Microporous Silica Based Membranes for Desalination
Abstract
:1. Introduction
2. Membrane Processing and Transport Mechanisms for Water Desalination
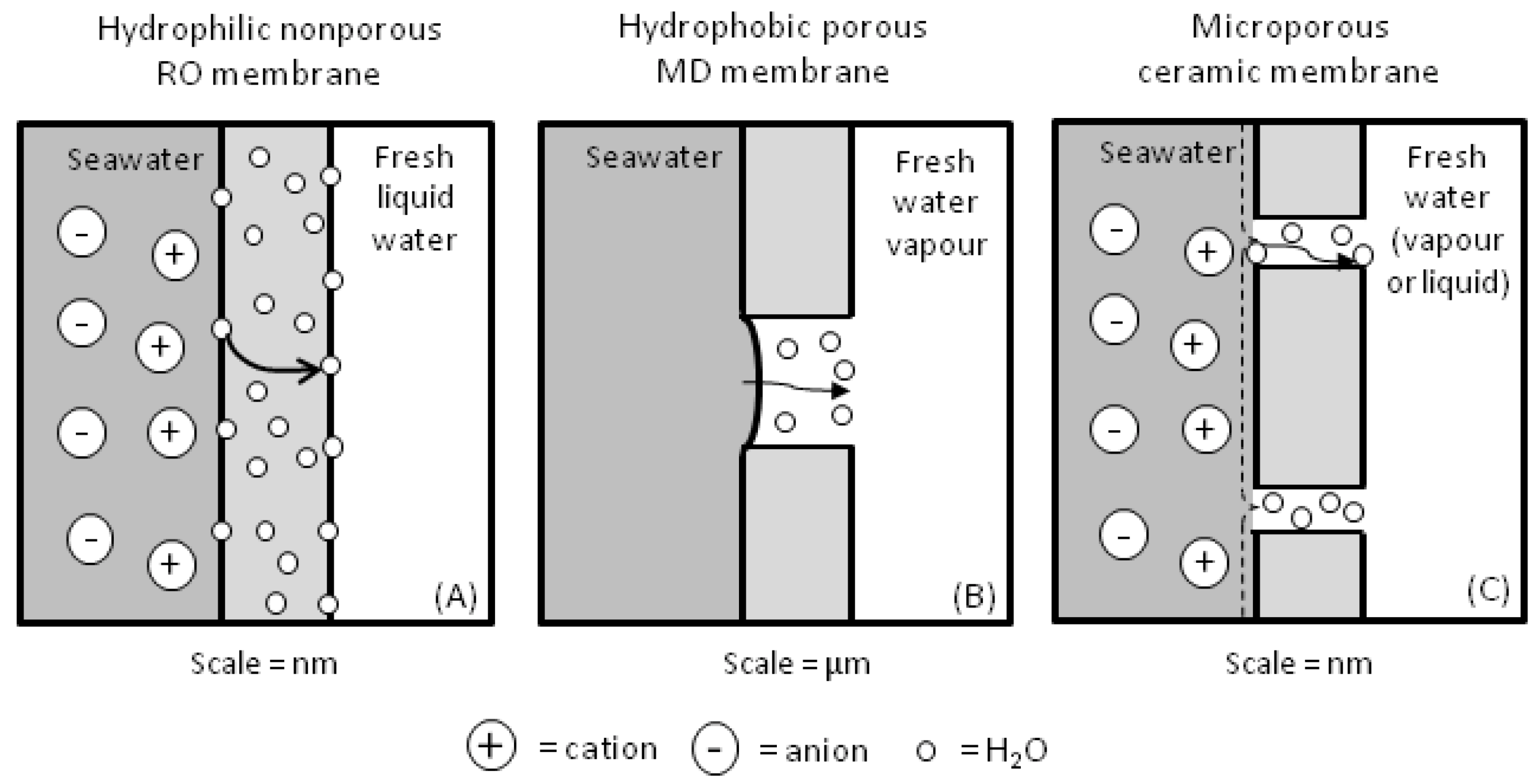
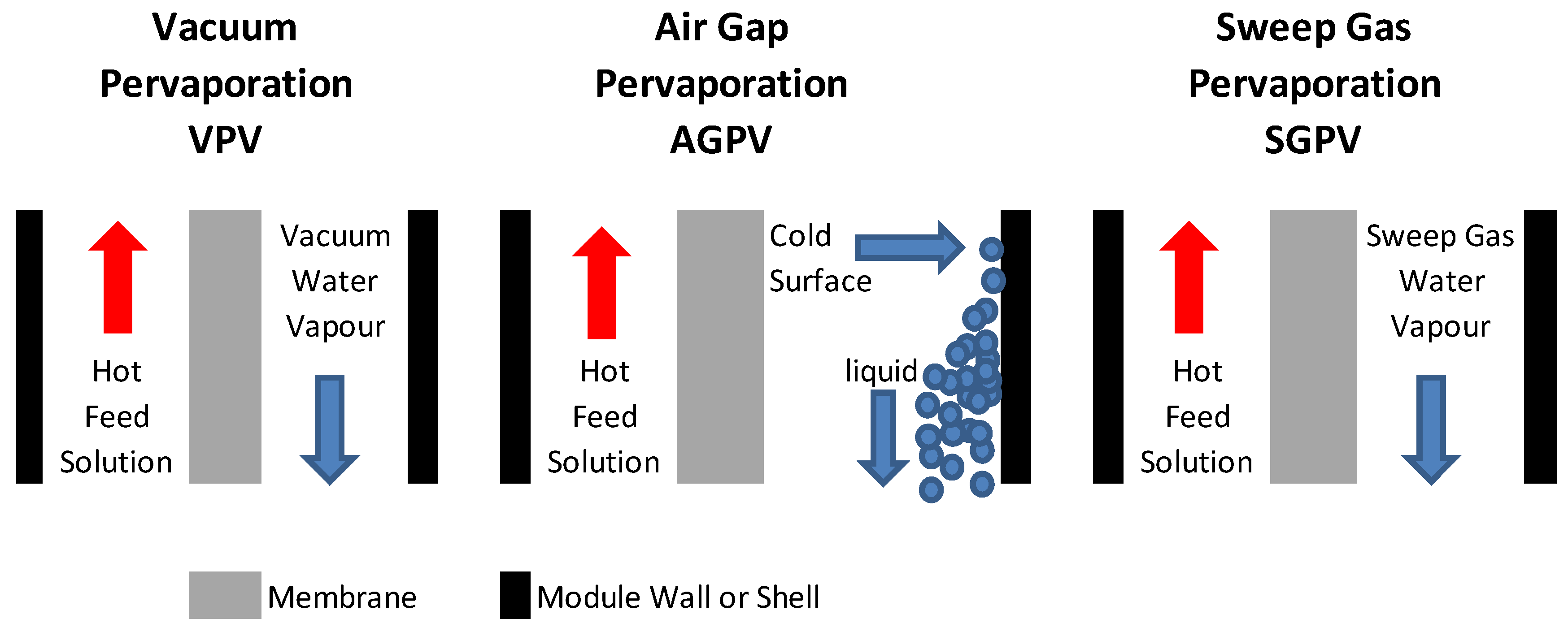
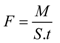
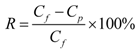
3. Features of Silica Based Membranes for Desalination
3.1. Features of Silica Based Membranes for Desalination
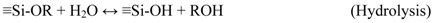
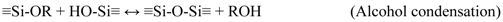
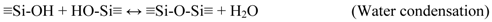
