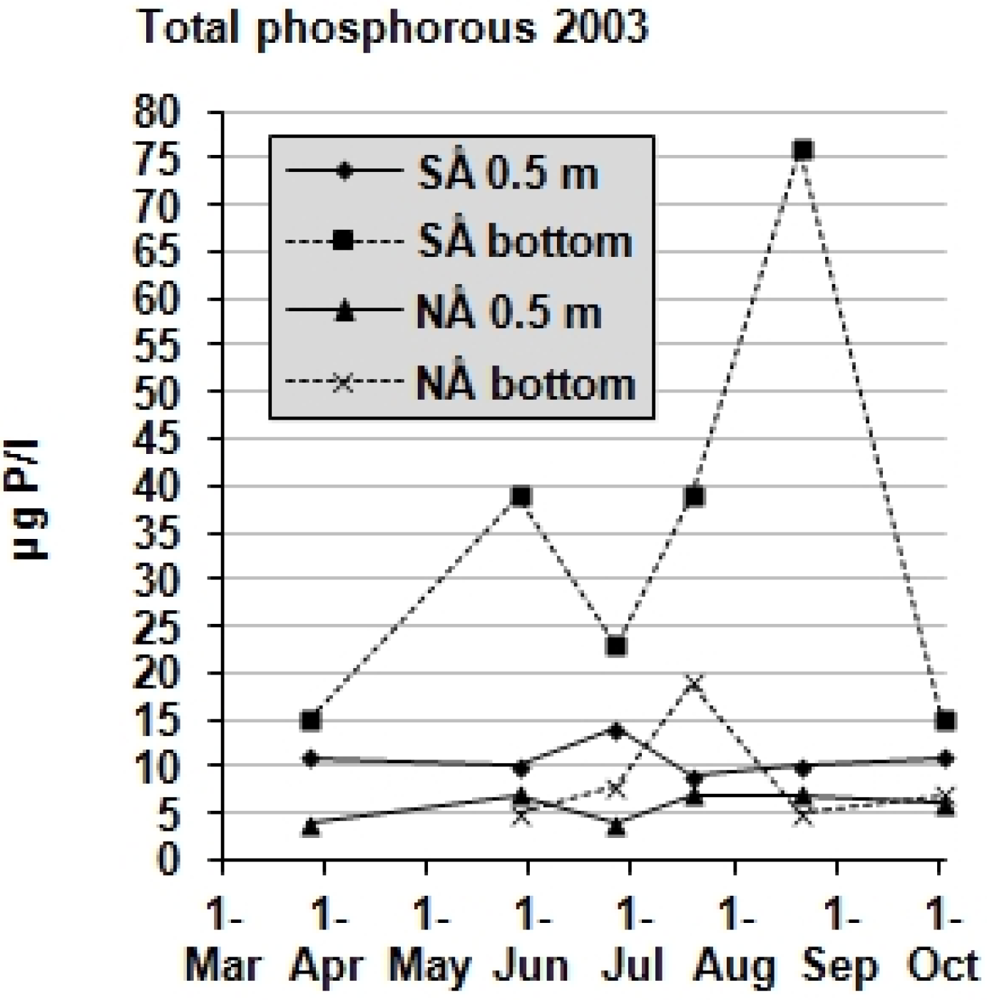
The total phosphorus in the surface water (0.5 m depth) and close to the bottom is shown in
Figure 4 for Lake South Åsvalltjärn (SÅ) and Lake North Åsvalltjärn (NÅ).
Figure 4.
Total phosphorus in the water (0.5 m), and close to the bottom (3–3.5 m for SÅ, and 2.5–2.7 m for NÅ) in Lake South Åsvalltjärn (SÅ), and Lake North Åsvalltjärn (NÅ). The detection limit was 5 µg/L and two samples are indicated below that limit.
Figure 4.
Total phosphorus in the water (0.5 m), and close to the bottom (3–3.5 m for SÅ, and 2.5–2.7 m for NÅ) in Lake South Åsvalltjärn (SÅ), and Lake North Åsvalltjärn (NÅ). The detection limit was 5 µg/L and two samples are indicated below that limit.
At 0.5 m total phosphorus concentrations in Lake South Åsvalltjärn varied between 9–14 µg P/L, and in Lake North Åsvalltjärn between the detection limit <5 µg P/L and 7 µg P/L. Close to the bottom the total phosphorus in Lake South Åsvalltjärn was above 15 µg P/L throughout the whole summer, with peak values in late May and mid-August. In Lake North Åsvalltjärn, the bottom total phosphorus values were close to the surface water values, except for a small peak of 20 µg P/L in mid July. The corresponding phosphate levels were low, and often under the detection limit of 5 µg P/L.
