Continuous Heterogeneous Fenton for Swine Wastewater Treatment: Converting an Industry Waste into a Wastewater Treatment Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Wastewater Preparation and Characterization
2.2. Coagulation/Adsorption and Heterogeneous Continuous Fenton
2.3. Toxicity Assessment
2.4. Analytical Methods
3. Results
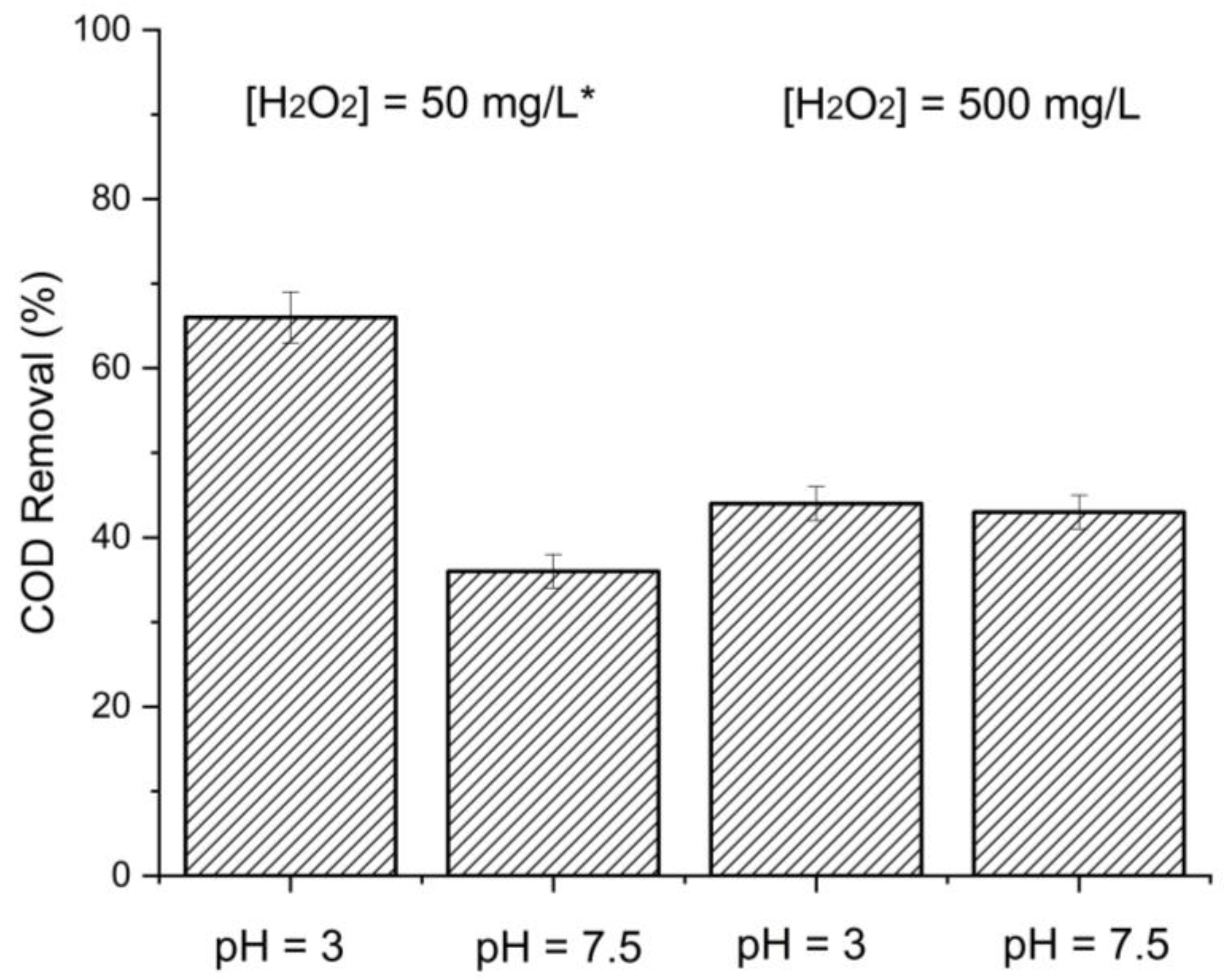
3.1. Batch Fenton Optimization
3.2. Continuous Fenton Reaction
3.2.1. Evaluation of Residence Time
3.2.2. Continuous Fenton Reaction Assessment
3.2.3. Toxicity Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, R.-F.; Wu, L.; Zhong, H.-T.; Liu, C.-X.; Qiao, W.; Wei, C.-H. Evaluation of Electrocoagulation Process for High-Strength Swine Wastewater Pretreatment. Sep. Purif. Technol. 2021, 272, 118900. [Google Scholar] [CrossRef]
- de Oliveira, J.F.; Fia, R.; Rodrigues, F.N.; Fia, F.R.L.; de Matos, M.P.; Siniscalchi, L.A.B.; Sanson, A.L. Quantification, Removal and Potential Ecological Risk of Emerging Contaminants in Different Organic Loads of Swine Wastewater Treated by Integrated Biological Reactors. Chemosphere 2020, 260, 127516. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2020. Overcoming Water Challenges in Agriculture; FAO: Rome, Italy, 2020. [Google Scholar]
- Garcia, B.B.; Lourinho, G.; Romano, P.; Brito, P.S.D. Photocatalytic Degradation of Swine Wastewater on Aqueous TiO2 Suspensions: Optimization and Modeling via Box-Behnken Design. Heliyon 2020, 6, e03293. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Kusmayadi, A.; Yen, H.-W.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Current Advances in Biological Swine Wastewater Treatment Using Microalgae-Based Processes. Bioresour. Technol. 2019, 289, 121718. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Narindri, B.; Chu, H.; Whang, L.-M. Recent Advancement on Biological Technologies and Strategies for Resource Recovery from Swine Wastewater. Bioresour. Technol. 2020, 303, 122861. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Silva-Núñez, A.; García-Perez, J.S.