3.2. Membrane Preparation
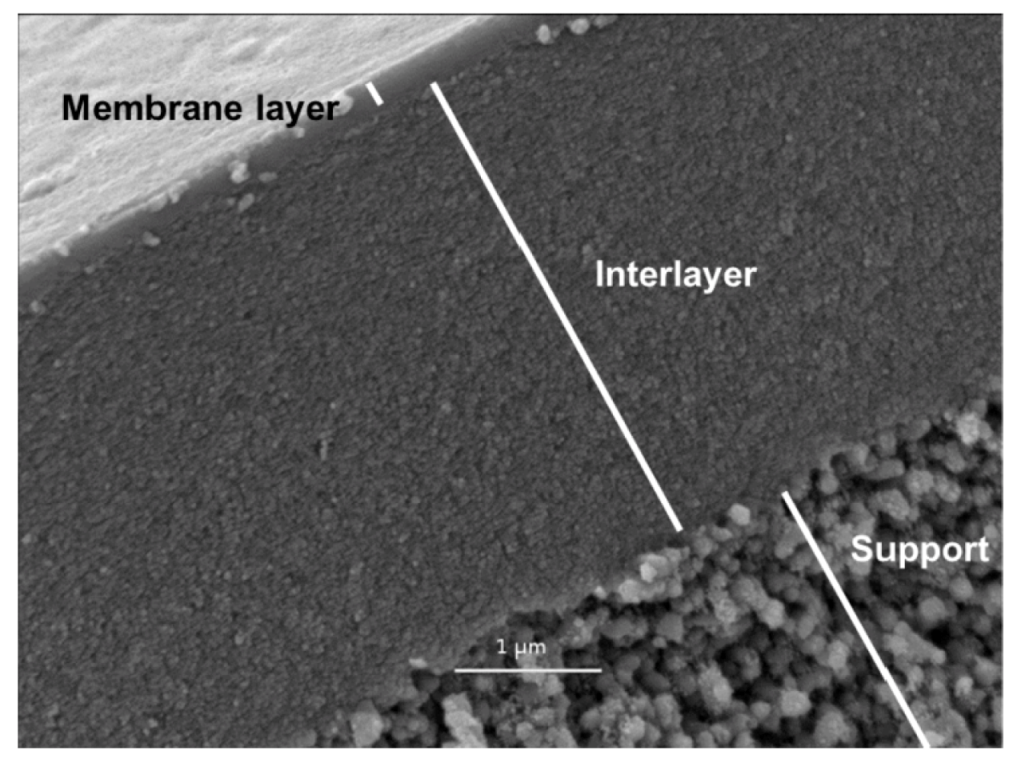
4. Novel Silica Based Membranes in Desalination
4.1. Hydro-stability and Current Strategies
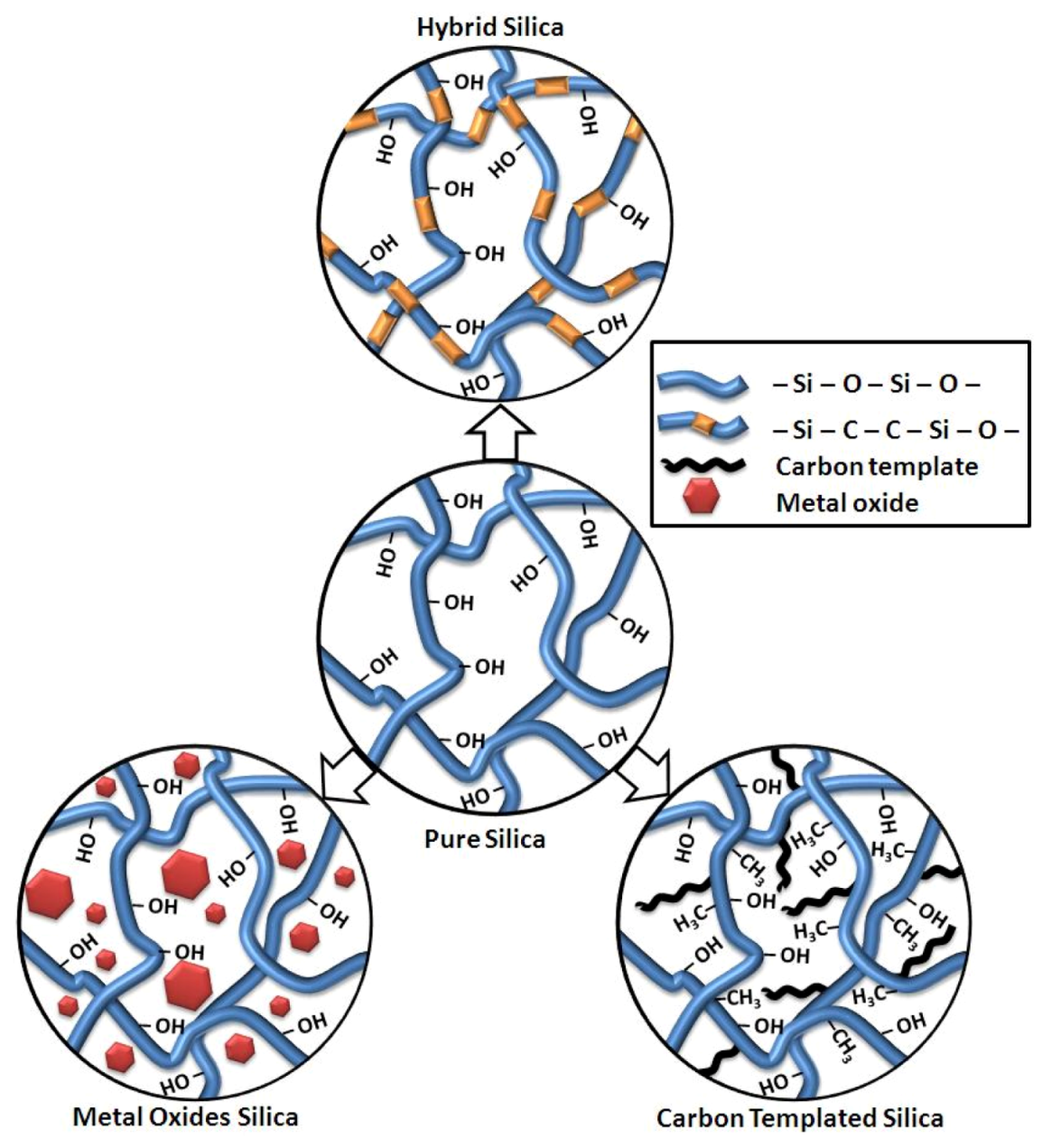
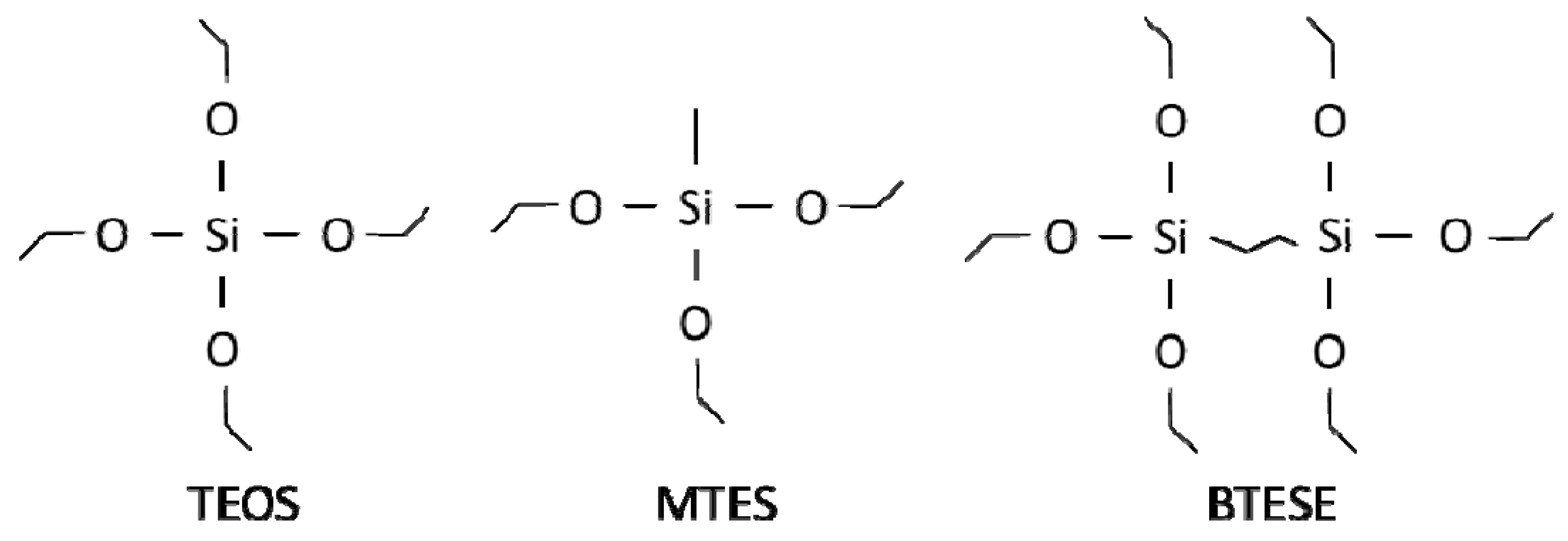
4.2. Membrane Performance: Effect of Testing Conditions
| Membrane type | Testing conditions (Temp., Pressure) | Feed conc. range (wt %) Lower/Higher | Water flux (kg m−2 h−1) | Rejection (%) | Stability Tests | Reference | |
| Carbonized template CTMSS | Ionic C6 | 20 °C, P = 7 bara |  | 99.9/98 | 5h | [19] | |