2.2.2. Oxygen, Temperature and pH
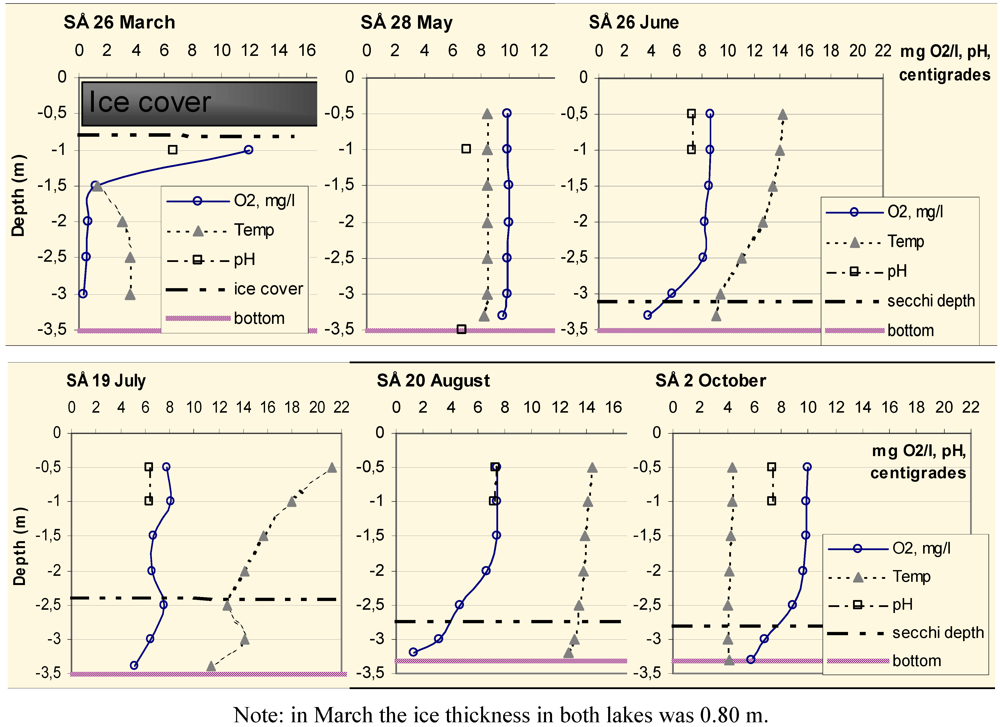
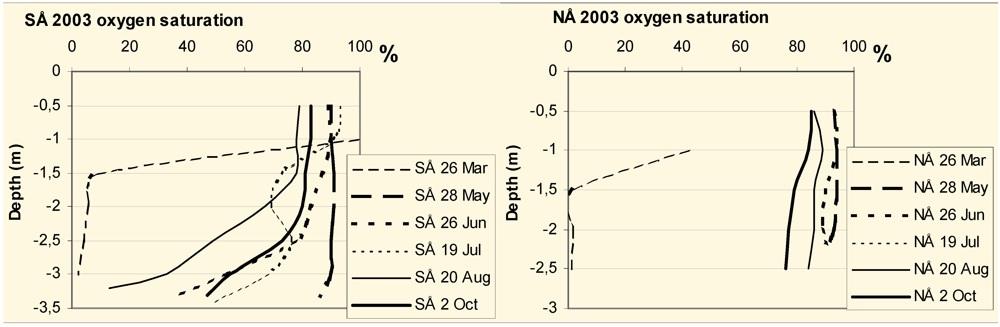
The oxygen, temperature and pH profiles of Lake South Åsvalltjärn (SÅ) and Lake North Åsvalltjärn (NÅ) at the sampling occasions are shown in
Figure 5,
Figure 6 and
Figure 7, where also Secchi depth, bottom depth at the sampling point, and the ice thickness for March is presented.
Figure 5.
Oxygen and temperature profiles, pH, depth of ice cover, Secchi depth and bottom depth at the sampling point in Lake South Åsvalltjärn (SÅ) 2003.
Figure 5.
Oxygen and temperature profiles, pH, depth of ice cover, Secchi depth and bottom depth at the sampling point in Lake South Åsvalltjärn (SÅ) 2003.
Figure 6.
Oxygen saturation profiles in the Lake South Åsvalltjärn (SÅ) and Lake North Åsvalltjärn (NÅ) 2003.
Figure 6.
Oxygen saturation profiles in the Lake South Åsvalltjärn (SÅ) and Lake North Åsvalltjärn (NÅ) 2003.
Figure 7.
Oxygen and temperature profiles, pH, depth of ice cover, Secchi depth and bottom depth at the sampling point in Lake North Åsvalltjärn (NÅ) from March to October 2003.
Figure 7.
Oxygen and temperature profiles, pH, depth of ice cover, Secchi depth and bottom depth at the sampling point in Lake North Åsvalltjärn (NÅ) from March to October 2003.
In Lake South Åsvalltjärn, the oxygen was almost depleted in March. During the rest of the sampling period, the oxygen was high in the surface water, but in June and August, it was lower close to the bottom. The Secchi depth decreased in June, was at its lowest in July, and thereafter increased again.
In Lake North Åsvalltjärn the oxygen was also almost depleted in March. During the rest of the sampling period, the oxygen was high all the way through the water column. A Secchi depth less than bottom depth was measured only in July.
The oxygen saturation in both lakes was high except for the March sample. In Lake South Åsvalltjärn the saturation decreased below a depth of 1.5–2 m from June and onwards.
2.2.3. Microalgae Samples
In Lake South Åsvalltjärn, a winter microalgae sample was collected on March 26. The microalgae biomass at that time was very low (0.36 mg/L), but within that low biomass, the haptophyte Chrysochromulina parva was dominating, along with green algae, mostly Chlamydomonas spp., but also Koliella spiralis. Also present was the dinophyte Gymnodinium uberrimum, and the cyanophyte Anabaena sp. (probably A. aequalis).
