Cleansing Tannery Effluent with Pleurotus opuntiae: A Green Solution for Environmental Restoration and Toxicity Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Sample
2.2. Characterization of Effluent
2.3. Fungal Culture Maintenance
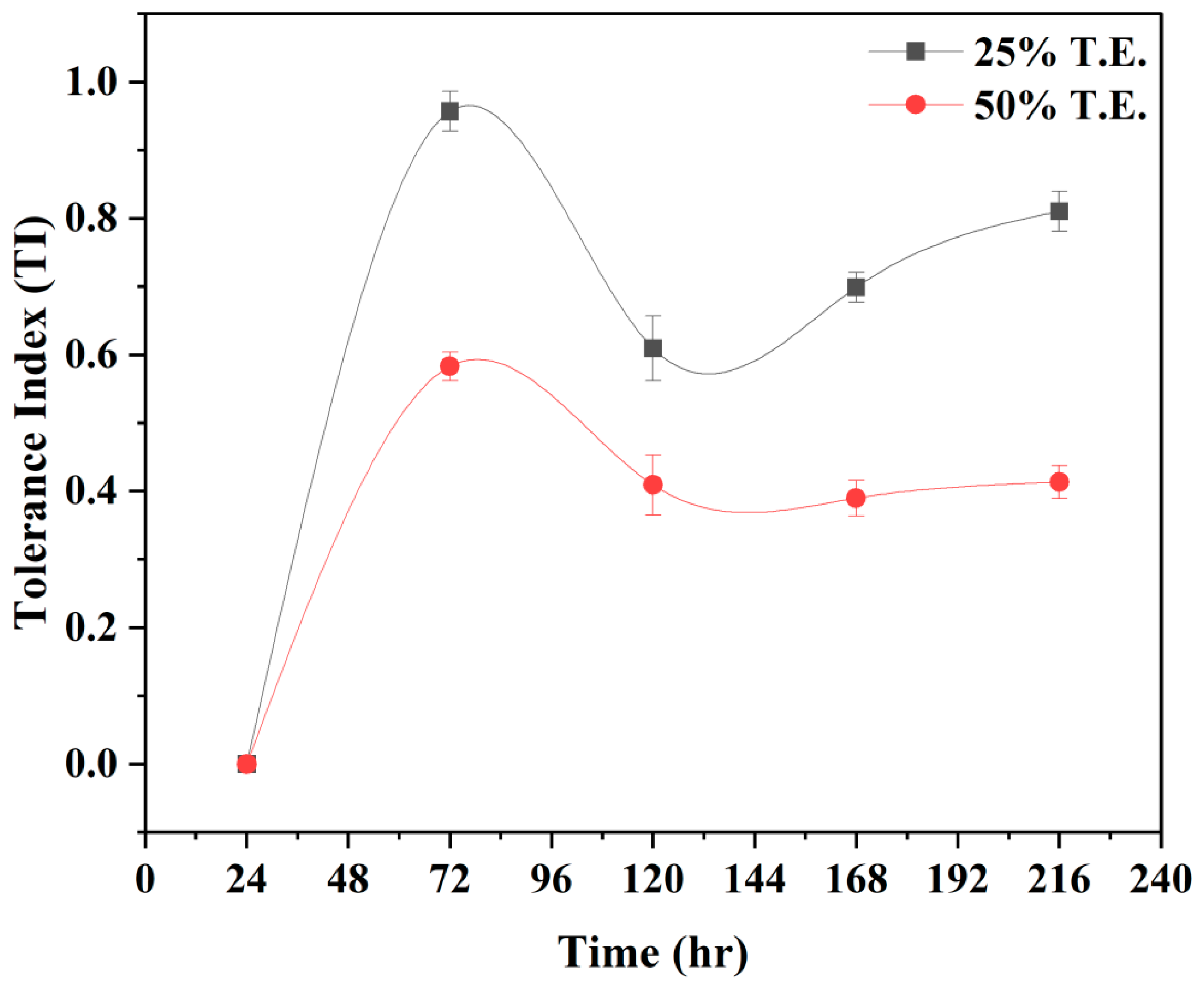
2.4. Establishing Tolerance Index of Pleurotus opuntiae
2.5. Experimental Design for Mycoremediation of Tannery Effluent
2.6. SEM, FTIR, and XRD Analysis
2.7. LC-MS Analysis
2.8. Analysis of Metallothionein Concentrations in Fungal Mycelium
2.9. Analysis of Antioxidants Enzymatic System in HM Containing Mycelia
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis of TE
3.2. Effect of TE Concentrations on P. opuntiae Growth
3.3. Quantifying Removal Rate and Bioaccumulation of Heavy Metals Using P. opuntiae
3.4. Analysis of Surface Morphology of Pleurotus opuntiae Mycelium after Mycoremediation
3.5. FTIR Analysis
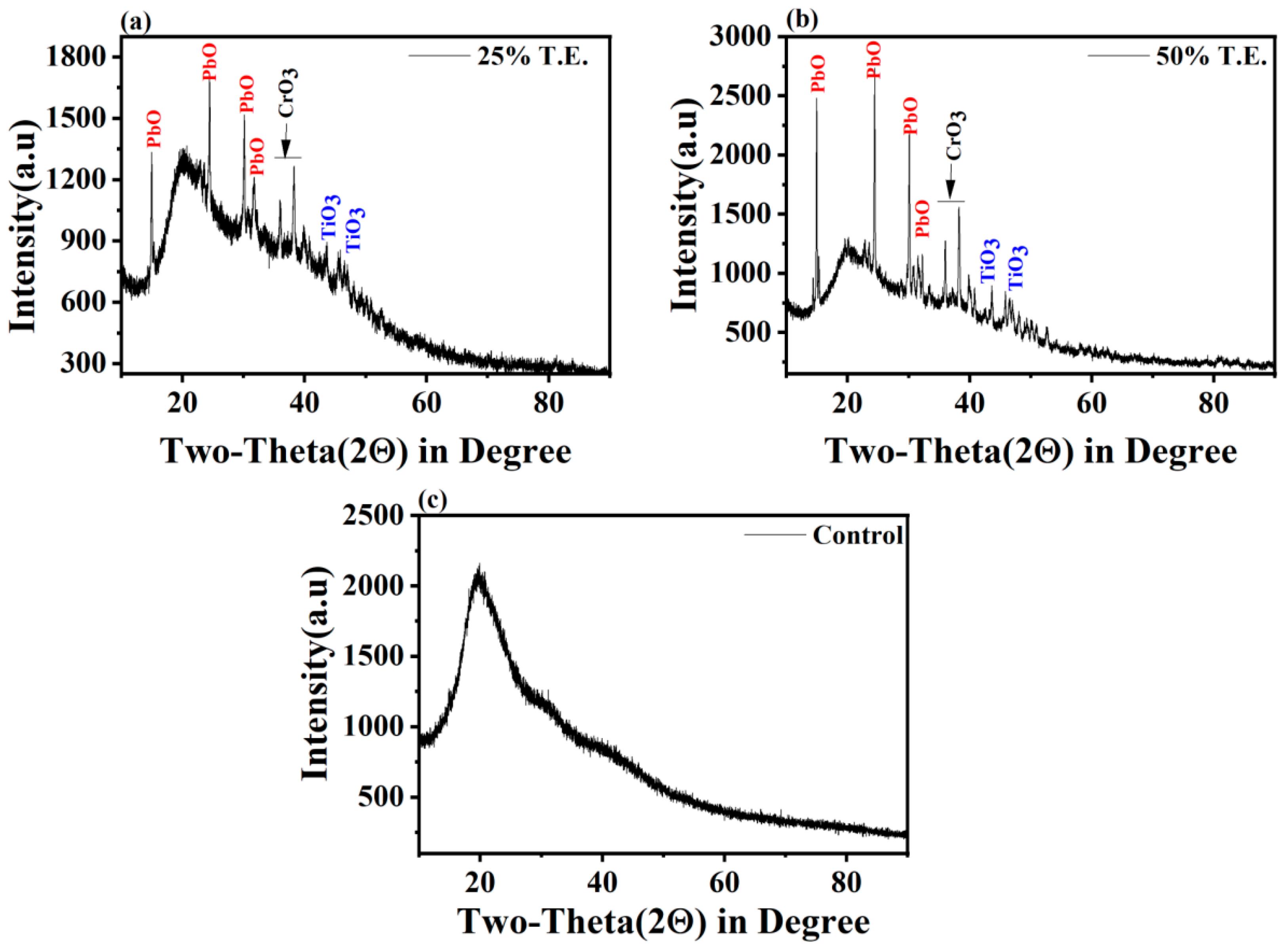
3.6. XRD Analysis
3.7. LC-MS Analysis
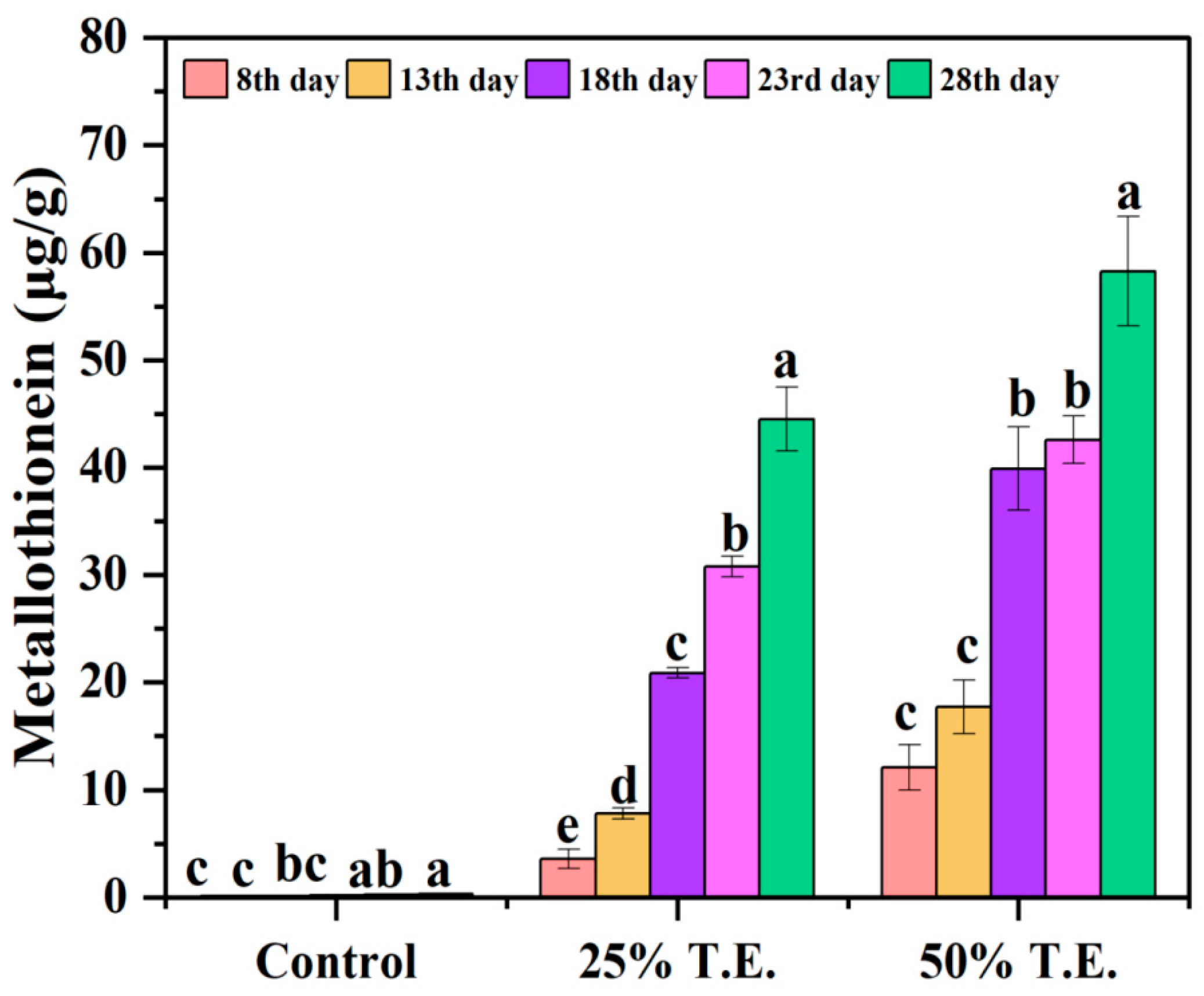
3.8. Metallothionein Concentration in P. opuntiae after Mycoremediation
3.9. Effect of TE Stress on Antioxidant Enzymatic System of Fungi
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tadesse, G.L.; Guya, T.K.; Walabu, M. Impacts of tannery effluent on environments and human health: A review article. Adv. Life Sci. Technol. 2017, 54, 10. [Google Scholar]
- Yadav, A.; Yadav, P.; Raj, A.; Ferreira, L.F.R.; Saratale, G.D.; Bharagava, R.N. Tannery wastewater: A major source of residual organic pollutants and pathogenic microbes and their treatment strategies. In Microbes in Agriculture and Environmental Development; CRC Press: Boca Raton, FL, USA, 2020; pp. 245–264. [Google Scholar]
- Yadav, A.; Mishra, S.; Kaithwas, G.; Raj, A.; Bharagava, R.N. Organic pollutants and pathogenic bacteria in tannery wastewater and their removal strategies. In Microbes and Environmental Management; Studium Press (India) Pvt. Ltd.: New Delhi, India, 2016; pp. 104–130. [Google Scholar]
- Raj, A.; Kumar, S.; Haq, I.; Kumar, M. Detection of tannery effluents induced DNA damage in mung bean by use of random amplified polymorphic DNA markers. Int. Sch. Res. Not. 2014, 2014, 727623. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, A.; Srivastava, J.K.; Raj, A. Reduction of pollution load of tannery effluent by cell immobilization approach using Ochrobactrum intermedium. J. Water Process Eng. 2021, 41, 102059. [Google Scholar] [CrossRef]