| Ionic C6 | 20 °C, ΔP < 1 barb | 0.3/3.5 | 3.2/1.4 | 86/92 | N/A | [66] | |
| Ionic C12 | 20 °C, ΔP < 1 barb | 0.3/3.5 | 2.8/1.6 | 84/94 | N/A | [66] | |
| Ionic C16 | 20 °C, ΔP < 1 barb | 0.3/3.5 | 3/2 | 91/97 | N/A | [66] | |
| 10 wt % PEG-PPG | 20 °C, ΔP < 1 barb | 0.3/3.5 | 1.5/1.5 | 90/99.8 | 12 h | [68] | |
| 20 wt % PEG-PPG | 20 °C, ΔP < 1 barb | 0.3/3.5 | 6.3/4.9 | 87/97 | 12 h | [68] | |
| Metal oxide | CoOxSi | 20 °C, ΔP < 1bar | 0.3/15 | 0.4–0.3 | 99.7/99.9 | 570 h | [21] |
| 50 °C, ΔP < 1bar | 0.3/15 | 0.9/0.35 | 99.5/99.9 | 570 h | [21] | ||
| 75 °C, ΔP < 1bar | 0.3/15 | 1.8/0.55 | 99.5/99.9 | 570 h | [21] | ||
| Hybrid | BTESE | 30 °C, ΔP < 1bar | 0.2 | 3 | 99 | N/A | [7] |
| BTESE | 90 °C, ΔP < 1bar | 0.2 | 34 | 99.9 | N/A | [7] | |
| MTES | 20 °C, P = 7 bara | 0.3/3.5* | 4.7/2.5 | 93.7/83 | 5 h | [19] | |

4.3. Future Challenges
5. Conclusions
Acknowledgments
References
- World Health Organization and United Nations International Children’s Fund, Progress on Sanitation and Drinking-Water in Joint Monitoring Programme for Water Supply and Sanitation; World Health Organization: Geneva, Switzerland, 2010.