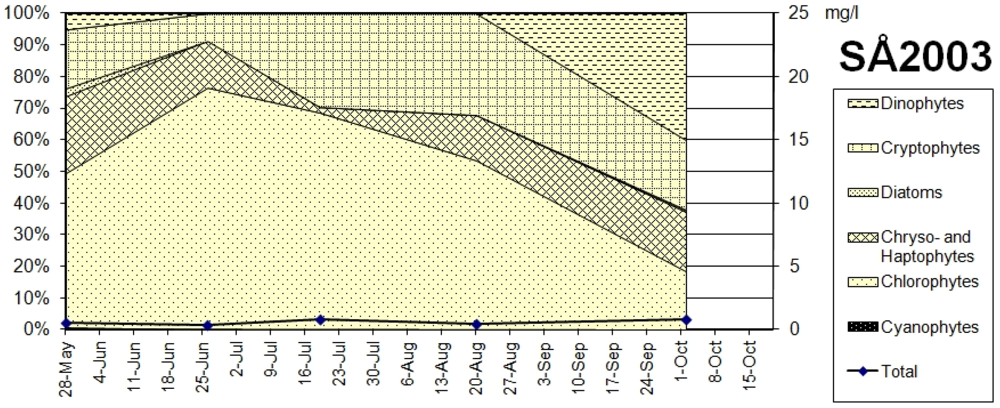
The biomass and microalgae taxa in the Lake South Åsvalltjärn spring, summer and autumn samples are shown in
Figure 8. The species and genera dominating were volvocalean green algae (
Chlamydomonas spp.), accompanied in the middle of the summer by some chlorococcalean green algae (mainly
Dictyosphaerium sp.). Present in significant amounts through the whole season were cryptophytes (
Cryptomonas spp.) and chryso- and haptophytes (
Cromulina sp.,
Chrysochromulina parva). Increasing in autumn was a dinophyte (
Gymnodinium lantzschii), which was also present in spring in small amounts together with a few diatoms (
Fragilaria spp.).
Figure 8.
Taxonomic groups of microalgae, and biomass in Lake South Åsvalltjärn (SÅ) 2003 (right axis: wet weight in mg/L).
Figure 8.
Taxonomic groups of microalgae, and biomass in Lake South Åsvalltjärn (SÅ) 2003 (right axis: wet weight in mg/L).
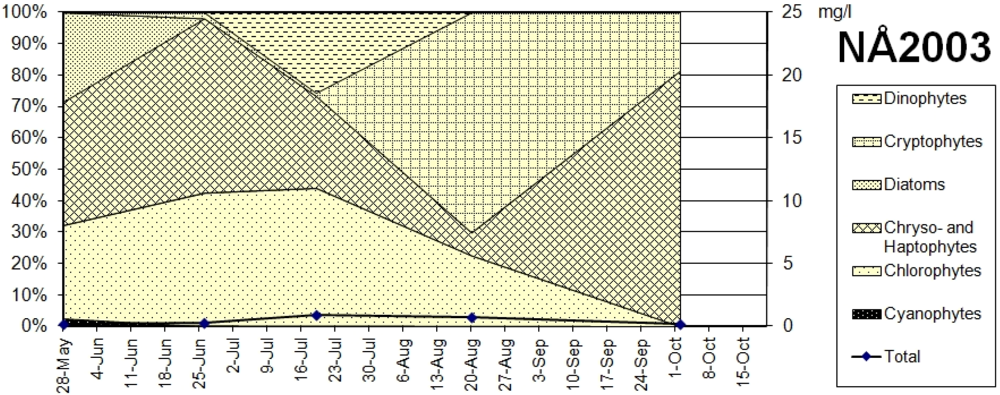
In Lake North Åsvalltjärn no winter sample was collected.
Figure 9 shows the biomass and microalgae taxa in the Lake North Åsvalltjärn spring, summer and autumn samples. During the productive period chryso- and haptophytes were present the full season (
Chrysochromulina parva,
Mallomonas akrokomos in early summer and October
, Dinobryon spp.,Chromulina spp. and
Ochromonas sp. only during summer), as were volvocal green algae except in October (
Chlamydomonas spp.). Diatoms were common in May (
Fragillaria ulna,
F. virescens,
F. capucina var. vaucheriae), cryptophytes dominated in August (
Cryptomonas spp.), and a dinophyte was common in July (
Gymnodinium uberrimum). Present in spring in small amounts were a few cyanophyte species (
Pseudanabaena limnetica, Anabaena inaequalis).
Figure 9.
Taxonomic groups of microalgae, and biomass in Lake North Åsvalltjärn (NÅ) 2003 (right axis: wet weight in mg/L).
Figure 9.
Taxonomic groups of microalgae, and biomass in Lake North Åsvalltjärn (NÅ) 2003 (right axis: wet weight in mg/L).
2.2.4. Fishing
A total of five Salvelinus alpinus, Arctic charr (mean weight 566 g), 29 Salvelinus fontinalis, brook trout (mean weight 240 g) and three Salmo trutta, brown trout (mean weight 333 g) were captured the night between 18 and 19 July 2003. Their total weight was 10.8 kg of which 60% were registered in Lake South Åsvalltjärn. The estimated total fish biomass was 116 kg, of which 31 kg was Arctic charr.
3. Comparison with the 1970s and 1980s Investigations
The investigations in Lake South Åsvalltjärn during the 1970s have been reported by Holmgren [
8]. Näslund [
15] studied the fish migration in 1985–1987 between the Åsvalltjärnarna lakes and the downstream Lake Visjön (
Figure 1). Many phytoplankton samples were collected in 1985 in order to establish the production base. From these 1985 samples, only estimates of the total biomass have previously been published [
9].
In general, the recovery of the Åsvalltjärnarna lakes after the introduction of advanced wastewater treatment in 1975 has been successful. The lakes have returned to an oligotrophic state, but not yet to the assumed pre-1964 situation. The total phosphorus concentrations are still a little higher than surrounding lakes, the likely result of leakage from sediments internally and from upstream Lake Topptjärn. Probably the most important difference from surrounding lakes is the very low oxygen still present in late winter. This bottleneck probably excludes many organisms from being year-round residents in the lakes, including the fish population in focus in this investigation. Details and trends are presented and discussed below.