- Saxena, G.; Chandra, R.; Bharagava, R.N. Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. Rev. Environ. Contam. Toxicol. Vol. 2017, 240, 31–69. [Google Scholar]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Mia, M.A.S.; Ahmad, F.; Rahman, M.M. An overview of chromium removal techniques from tannery effluent. Appl. Water Sci. 2020, 10, 205. [Google Scholar] [CrossRef]
- Das, C.; Naseera, K.; Ram, A.; Meena, R.M.; Ramaiah, N. Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 235–243. [Google Scholar] [CrossRef]
- Sao, K.; Pandey, M.; Pandey, P.K.; Khan, F. Highly efficient biosorptive removal of lead from industrial effluent. Environ. Sci. Pollut. Res. 2017, 24, 18410–18420. [Google Scholar] [CrossRef]
- Wingenfelder, U.; Hansen, C.; Furrer, G.; Schulin, R. Removal of heavy metals from mine waters by natural zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef]
- Miransari, M. Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol. Adv. 2011, 29, 645–653. [Google Scholar] [CrossRef]
- Poornima, M.; Kumar, R.S.; Thomas, P.D. Isolation and molecular characterization of bacterial strains from tannery effluent and reduction of chromium. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 530–538. [Google Scholar]
- Jiang, J.; Liu, H.; Li, Q.; Gao, N.; Yao, Y.; Xu, H. Combined remediation of Cd–phenanthrene co-contaminated soil by Pleurotus cornucopiae and Bacillus thuringiensis FQ1 and the antioxidant responses in Pleurotus cornucopiae. Ecotoxicol. Environ. Saf. 2015, 120, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Zeng, G.M.; Niu, Q.Y.; Liu, Y.; Zhou, L.; Jiang, L.H.; Tan, X.F.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Hussain, J.; Akbar, A.; Mehmood, K.; Anwar, M.; Hasni, M.S.; Ullah, S.; Sajid, S.; Ali, I. Biosorption of heavy metals by obligate halophilic fungi. Chemosphere 2018, 199, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Piccini, M.; Raikova, S.; Allen, M.J.; Chuck, C.J. A synergistic use of microalgae and macroalgae for heavy metal bioremediation and bioenergy production through hydrothermal liquefaction. Sustain. Energy Fuels 2019, 3, 292–301. [Google Scholar] [CrossRef]