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination-development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Duke, M.C.; O’Brien-Abraham, J.; Milne, N.; Zhu, B.; Lin, J.Y.S.; Diniz da Costa, J.C. Seawater desalination performance of mfi type membranes made by secondary growth. Sep. Purif. Technol. 2009, 68, 343–350. [Google Scholar]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Li, D.; Wang, H. Recent developments in reverse osmosis desalination membranes. J. Mater. Chem. 2010, 20, 4551–4566. [Google Scholar]
- Igi, R.; Yoshioka, T.; Ikuhara, Y.H.; Iwamoto, Y.; Tsuru, T. Characterization of co-doped silica for improved hydrothermal stability and application to hydrogen separation membranes at high temperatures. J. Am. Ceram. Soc. 2008, 91, 2975–2981. [Google Scholar]
- Tsuru, T.; Igi, R.; Kanezashi, M.; Yoshioka, T.; Fujisaki, S.; Iwamoto, Y. Permeation properties of hydrogen and water vapor through porous silica membranes at high temperatures. AIChE J. 2011, 57, 618–629. [Google Scholar]
- Uhlmann, D.; Smart, S.; Diniz da Costa, J.C. H2S stability and separation performance of cobalt oxide silica membranes. J. Membr. Sci. 2011, 380, 48–54. [Google Scholar] [CrossRef]
- Yacou, C.; Smart, S.; Diniz da Costa, J.C. Long term performance cobalt oxide silica membrane module for high temperature H2 separation. Energy Environ. Sci. 2012, 5, 5820–5832. [Google Scholar] [CrossRef]
- Li, L.X.; Dong, J.H.; Nenoff, T.M.; Lee, R. Reverse osmosis of ionic aqueous solutions on a mfi zeolite membrane. Desalination 2004, 170, 309–316. [Google Scholar] [CrossRef]
- Lin, J.; Murad, S. A computer simulation study of the separation of aqueous solutions using thin zeolite membranes. Mol. Phys. 2001, 99, 1175–1181. [Google Scholar]
- Leboda, R.; Mendyk, E. Hydrothermal modification of porous structure of silica adsorbents. Mater. Chem. Phys. 1991, 27, 189–212. [Google Scholar] [CrossRef]
- Wang, L.K.; Chen, J.P.; Hung, Y.-T.; Shammas, N.K. Handbook of Environmental Engineering: Membrane and Desalination Technology; Humana Press: New York, NY, USA, 2011; Volume 13. [Google Scholar]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Pina, M.P.; Mallada, R.; Arruebo, M.; Urbiztondo, M.; Navascués, N.; de la Iglesia, O.; Santamaria, J. Zeolite films and membranes. Emerging applications. Microporous Mesoporous Mater. 2011, 144, 19–27. [Google Scholar]
- Yang, W.; Li, Y. Zeolite membranes. In Inorganic Membranes for Energy and Environmental Applications; Bose, A.C., Ed.; Springer: New York, NY, USA, 2009; pp. 275–286. [Google Scholar]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Bolto, B.; Hoang, M.; Xie, Z. A review of membrane selection for the dehydration of aqueous ethanol by pervaporation. Chem. Eng. Process. Process Intensif. 2011, 50, 227–235. [Google Scholar] [CrossRef]
- Duke, M.C.; Mee, S.; Diniz da Costa, J.C. Performance of porous inorganic membranes in non-osmotic desalination. Water Res. 2007, 41, 3998–4004. [Google Scholar] [CrossRef]
- Wee, S.L.; Tye, C.T.; Bhatia, S. Membrane separation process-pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Lin, C.X.C.; Ding, L.P.; Smart, S.; Diniz da Costa, J.C. Cobalt oxide silica membranes for desalination. J. Colloid Interface Sci. 2012, 368, 70–76. [Google Scholar]
- Drobek, M.; Motuzas, J.; Julbe, A.; Ding, L.; Yacou, C.; Diniz da Costa, J.C. Long term pervaporation desalination of tubular mfi zeolite membranes. J. Membr. Sci. 2012, 415–416, 816–823. [Google Scholar]
- De Lange, R.S.A.; Keizer, K.; Burggraaf, A.J. Analysis and theory of gas transport in microporous sol-gel derived ceramic membranes. J. Membr. Sci. 1995, 104, 81–100. [Google Scholar] [CrossRef]
- Basile, A.; Gallucci, F. Membranes for Membrane Reactors; John Wiley and Sons: Singapore, 2011; Volume 1. [Google Scholar]
- Kesmez, Ö.; Burunkaya, E.; Kiraz, N.; Çamurlu, H.E.; Asiltürk, M.; Arpaç, E. Effect of acid, water and alcohol ratios on sol-gel preparation of antireflective amorphous SiO2 coatings. J. Non-Cryst. Solids 2011, 357, 3130–3135. [Google Scholar]
- Klein, L.C. Sol-Gel Technology for Thin Films, Fibers, Preforms, Electronics and Specialty Shapes; William Andrew Publishing/Noyes: Park Ridge, NJ, USA, 1988. [Google Scholar]
- Kreiter, R. Hydrothermal stability of silica-based membranes. In Proceedings of Sol-Gel Conference, Porto de Galinhas, Brasil, 22–27 August 2009; Energy Reasearch Center of the Netherland: Porto de Galinhas, Brasil, 2009. [Google Scholar]
- Kreiter, R.; Rietkerk, M.; Bonekamp, B.; van Veen, H.; Kessler, V.; Vente, J. Sol-gel routes for microporous zirconia and titania membranes. J. Sol Gel Sci. Technol. 2008, 48, 203–211. [Google Scholar]