3.1. Phosphorus and Oxygen
Lake South Åsvalltjärn was part of a national survey from 1972–1979, with the purpose of evaluating effects in the recipients from the large expansion of wastewater treatment plants in Sweden during the 1970s, especially with a focus on phosphorus chemical precipitation [
3]. Lake South Åsvalltjärn was among the 15 of 23 lakes evaluated responded with decreasing phosphorus concentrations. The average total phosphorus decreased from hypereutrophic concentrations, 168 µg P/L before 1975, to eutrophic concentrations, 74 µg P/L after 1975; phosphate levels decreased from 54 µg P/L to 16 µg P/L (
Table 2). Total phosphorus was also measured in the late 1980s [
9] and had then further decreased to approximately 30–40 µg P/L, which is still categorized as eutrophic [
3]. In 2003 the concentration was below 15 µg P/L, and an oligotrophic situation was achieved. In Lake North Åsvalltjärn the levels of phosphorus reached in the water (
Figure 4) was close to what is expected to have been there before 1964.
Table 2.
Trends in Lake South Åsvalltjärn during the four periods of samplings.
Table 2.
Trends in Lake South Åsvalltjärn during the four periods of samplings.
| Parameter | 1972–1974* | 1978–1980* | 1985–1988 | 2003*** |
|---|
| Total phosphorus (µg/L) | 168 | 74 | 30–40** | 9–14 |
| Phosphate (PO4-P) (µg/L) | 54 | 16 | | <5–11 |
| Secchi depth (m) | 1.3 | 2.1 | | 2.8 |
| Phytoplankton average biomass (mg/L) | 1973:11.2 | 1977:6.3 | 1985:2.4** | 0.6 (March value excluded) |
| 1978:4.6 |
| 1980:2.9 |
| Phytoplankton peak biomass (mg/L) | 1973:32.0 | 1977:18.8 | 1985:5.3*** | 0.8 (March 0.9 mg/L excluded) |
| 1978:11.9 |
| 1980:23.7 |
Oxygen was almost depleted in both lakes in late March 2003. During spring to autumn 2003, there was always plenty of oxygen, at least in the upper 2 m with oxygen saturation values of 67%–100% (
Figure 6). In Lake South Åsvalltjärn 2003 oxygen was lower in the lowest meter close to the bottom from June an onwards, but Lake North Åsvalltjärn was characterized by “straight” oxygen curves during the whole summer. This can be explained by the different morphology of the lakes. Lake North Åsvalltjärn is shallow, has an elongated shape and is wind exposed, while Lake South Åsvalltjärn is circular and wind protected, which are prerequisites for stagnation and stratification.
The single 2003 total phosphorus samples close to the bottom in the Åsvalltjärnarna lakes (
Figure 4) are probably not very reliable. Sediment particles are easily set in motion by the Ruttner sampler, and may increase the phosphorus content in the samples. However, together with the oxygen results some conclusions may be drawn. Phosphorus is probably released from the sediment when oxygen is low. Bottom total phosphorus values in Lake South Åsvalltjärn may therefore be expected to be higher than in Lake North Åsvalltjärn, which was the case on average.
A surprise was the decreasing oxygen with depth in October 2003 accompanied by a full depth 4 °C water (
Figure 5). Perhaps this is an indication of bioactivity in the sediments.
The current estimated load of 70–80 kg P/year to the groundwater between Lake Topptjärn and Lake South Åsvalltjärn may suggest that the phosphorus load has increased, since the estimations for the late 1970s was a load of approximately 20 kg P/year to the drainage area. The change of treatment method in 1991 was partly due to malfunctions in the old plant, which suggests that the approximation from the late 1970s was too low. The difference between the two approximated loads may be much less than 50–60 kg P/year. The trend of phosphorus retention in the Topptjärnbäcken Creek (
Figure 3), although the major part of the groundwater load is transported south towards the creek (
Table 1), also suggests that the main part of the P reaching Lake South Åsvalltjärn comes from sediment release in Lake Topptjärn and internal load in Lake South Åsvalltjärn, and to some extent also in Lake North Åsvalltjärn.
3.2. Microalgae and Secchi Depth
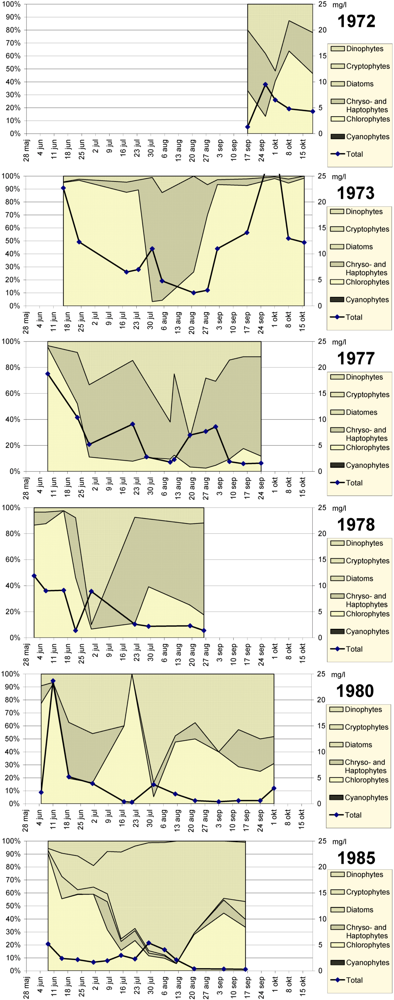
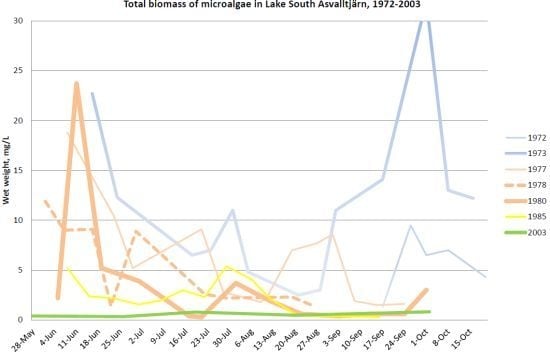
Holmgren [
8] reported microalgae biomass and composition for the years 1972–1980 in Lake South Åsvalltjärn, redrawn here in
Figure 10. The average phytoplankton biomass decreased during that period from 11.2 mg/L to 2.9 mg/L (
Table 2). In 1985 the biomass had decreased further to 2.4 µg/L [
9]. In 2003 the microalgae biomass was 0.6 µg/L. From the pre-1975 situation to 2003, the average microalgae biomass decreased twentyfold, and from 1985 to 2003, it was fourfold. The decrease in peak biomass is lower, fourfold since the situation before 1975. The Secchi depth followed the microalgae densities, and increased during the 1970s from average 1.3 m to 2.1 m, and by 2003 increased further to 2.8 m.