- Ezzouhri, L.; Castro, E.; Moya, M.; Espinola, F.; Lairini, K. Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier, Morocco. Afr. J. Microbiol. Res. 2009, 3, 35–48. [Google Scholar]
- Damodaran, D.; Balakrishnan, R.M.; Shetty, V.K. The uptake mechanism of Cd (II), Cr (VI), Cu (II), Pb (II), and Zn (II) by mycelia and fruiting bodies of Galerina vittiformis. BioMed Res. Int. 2013, 2013, 149120. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, S.; Mathur, N.; Bhatnagar, P. Mushroom as a product and their role in mycoremediation. AMB Express 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Larou, E.; Mountzouris, K.C.; Zervakis, G.I. Detoxification of olive mill wastewater and bioconversion of olive crop residues into high-value-added biomass by the choice edible mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, M.P. Bioremediation of vegetable and agrowastes by Pleurotus ostreatus: A novel strategy to produce edible mushroom with enhanced yield and nutrition. Cell. Mol. Biol. 2014, 60, 2–6. [Google Scholar]
- Yadav, P.; Mishra, V.; Kumar, T.; Rai, A.K.; Gaur, A.; Singh, M.P. An Approach to Evaluate Pb Tolerance and Its Removal Mechanisms by Pleurotus opuntiae. J. Fungi 2023, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Washington, DC, USA; Water Environmental Federation: Washington, DC, USA, 1998. [Google Scholar]
- Valix, M.; Tang, J.Y.; Cheung, W.H. The effects of mineralogy on the biological leaching of nickel laterite ores. Miner. Eng. 2001, 14, 1629–1635. [Google Scholar] [CrossRef]
- Valix, M.; Loon, L.O. Adaptive tolerance behaviour of fungi in heavy metals. Miner. Eng. 2003, 16, 193–198. [Google Scholar] [CrossRef]
- Chen, S.H.; Ng, S.L.; Cheow, Y.L.; Ting, A.S.Y. A novel study based on adaptive metal tolerance behavior in fungi and SEM-EDX analysis. J. Hazard. Mater. 2017, 334, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Gola, D.; Dey, P.; Bhattacharya, A.; Mishra, A.; Malik, A.; Namburath, M.; Ahammad, S.Z. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour. Technol. 2016, 218, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Ponzano, E.; Dondero, F.; Fabbri, R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to Mediterranean and Antarctic molluscs. Mar. Environ. Res. 1997, 44, 69–84. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.G.; Farr, A.L.; Randall, R.G. Protein measurements with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Samanta, L.; Chainy, G.B.N. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Ind. J. Biochem. Biophys. 2000, 37, 201–204. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods Enzymology; Colowick, S.P., Kaplane, N.O., Eds.; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–127. [Google Scholar]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lungs and liver. Biochem. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Environmental Protection Act, Standards for Effluent Discharge Regulations. General Notice No. 44. of 2003. 2002. Available online: http://faolex.fao.org/docs/texts/mat52519.doc (accessed on 13 March 2023).
- Central Pollution Control Board. Pollution Assessment: River Ganga. Status of Grossly Polluting Industries, GPI; Central Pollution Control Board: New Delhi, India, 2013. [Google Scholar]
- Bosnic, M.; Buljan, J.; Daniels, R.P. Pollutants in Tannery Effluents, Regional Program for Pollution Control in the Tanning Industry in South-East Asia S. RAS/92/120; United Nations Industrial Development Organization: Vienna, Austria, 2000; pp. 1–567. [Google Scholar]
- Khan, F.A.; Ansari, A.A. Eutrophication: An ecological vision. Bot. Rev. 2005, 71, 449–482. [Google Scholar] [CrossRef]
- Sugasini, A.; Rajagopal, K. Characterization of physicochemical parameters and heavy metal analysis of tannery effluent. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 349–359. [Google Scholar]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Yadav, A.; Singh, R.; Chandra, R.; Singh, D.P.; Raj, A.; Bharagava, R.N. Stress response of Triticum aestivum L. and Brassica juncea L. against heavy metals growing at distillery and tannery wastewater contaminated site. Chemosphere 2018, 206, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bharagava, R.N. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Health Part C 2016, 34, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Vaseem, H.; Singh, V.K.; Singh, M.P. Heavy metal pollution due to coal washery effluent and its decontamination using a macrofungus, Pleurotus ostreatus. Ecotoxicol. Environ. Saf. 2017, 145, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, X.; Shen, Y.; Chang, C.; Guo, E.; La, G.; Zhao, Y.; Li, X. Tolerance and removal mechanisms of heavy metals by fungus Pleurotus ostreatus Haas. Water Air Soil Pollut. 2017, 228, 1–9. [Google Scholar] [CrossRef]