- Lange, R.S.A.; Kumar, K.N.P.; Hekkink, J.H.A.; Velde, G.M.H.; Keizer, K.; Burggraaf, A.J.; Dokter, W.H.; Garderen, H.F.; Beelen, T.P.M. Microporous SiO2 and SiO2/MOx (M = Ti, Zr, Al) for ceramic membrane applications: A microstructural study of the sol-stage and the consolidated state. J. Sol Gel Sci. Technol. 1994, 2, 489–495. [Google Scholar] [CrossRef]
- Livage, J.; Sanchez, C. Sol-gel chemistry. J. Non Cryst. Solids 1992, 145, 11–19. [Google Scholar]
- Nair, B.N.; Yamaguchi, T.; Okubo, T.; Suematsu, H.; Keizer, K.; Nakao, S.-I. Sol-gel synthesis of molecular sieving silica membranes. J. Membr. Sci. 1997, 135, 237–243. [Google Scholar]
- Favennec, L.; Jousseaume, V.; Rouessac, V.; Fusalba, F.; Durand, J.; Passemard, G. Porous extreme low κ (Elκ) dielectrics using a PECVD porogen approach. Mater. Sci. Semicond. Process. 2004, 7, 277–282. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T.; de Souza, K.R.; Noronha, F.B. Chapter 2—Review of silica membranes for hydrogen separation prepared by chemical vapor deposition. In Membrane Science and Technology; Oyama, S.T., Susan, M.S.-W., Eds.; Elsevier: New York, NY, USA, 2011; Volume 14, pp. 25–60. [Google Scholar]
- Nomura, M.; Aida, H.; Gopalakrishnan, S.; Sugawara, T.; Nakao, S.-I.; Yamazaki, S.; Inada, T.; Iwamoto, Y. Steam stability of a silica membrane prepared by counterdiffusion chemical vapor deposition. Desalination 2006, 193, 1–7. [Google Scholar] [CrossRef]
- Nomura, M.; Ono, K.; Gopalakrishnan, S.; Sugawara, T.; Nakao, S.-I. Preparation of a stable silica membrane by a counter diffusion chemical vapor deposition method. J. Membr. Sci. 2005, 251, 151–158. [Google Scholar] [CrossRef]
- Wojcik, A.B.; Klein, L.C. Transparent inorganic/organic copolymers by the sol-gel process: Copolymers of tetraethyl orthosilicate (TEOS), vinyl triethoxysilane (VTES) and (meth)acrylate monomers. J. Sol Gel Sci. Technol. 1995, 4, 57–66. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-gel-glass: I. Gelation and gel structure. Non Cryst. Solids 1985, 70, 301–322. [Google Scholar]
- Boonstra, A.H.; Baken, J.M.E. Relation between the acidity and reactivity of a teos, ethanol and water mixture. J. Non Cryst. Solids 1990, 122, 171–182. [Google Scholar] [CrossRef]
- Chang, S.Y.; Ring, T.A. Map of gel times for three phase region tetraethoxysilane, ethanol and water. J. Non Cryst. Solids 1992, 147–148, 56–61. [Google Scholar]
- Estella, J.; Echeverría, J.C.; Laguna, M.; Garrido, J.J. Silica xerogels of tailored porosity as support matrix for optical chemical sensors. Simultaneous effect of ph, ethanol:Teos and water:Teos molar ratios, and synthesis temperature on gelation time, and textural and structural properties. J. Non Cryst. Solids 2007, 353, 286–294. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Bonekamp, B.C. Preparation of asymmetric ceramic membrane supports by dip-coating. In Fundamentals of Inorganic Membrane Science and Technology; Burggraaf, A.J., Cot, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 141–226. [Google Scholar]
- Bonekamp, B.C.; van Horssen, A.; Correia, L.A.; Vente, J.F.; Haije, W.G. Macroporous support coatings for molecular separation membranes having a minimum defect density. J. Membr. Sci. 2006, 278, 349–356. [Google Scholar] [CrossRef]
- Van Gestel, T.; Vandecasteele, C.; Buekenhoudt, A.; Dotremont, C.; Luyten, J.; van der Bruggen, B.; Maes, G. Corrosion properties of alumina and titania NF membranes. J. Membr. Sci. 2003, 214, 21–29. [Google Scholar]
- Tsuru, T.; Izumi, S.; Yoshioka, T.; Asaeda, M. Temperature effect on transport performance by inorganic nanofiltration membranes. AIChE J. 2000, 46, 565–574. [Google Scholar]
- Tsuru, T.; Wada, S.I.; Izumi, S.; Asaeda, M. Silica-zirconia membranes for nanofiltration. J. Membr. Sci. 1998, 149, 127–135. [Google Scholar] [CrossRef]
- Scriven, L.E. Physics and applications of dip coating and spin coating. In Proceedings of Better Ceramics through Chemistry III, Reno, NV, USA, 5–8 April 1988; Brinker, C.J., Clark, D.E., Ulrich, D.R., Society., M.R., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1988; pp. 717–729. [Google Scholar]
- Brinker, C.J.; Hurd, A.J.; Schunk, P.R.; Frye, G.C.; Ashley, C.S. Review of sol-gel thin film formation. J. Non Cryst. Solids 1992, 147, 424–436. [Google Scholar]
- Brinker, C.J.; Clark, D.E.; Ulrich, D.R.; Society, M.R. Symposium on Better Ceramics through Chemistry III; Proceedings of Better Ceramics through Chemistry III, Reno, NV, USA, 5–8 April 1988; Materials Research Society: Pittsburgh, PA, USA, 1988; pp. 1–847.