Figure 10.
Spring to autumn development, May–September of total phytoplankton in Lake South Åsvalltjärn 1972–1985. 1972–1980 data from Holmgren [
8], 1985 data from Holmgren unpublished data. Right axis: wet weight in mg/L.
Figure 10.
Spring to autumn development, May–September of total phytoplankton in Lake South Åsvalltjärn 1972–1985. 1972–1980 data from Holmgren [
8], 1985 data from Holmgren unpublished data. Right axis: wet weight in mg/L.
The microalgae development in Lake South Åsvalltjärn during the productive season from 1972 to 1985 is presented in
Figure 10, and for 2003 in
Figure 8. The development in Lake North Åsvalltjärn in 2003 is presented in
Figure 9. The general pattern can be summarized in the following way [
16]: The hypereutrophic state before 1975 was mainly characterized by small chlorococcal green algae. During the eutrophic phase of recovery cryptophytes became more and more dominant. The pre-1964 pristine lake oligotrophic state was probably characterized by a dominance of chryso- and haptophytes, which is what was found in Lake North Åsvalltjärn in 2003. In Lake South Åsvalltjärn in 2003 chryso- and haptophytes were not as important. Instead, small chlorococcal green algae were still present indicating a higher nutrient status.
The biodiversity is also expected to be higher in the pristine-like state than in the more nutrient rich states. The impression in the 2003 samples was that more species and genera were present in Lake North Åsvalltjärn though some genera were not resolved at species level (e.g., Cryptomonas and Chlamydomonas).
The species and genera composition in 1972–1980, following the aforementioned pattern has been reported in Holmgren [
8]. The 1985 investigation has not been previously reported [
17] and will therefore be given some attention in the following. The taxa and biomass development are shown in
Figure 10. The initial green algae dominance consisted of volvocalean (
Chlamydomonas spp.) and chlorococcal green algae
(species of Scenedesmus, Planctococcus, Nephrocyticum), the latter increasing in the early summer, together with cryptophytes (
Cryptomonas spp.) and a dinoflagellate (
Gymnodinium sp.). The late summer was dominated by a cryptophyte (
Cryptomonas marssonii), together with an increasing part of chlorococcalean green algae (
Dictyosphaerium), and finally in autumn some significance also of other cryptophytes (
Cryptomonas spp.,
Rhodomonas lacustris) and a diatom (
Tabellaria flocculosa). Compared to the earlier years in
Figure 10, cryptophytes were dominating, and small chlorococcal green algae were still present to some extent. Chryso- and haptophytes had low significance.
In warmer climates cyanophytes would have been expected to be more significant than was the case in the Åsvalltjärnarna lakes. In studies of fertilized and polluted subarctic lakes Holmgren [
18] found that cyanobacteria never had any quantitative importance in any of the studied lakes. He attributed this to the microalgae being sensitive to the combination of low water temperatures and long days at high latitudes, basing his conclusions on investigations by Abeliovich and Shilo [
19] on photooxidation.
The relation between phosphorus and microalgae biomass was investigated in the national survey 1972–1979 mentioned above [
3]. Of 15 lakes with decreasing phosphorus concentrations, microalgae (measured as chlorophyll
a) decreased only in seven of the lakes. Secchi depth increased only in six of them. Lake South Åsvalltjärn was one of the lakes which responded with both decreasing microalgae biomass (
Figure 10) and increasing Secchi depth (
Table 2). The reason for this was attributed to the concentrations where phosphorus became the limiting factor for microalgae growth. Using a laboratory algae growth potential test (AGP-test) it was established that below 100 µg P/L phosphorus was the limiting factor, and in the range of 100–200 µg P/L nitrogen, carbon dioxide, light, or combinations of these, started to take over as limiting factors. Lake South Åsvalltjärn was one of the lakes in the investigation shifting to the range of clear phosphorus limitation after introduction of wastewater treatment with chemical precipitation. Two other polluted lakes where wastewater treatment had decreased the nutrient load was evaluated by Forsberg [
2]. One of them, Lake Boren, recovered in a similar way as Lake South Åsvalltjärn during the 1970s, but in the other, Lake Uttran, the nutrient load increased after reduced nutrient load. Forsberg [
2] attributed this to two important differences between the lakes. First, the flushing rate, where Lake Boren was flushed every second month on average, while Lake Uttran took 50 month to flush. One other factor that seemed important in Lake Uttran was the predominant winds causing occasional resuspension of the sediments in the lake. Lake South Åsvalltjärn has a flushing rate of approximately one month as an annual average, which is more similar to Lake Boren than Lake Uttran.