- Turkekul, I.; Elmastas, M.; Tüzen, M. Determination of iron, copper, manganese, zinc, lead, and cadmium in mushroom samples from Tokat, Turkey. Food Chem. 2004, 84, 389–392. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Aprile, G.; Conte, B.; Cobianchi, R.C. Comparison of the heavy metal bioaccumulation capacity of an epiphytic moss and an epiphytic lichen. Environ. Pollut. 2008, 151, 401–407. [Google Scholar] [CrossRef]
- Sazanova, K.; Osmolovskaya, N.; Schiparev, S.; Yakkonen, K.; Kuchaeva, L.; Vlasov, D. Organic acids induce tolerance to zinc-and copper-exposed fungi under various growth conditions. Curr. Microbiol. 2015, 70, 520–527. [Google Scholar] [CrossRef]
- Morcillo, P.; Esteban, M.Á.; Cuesta, A. Heavy metals produce toxicity, oxidative stress and apoptosis in the marine teleost fish SAF-1 cell line. Chemosphere 2016, 144, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Zeng, G.M.; Zhong, H.; Yuan, X.Z.; Jiang, L.L.; Fu, H.Y.; Ma, X.L.; Zhang, J.C. Effect of saponins on cell surface properties of Penicillium simplicissimum: Performance on adsorption of cadmium (II). Colloids Surf. B Biointerfaces 2011, 86, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Cheow, Y.L.; Ng, S.L.; Ting, A.S.Y. Mechanisms for metal removal established via electron microscopy and spectroscopy: A case study on metal tolerant fungi Penicillium simplicissimum. J. Hazard. Mater. 2019, 362, 394–402. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Jiang, L.L.; Zeng, G.M.; Liu, Z.F.; Zhong, H.; Huang, H.J.; Zhou, M.F.; Cu, K.L. Effect of rhamnolipids on cadmium adsorption by Penicillium simplicissimum. J. Cent. South Univ. 2012, 19, 1073–1080. [Google Scholar] [CrossRef]
- Walker, G.M.; Weatherley, L.R. Adsorption of dyes from aqueous solution—The effect of adsorbent pore size distribution and dye aggregation. Chem. Eng. J. 2001, 83, 201–206. [Google Scholar] [CrossRef]