- Smart, S.; Lin, C.X.C.; Ding, L.; Thambimuthu, K.; Diniz da Costa, J.C. Ceramic membranes for gas processing in coal gasification. Energy and Environmental Science 2010, 3, 268–278. [Google Scholar]
- Brinker, C.J.; Scherer, G.W. Sol-gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990; pp. 787–838. [Google Scholar]
- Kappert, E.J.; Nijmeijer, A.; Benes, N.E. Expeditious calcination of inorganic membranes by an instant temperature increment. Microporous Mesoporous Mater. 2012, 151, 211–215. [Google Scholar]
- Schillo, M.C.; Park, I.S.; Chiu, W.V.; Verweij, H. Rapid thermal processing of inorganic membranes. J. Membr. Sci. 2010, 362, 127–133. [Google Scholar] [CrossRef]
- De Vos, R.M.; Verweij, H. Improved performance of silica membranes for gas separation. J. Membr. Sci. 1998, 143, 37–51. [Google Scholar] [CrossRef]
- Giessler, S.; Jordan, L.; Diniz da Costa, J.C.; Lu, G.Q. Performance of hydrophobic and hydrophilic silica membrane reactors for the water gas shift reaction. Sep. Purif. Technol. 2003, 32, 255–264. [Google Scholar] [CrossRef]
- Iler, R.K. Chemistry of Silica; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Young, G.J. Interaction of water vapor with silica surfaces. J. Colloid Sci. 1958, 13, 67–85. [Google Scholar] [CrossRef]
- Burneau, A.E.; Gallas, J.-P. Vibrational Spectroscopies—Hydroxyl groups on silica surfaces. In The Surface Properties of Silicas; Legrand, A.P., Ed.; John Wiley: Chichester, NY, USA, 1998; pp. 147–234. [Google Scholar]
- Duke, M.C.; Diniz da Costa, J.C.; Do, D.D.; Gray, P.G.; Lu, G.Q. Hydrothermally robust molecular sieve silica for wet gas separation. Adv. Funct. Mater. 2006, 16, 1215–1220. [Google Scholar]
- Fournier, R.O.; Rowe, J.J. Solubility of amorphous silica in water at high-temperatures and high-pressures. Am. Mineral. 1977, 62, 1052–1056. [Google Scholar]
- Raman, N.K.; Brinker, C.J. Organic “template” approach to molecular sieving silica membranes. J. Membr. Sci. 1995, 105, 273–279. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Naik, S.; Braunbarth, C.M.; Cornelius, C.J.; Pardey, R.; Brinker, C.J. Organic-templated silica membranes: I. Gas and vapor transport properties. J. Membr. Sci. 2003, 215, 225–233. [Google Scholar] [CrossRef]
- Giessler, S.; Diniz da Costa, J.C.; Lu, G.Q. Hydrophobicity of templated silica xerogels for molecular sieving applications. J. Nanosci. Nanotechnol. 2001, 1, 331–336. [Google Scholar] [CrossRef]
- Duke, M.C.; Diniz da Costa, J.C.; Lu, G.Q.; Petch, M.; Gray, P. Carbonised template molecular sieve silica membranes in fuel processing systems: Permeation, hydrostability and regeneration. J. Membr. Sci. 2004, 241, 325–333. [Google Scholar] [CrossRef]
- Wijaya, S.; Duke, M.C.; Diniz da Costa, J.C. Carbonised template silica membranes for desalination. Desalination 2009, 236, 291–298. [Google Scholar] [CrossRef]
- Raman, N.K.; Anderson, M.T.; Brinker, C.J. Template-based approaches to the preparation of amorphous, nanoporous silicas. Chem. Mater. 1996, 8, 1682–1701. [Google Scholar]
- Ladewig, B.P.; Tan, Y.H.; Lin, C.X.C.; Ladewig, K.; Diniz da Costa, J.C.; Smart, S. Preparation, characterization and performance of templated silica membranes in non-osmotic desalination. Materials 2011, 4, 845–856. [Google Scholar]
- De Vos, R.M.; Maier, W.F.; Verweij, H. Hydrophobic silica membranes for gas separation. J. Membr. Sci. 1999, 158, 277–288. [Google Scholar] [CrossRef]
- Campaniello, J.; Engelen, C.W.R.; Haije, W.G.; Pex, P.P.A.C.; Vente, J.F. Long-term pervaporation performance of microporous methylated silica membranes. Chem. Commun. 2004, 10, 834–835. [Google Scholar]