3.3. Fish
Näslund [
15] studied the fish migration in 1985–1987 between the Åsvalltjärnarna lakes and the downstream Lake Visjön (
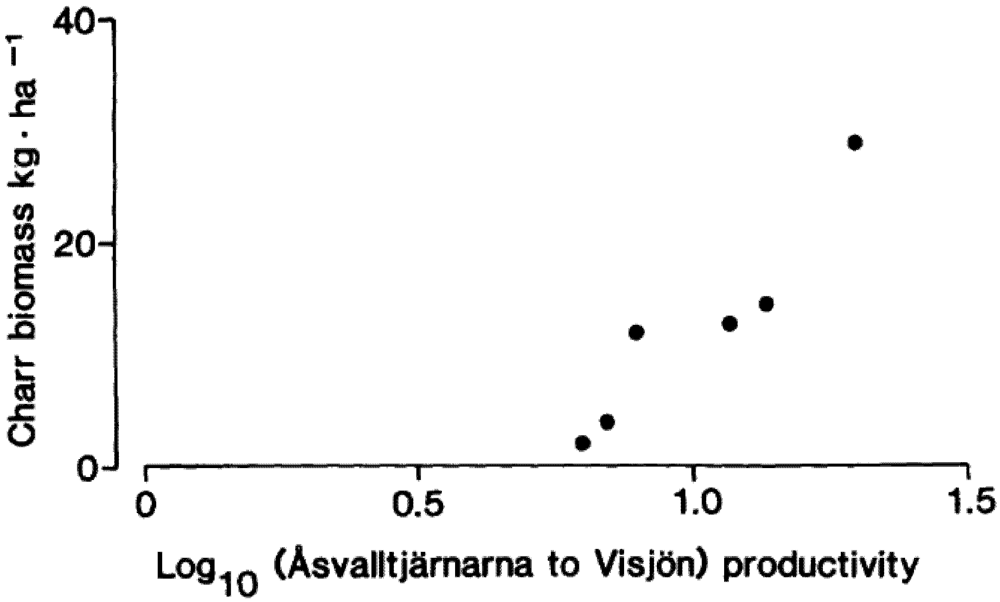
Figure 1), and found that the intensity in the migration was proportional to the productivity difference between the Åsvalltjärnarna lakes and Lake Visjön (
Figure 11).
Figure 11.
Log
10 relative productivity (based on total phosphorus) of the Åsvalltjärnarna lakes/Lake Visjön versus the Arctic charr biomass (kg/ha) in Åsvalltjärnarna [
9]. The 2003 value was: relative productivity 0.084, and charr biomass 5.6 kg/ha.
Figure 11.
Log
10 relative productivity (based on total phosphorus) of the Åsvalltjärnarna lakes/Lake Visjön versus the Arctic charr biomass (kg/ha) in Åsvalltjärnarna [
9]. The 2003 value was: relative productivity 0.084, and charr biomass 5.6 kg/ha.
In more detail, the fish migration study described how a proportion of an Arctic charr population migrated upstream into small tributaries during spring. The migration was initiated by a rise in water temperature, guided by water temperature gradients and confined to a period of two to three weeks during the snow-melt period. The majority of the migrants were juveniles, 15–20 cm in length. An effect of competition on the migratory behaviour was suggested, since charr in this size interval grew very slowly in Lake Visjön because of a competitive bottleneck. The migrants were shown to have a lower condition factor than the fish resident in Lake Visjön. Most of the migrants ended up in infertile habitats such as springs and bogs and returned downstream within a few days. However, some of them found their way to the productive Åsvalltjärnarna lakes. In contrast to charr that moved into most of the other tributaries of Lake Visjön, the charr entering the Åsvalltjärnarna lakes remained in the new habitat over the entire summer. Due to the abundant supply of zooplankton and zoo-benthos, fish in the Åsvalltjärnarna lakes grew considerably faster than charr resident in Lake Visjön. In fact, charr in the size interval 15–20 cm increased fourfold in weight over the three months in the lakes, which is very close to what is achieved under intense farming conditions in cage-cultures. Return migrations for spawning and over-wintering in Visjön occurred in September to October. Over-wintering in the Åsvalltjärnarna lakes was impossible due to oxygen depletion from early December. Migrants to the Åsvalltjärnarna lakes repeated the habitat shift annually for three to four years, whereafter they took up residency in Lake Visjön. The cessation of migration for larger/older individuals was probably a consequence of the fact that the relatively small food items in the Åsvalltjärnarna lakes (mainly zooplankton) became less beneficial from an energetic perspective with increasing fish size. In addition, large fish have better opportunities to compete for food and avoid predation in Lake Visjön. The incidence of repeat migration was regulated via the responses of migrants to changes in productivity in the distant habitat. As a result of the decreased phosphorus load, with reduced productivity, repeat migration and also fish biomass in the Åsvalltjärnarna lakes decreased with time. It was concluded that the migrations represent attempts to exploit seasonally favourable habitats rather than efforts to colonise new areas. It was evident that population of Arctic charr did express an exploratory behaviour and may, due to a learning capacity, develop a system with seasonal habitat shifts. Prerequisites are the flexible life history of this species and the availability of relatively more productive, distant habitats. As a result of the habitat shifts migrants grew faster and matured earlier (but at the same size) as resident fish. It was also evident that there was a relationship between relative productivity and fish biomass in the distant habitat. These findings indicate that, from a nutrient enhancement perspective, it would be possible to increase fish production not only in the population present in the actual lake, but also for other population of the watershed. This if the enriched habitat is accessible and the population holds the characteristics necessary for the development of seasonal habitat shifts.