- Aytar, P.N.A.R.; Gedikli, S.E.R.A.P.; Buruk, Y.E.L.I.Z.; Cabuk, A.; Burnak, N. Lead and nickel biosorption with a fungal biomass isolated from metal mine drainage: Box–Behnken experimental design. Int. J. Environ. Sci. Technol. 2014, 11, 1631–1640. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Hexavalent chromium reduction ability and bioremediation potential of Aspergillus flavus CR500 isolated from electroplating wastewater. Chemosphere 2019, 237, 124567. [Google Scholar] [CrossRef]
- Dhal, B.; Pandey, B.D. Mechanism elucidation and adsorbent characterization for removal of Cr (VI) by native fungal adsorbent. Sustain. Environ. Res. 2018, 28, 289–297. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zhu, G.; Daugulis, A.J.; Chen, Q.; Ma, H.Y.; Zheng, P.; Liang, J.; Ma, X.K. Removal and biomineralization of Pb2+ in water by fungus Phanerochaete chrysoporium. J. Clean. Prod. 2020, 260, 120980. [Google Scholar] [CrossRef]
- Xu, X.; Hao, R.; Xu, H.; Lu, A. Removal mechanism of Pb (II) by Penicillium polonicum: Immobilization, adsorption, and bioaccumulation. Sci. Rep. 2020, 10, 9079. [Google Scholar] [CrossRef]
- Damodaran, D.; Shetty, K.V.; Mohan, B.R. Effect of chelaters on bioaccumulation of Cd (II), Cu (II), Cr (VI), Pb (II) and Zn (II) in Galerina vittiformis from soil. Int. Biodeterior. Biodegrad. 2013, 85, 182–188. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Ramesh, G.; Podila, G.K.; Gay, G.; Marmeisse, R.; Reddy, M.S. Different patterns of regulation for the copper and cadmium metallothioneins of the ectomycorrhizal fungus Hebeloma cylindrosporum. Appl. Environ. Microbiol. 2009, 75, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Kameo, S.; Iwahashi, H.; Kojima, Y.; Satoh, H. Induction of metallothioneins in the heavy metal resistant fungus Beauveria bassiana exposed to copper or cadmium. Analusis 2000, 28, 382–385. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Wang, Y.S.; Lou, Z.P.; Dong, J.D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, Y.; Ge, Q.; Li, Y.; Sun, J.; Zhang, Y.; Liu, X. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012, 196, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Saudagar, P.; Sundar, S.; Dubey, V.K. Miltefosine-unresponsive Leishmania donovani has a greater ability than miltefosine-responsive L. donovani to resist reactive oxygen species. FEBS J. 2013, 280, 4807–4815. [Google Scholar] [CrossRef]