- Castricum, H.L.; Sah, A.; Kreiter, R.; Blank, D.H.A.; Vente, J.F.; ten Elshof, J.E. Hydrothermally stable molecular separation membranes from organically linked silica. J. Mater. Chem. 2008, 18, 2150–2158. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Development of robust organosilica membranes for reverse osmosis. Langmuir 2011, 27, 13996–13999. [Google Scholar]
- Battersby, S.; Smart, S.; Ladewig, B.; Liu, S.; Duke, M.C.; Rudolph, V.; Diniz da Costa, J.C. Hydrothermal stability of cobalt silica membranes in a water gas shift membrane reactor. Sep. Purif. Technol. 2009, 66, 299–305. [Google Scholar]
- Kanezashi, M.; Asaeda, M. Stability of H2-permeselective Ni-doped silica membranes in steam at high temperature. J. Chem. Eng. Jpn. 2005, 38, 908–912. [Google Scholar] [CrossRef]
- Kanezashi, M.; Asaeda, M. Hydrogen permeation characteristics and stability of Ni-doped silica membranes in steam at high temperature. J. Membr. Sci. 2006, 271, 86–93. [Google Scholar] [CrossRef]
- Uhlmann, D.; Liu, S.; Ladewig, B.P.; Diniz da Costa, J.C. Cobalt-doped silica membranes for gas separation. J. Membr. Sci. 2009, 326, 316–321. [Google Scholar] [CrossRef]
- Ikuhara, Y.H.; Mori, H.; Saito, T.; Iwamoto, Y. High-temperature hydrogen adsorption properties of precursor-derived nickel nanoparticle-dispersed amorphous silica. J. Am. Ceram. Soc. 2007, 90, 546–552. [Google Scholar]
- Sparrow, B.S. Empirical equations for the thermodynamic properties of aqueous sodium chloride. Desalination 2003, 159, 161–170. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.-D.; Duke, M.; Xie, Z.; Gray, S. Performance of asymmetric hollow fibre membranes in membrane distillation under various configurations and vacuum enhancement. J. Membr. Sci. 2010, 362, 517–528. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N. Economic evaluation of desalination by small-scale autonomous solar-powered membrane distillation units. Desalination 2008, 220, 566–573. [Google Scholar] [CrossRef]
- Blanco Gálvez, J.; García-Rodríguez, L.; Martín-Mateos, I. Seawater desalination by an innovative solar-powered membrane distillation system: The medesol project. Desalination 2009, 246, 567–576. [Google Scholar] [CrossRef]
- Blanco, J.; Malato, S.; Fernández-Ibañez, P.; Alarcón, D.; Gernjak, W.; Maldonado, M.I. Review of feasible solar energy applications to water processes. Renew. Sustain. Energy Rev. 2009, 13, 1437–1445. [Google Scholar] [CrossRef]
- Chen, T.-C.; Ho, C.-D. Immediate assisted solar direct contact membrane distillation in saline water desalination. J. Membr. Sci. 2010, 358, 122–130. [Google Scholar] [CrossRef]
- Mericq, J.-P.; Laborie, S.; Cabassud, C. Evaluation of systems coupling vacuum membrane distillation and solar energy for seawater desalination. Chem. Eng. J. 2011, 166, 596–606. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal interactions and fouling of nf and ro membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Elma, M.; Yacou, C.; Wang, D.K.; Smart, S.; Diniz da Costa, J.C. Microporous Silica Based Membranes for Desalination. Water 2012, 4, 629-649. https://doi.org/10.3390/w4030629
Elma M, Yacou C, Wang DK, Smart S, Diniz da Costa JC. Microporous Silica Based Membranes for Desalination. Water. 2012; 4(3):629-649. https://doi.org/10.3390/w4030629
Chicago/Turabian StyleElma, Muthia, Christelle Yacou, David K. Wang, Simon Smart, and João C. Diniz da Costa. 2012. "Microporous Silica Based Membranes for Desalination" Water 4, no. 3: 629-649. https://doi.org/10.3390/w4030629
APA StyleElma, M., Yacou, C., Wang, D. K., Smart, S., & Diniz da Costa, J. C. (2012). Microporous Silica Based Membranes for Desalination. Water, 4(3), 629-649. https://doi.org/10.3390/w4030629