Näslund
et al. [
9] saw a decreasing trend during 1985–1987 with decreasing Arctic charr biomass (
Table 3) and less repeats in migration behaviour. The samples in 2003 indicate that this decrease did not continue for Arctic charr, or may even have been reversed (
Table 3). The total fish biomass seems to have increased back to the estimated levels in 1980. The other fishes, brook and brown trout mainly habitating the downstream river system, had increased, possibly due to lower competition pressure from Arctic charr. The fish stock in 2003 was higher than expected, and did not follow the same decreasing trend as phosphorus and phytoplankton. Especially the phytoplankton trend indicates that even though a higher stock, the growth of the population might be lower than in the mid-1980s. It must be remembered, though, that the 2003 sample is based only on four catch opportunities during one night of fishing.
The low oxygen in late winter prohibits the fish populations to be resident in the Åsvalltjärnarna lakes. Free-swimming trout are believed to tolerate oxygen levels as low as 5–5.5 mg/L, but the ideal is considered to be more than 80% saturation [
20]. In Lake North Åsvalltjärn, oxygen (
Figure 7) is optimal in the surface water and well above 5 mg/L full depth during summer, but too low during winter. In Lake South Åsvalltjärn the oxygen (
Figure 5) was below 5 mg/L in bottom water in June and August samples, but higher in surface water, and there were always upper parts with above 80% saturation (
Figure 6).
Table 3.
Trends in fish biomass and proportion of Arctic charr in the Åsvalltjärnarna lakes. Data from 1977–1987 in Näslund [
15] and from 2003 in the present study.
Table 3.
Trends in fish biomass and proportion of Arctic charr in the Åsvalltjärnarna lakes. Data from 1977–1987 in Näslund [15] and from 2003 in the present study.
| Year a | Total fish biomass (kg) | Arctic charr biomass (kg) | Percent Arctic charr (%) |
|---|
| 1977 | 208 | 162 | 78 |
| 1979 | 90 | 76 | 84 |
| 1980 | 94 | 65 | 70 |
| 1985 | 75 | 63 | 84 |
| 1986 | 30 | 20 | 67 |
| 1987 | 34 | 11 | 32 |
| 2003 | 116 | 31 | 26 |
4. Options for Future Management of the Åsvalltjärnarna Lakes
In this section, possible future development options for the Åsvalltjärnarna lakes are discussed. There are three possible management strategies for the last phase of recovery in the lakes, namely continued development (1) as implicit in current management; (2) to a pristine-like state; and (3) to maintenance of the developed fish migration pattern.
The first alternative is a “zero alternative”, according to environmental impact assessment (EIA) terminology, which means not changing anything but continuing the current management. This will probably leave the lakes in a higher nutrient status than the surrounding lakes for a long time, not necessarily because of insufficient wastewater treatment, but due to phosphorus release from the sediments in the Åsvalltjärnarna lakes, and in the upstream Lake Topptjärn. The winter oxygen depletion will probably also remain for a long time.
The second alternative is to decrease the nutrient levels even more and reach a pristine-like state. This will probably require some action regarding the sediments, like removal (dredging) or sealing. It is unclear to what extent it also means higher demands on the wastewater treatment.
The third alternative is to maintain the spontaneously developed fish migration pattern. This will probably require allowing an increasing amount of nutrients to again pass through the wastewater treatment plant. This would be a unique decision in the history of wastewater treatment in Sweden. The results from the 2003 investigation suggest that the nutrient and microalgae base for the fish population have significantly decreased since the mid-1980s, which means that from a downstream fish production and population resilience perspective, the wastewater treatment is now too effective. For the downstream fish population, the concentrations of phosphorus and microalgae were probably better in the mid-1980s than today. However, higher nutrient levels in the Åsvalltjärnarna lakes will continue to affect the downstream creeks, which will have a higher nutrient state than surrounding water systems. Such influence may be considered the outer limit downstream of allowed nutrient discharge from the wastewater treatment. In principle, the wastewater treatment will include the downstream creeks. When the water enters Lake Visjön, it should be clean enough to not change the pristine-like state there.
The first two management options can be considered as established options. The third option, however, has so far been rarely elucidated in the context of environmental management options. It carries some interesting features regarding nutrient recycling not very often recognized, and will therefore be given special attention in the following.
An Interesting Example of Regional Nutrient Management?
A maintained fish production moves the focus regarding wastewater treatment from a “protecting the recipient” view to a view of finding cooperation between wastewater treatment and productive benefits downstream, a type of regional nutrient management. There are three possible reasons for a more general use of such a regional nutrient management view.
The first reason is the above-mentioned: if there are identifiable benefits of discharging nutrients, it should be considered. In the Storlien case, the benefits are twofold. The economically beneficial fish production in the downstream rivers and Lake Visjön is obvious. The other benefit is a broader and more diverse nutrient and energy base for the fish population in Lake Visjön. Reasons to favour a population can, in addition to economic benefits, be nature conservation reasons, where there is a goal to preserve the fish population, and the extra production opportunity will enhance the resilience of the population.
A second reason may be what is called cultural oligotrophication [
21,
22,
23], which is a term for when the slow natural oligotrophication processes of highland areas, are speeded up due to human actions. Such human actions can be reservoirs and impoundments causing wash-out effects on shorelines, or logging causing higher nutrient loss from the soils, etc
. Wastewater treatment may then be a cheap and acceptable source of nutrients to counteract such processes in a region. In this Storlien case study, cultural oligotrophication may not be present, since there are no such processes obvious in the watershed. On the contrary, there are probably other human eutrophication sources present than wastewater. However, on the larger regional scale and in other locations, it may be a factor to consider.
A third reason may be a desired goal of nutrient recirculation in society, as mentioned above. The discussion in Sweden has so far focused very much on terrestrial use, mostly agriculture. Aquaculture, however, in this case through natural aquatic ecosystems, may be an option that should be further investigated.