- Scaeteanu, G.V.; Săndulescu, E.B.; Alistar, C.F.; Croitoru, C.M.; Madjar, R.M.; Alistar, A.; Gîlea, G.C.; Stavrescu, M. A short note on water quality and some biodiversity components in Gurban valley, Giurgiu country. AgroLife Sci. J. 2023, 12, 167–180. [Google Scholar]






| Physicochemical Parameters | Tannery Effluent | USEPA (2002) a | WHO (2008) b | CPCB (2013) c |
|---|---|---|---|---|
| pH | 6.5 ± 0.42 | 5.0–9.0 | 6.0–9.0 | 6.0–9.0 |
| Total solids (mg/L) | 2097 ± 43.86 | - | 1500 | - |
| Total suspended solids (mg/L) | 473.33 ± 23.02 | 35 | - | 100 |
| Total dissolved solids (mg/L) | 658.33 ± 6.80 | - | - | 2100 |
| Turbidity (NTU) | 27.43 ± 0.70 | - | - | - |
| Electrical conductivity (S/cm) | 1.23 ± 0.25 | - | - | - |
| Alkanity (mg/L) | 540.96 ± 30.0 | - | - | - |
| Total hardness (mg/L) | 1410 ± 15.52 | - | - | - |
| Phosphate (mg/L) | 28.17 ± 0.34 | 1.0 | - | 5 |
| Nitrate (mg/L) | 505.89 ± 10.90 | 10 | 50.0 | 10 |
| Ammonia (mg/L) | 126.5 ± 1.67 | 1.0 | 1.50 | - |
| Sulphate (mg/L) | 36.95 ± 0.17 | - | - | 1000.0 |
| Chloride (mg/L) | 947.2 ± 23.4 | - | - | 600 |
| BOD (mg/L) | 751.13 ± 21.61 | - | - | 30.0 |
| Mg (mg/L) | 149.25 ± 0.88 | - | - | - |
| Cr (mg/L) | 58.2 ± 0.07 | - | 0.05 | 2.0 |
| Pb (mg/L) | 7.06 ± 0.032 | 0.05 | 0.01 | 0.1 |
| Zn (mg/L) | 3.52 ± 0.005 | 2.0 | 0.05 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.; Mishra, V.; Kumar, T.; Singh, U.K.; Vamanu, E.; Singh, M.P. Cleansing Tannery Effluent with Pleurotus opuntiae: A Green Solution for Environmental Restoration and Toxicity Evaluation. Water 2024, 16, 1313. https://doi.org/10.3390/w16091313
Yadav P, Mishra V, Kumar T, Singh UK, Vamanu E, Singh MP. Cleansing Tannery Effluent with Pleurotus opuntiae: A Green Solution for Environmental Restoration and Toxicity Evaluation. Water. 2024; 16(9):1313. https://doi.org/10.3390/w16091313
Chicago/Turabian StyleYadav, Priyanka, Vartika Mishra, Tejmani Kumar, Umesh Kumar Singh, Emanuel Vamanu, and Mohan Prasad Singh. 2024. "Cleansing Tannery Effluent with Pleurotus opuntiae: A Green Solution for Environmental Restoration and Toxicity Evaluation" Water 16, no. 9: 1313. https://doi.org/10.3390/w16091313