Possibilities and Problems
The positive downstream effects in the Storlien case developed without planning, by the self-organizing power of the downstream ecosystems. Can such effects be achieved in a more planned way? If the Storlien case had been planned, one important question would have been whether it its acceptable to change the ecosystems in the Åsvalltjärnarna lakes to a higher nutrient state as an interface ecosystem between the wastewater treatment and the natural ecosystems. One way to avoid this dilemma would be to, within the wastewater treatment plant, design for these interface ponds, added to what is necessary from hygienic and hazardous substances reasons. Access to these ponds (but not to the ordinary wastewater treatment facility) for recipient fish populations should be arranged by, for example, fish ladders during the full productive season or, as in the Åsvalltjärnarna lakes, just in spring and autumn. Another option is to plant juveniles in the aquaculture ponds, and let them out in the autumn. A problem could be that normal wastewater treatment plant design often has the outlet in the recipient lake some distance from the shore and some meters below the surface. This arrangement is not likely to allow for fish spontaneously finding their way to the aquaculture ponds.
Stabilization pond systems are dimensioned for the coldest design period. The size and number of ponds needed to meet the winter treatment conditions can be compared with the smaller size needed for summer conditions, and the difference can be the size of the last maturation pond. This last pond, or several subponds, can then be given access to migrating fish during the productive period. The hygienic and eventually toxicological aspects must regulate what is possible.
Another combination is with fellingsdams [
24], a treatment type that is currently expanding in the rural northern part of Sweden. These were often a series of previous stabilization ponds, which in the 1970s were considered as not performing well enough regarding nutrient reduction, and therefore at that time were replaced by chemical precipitation in so-called “package plants.” These lacked robust function in the context of a high flow variation and low manual operation intensity. Instead, in the fellingdams, the chemical precipitation is combined with two of the old stabilization ponds, the first as a flow buffer pond also providing primary treatment. Precipitant is added in a pump house pit between the ponds, and precipitation and settling is performed in the second pond. Often there are more than two old stabilization ponds present, which are then sometimes used as extra maturation ponds. These extra ponds can easily be used as aquaculture maturation ponds, if the problem of access for fish from the downstream recipient could be solved.
One feature of the Storlien example is that there were no other fish populations in the system besides fish considered game fish by humans. In other places, the aquaculture may take other paths of food chains, which cannot always be predicted, as is a general lesson of manipulating natural systems.
The hygienic and toxicity issues are key questions to cope with. Currently, for instance, residues from medication are an issue in the wastewater treatment debate. This is an important concern, and the general answer is given above: the wastewater should be sufficiently treated concerning hygienic risks and hazardous substances including residues from medication. This question is, in addition, not only connected to pure risk assessment, but also to image questions of products or the attractiveness of regions. In sparsely populated areas of cold climates, the image is often connected to buzzwords such as “pure,” “natural,” and “pristine.” Charr and trout connected with the words “wastewater aquaculture” do not have the same image as connected with the previous words. One solution to this can be to use only certain fractions of the wastewater for this type of summer migration aquaculture ponds. This can be source-separated urine, greywater only, or dairy industrial wastewater only. Another option is to use drainage water ponds collecting the diffuse leakage of fertilizers from ecological farming (e.g., without pesticides).
5. Conclusions
Two small subarctic lakes, eutrophicated due to wastewater discharge from 1964, have recovered significantly since 1975 when an advanced wastewater treatment plant was originally built. In 2003, the lakes were found to have returned to an oligotrophic state, but not fully to a pre-sewage situation. In the upper lake, more heavily polluted, the total phosphorous levels had decreased from an average of 168 µg P/L in 1972–1974 to an average of 12 µg P/L in 2003. The phytoplankton biomass had decreased twentyfold during the same period. The introduction of the wastewater treatment plant has been successful.
In the 1980s, investigations concluded that a population of downstream Arctic charr had developed a migration behaviour utilizing the nutrients in the lakes. Since then, the phosphorous concentration and phytoplankton biomass have decreased almost an order of magnitude, suggesting fish production should also be lower. However, a few multiple mesh gillnetting samples indicated the standing biomass to be the same as in the mid-1980s, but shed no further light on the actual production of fish biomass.
Three possible management strategies for the last phase of recovery were suggested, namely continued development (1) as implicit in current management; (2) to a pristine-like state; and (3) to maintain the developed fish migration pattern. The state and art of the sediments upstreams are crucial for the outcome of these management strategies. This has not been directly investigated, but there are several indications of it. A hypothesis is that with current management the storage of sediment nutrients are believed to maintain the lakes in an oligotrophic state but with higher nutrient status than surrounding lakes. Oxygen depletion in late winter will continue to profoundly affect the composition of residential organisms in the lakes. A pristine-like state probably requires removal or sealing of the sediments. Maintenance of the fish migration behaviour with production levels of the 1980s probably requires a higher nutrient load on the lakes again.
Future Research
The perspective of the Åsvallstjärnarna lakes’ function as an ecotone interface between the wastewater treatment and the recipient Lake Visjön may inspire future development of the pond technology. The wastewater treatment plant could contain this interface with access for downstream fish populations during the productive summer period.
The problem discussed above with the wastewater often being discharged in the recipient through a underwater tube, problematic for fish entrance, may be solved in cooperation between fish biologists and engineers.
In nutrient-poor regions, which are generally highland regions, the general guidelines for wastewater treatment may be re-evaluated: the old focus on hygienic and toxicological aspects should still be a main focus, together with the continuing development of focus on the group of substances that can be summarized as “foreign to nature”-substances. The nutrients, however, especially phosphorus, may be absorbed at low levels in the treatment plant, and instead planned for downstream utilization of fish or other aquatic benefits, or maybe as fertilizer in irrigation systems. Separation of phosphorus for agricultural use is still an option but will probably always be connected with higher cost of both money and resources for its separation and transportation.