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Castillo-Zacarías, C.; Afewerki, S.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Phyco-Remediation of Swine Wastewater as a Sustainable Model Based on Circular Economy. J. Environ. Manag. 2021, 278, 111534. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Ryu, H.-D.; Chung, E.-G.; Oa, S.-W.; Kim, Y.-S. TOC Standards for Sustainably Managing Refractory Organic Matter in Swine Wastewater Effluent. Sustainability 2022, 14, 10092. [Google Scholar] [CrossRef]
- Emerick, T.; Vieira, J.L.; Silveira, M.H.L.; João, J.J. Ultrasound-Assisted Electrocoagulation Process Applied to the Treatment and Reuse of Swine Slaughterhouse Wastewater. J. Environ. Chem. Eng. 2020, 8, 104308. [Google Scholar] [CrossRef]
- Dong, L.; Qi, Z.; Li, M.; Zhang, Y.; Chen, Y.; Qi, Y.; Wu, H. Organics and Nutrient Removal from Swine Wastewater by Constructed Wetlands Using Ceramsite and Magnetite as Substrates. J. Environ. Chem. Eng. 2021, 9, 104739. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- Chan, R.; Chiemchaisri, C.; Chiemchaisri, W.; Boonsoongnern, A.; Tulayakul, P. Occurrence of antibiotics in typical pig farming and its wastewater treatment in Thailand. Emerg. Contam. 2022, 8, 21–29. [Google Scholar] [CrossRef]
- Hashim, K.; Saad, W.I.; Saffa, K.; Al-Janabi, A. Effects of organic matter on the performance of water and wastewater treatment: Electrocoagulation a case study. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1184, 012018. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Li, J.; Liu, Y.; Zhang, X.; Jia, H. Insight into chemical phosphate recovery from municipal wastewater. Sci. Total Environ. 2017, 576, 159–171. [Google Scholar] [CrossRef]
- Yang, S.; Peng, S.; Xu, J.; He, Y.; Wang, Y. Effects of water saving irrigation and controlled release nitrogen fertilizer managements on nitrogen losses from paddy fields. Paddy Water Environ. 2015, 13, 71–80. [Google Scholar] [CrossRef]
- Prokhorova, A.; Kainuma, M.; Hiyane, R.; Boerner, S.; Goryanin, I. Concurrent treatment of raw and aerated swine wastewater using an electrotrophic denitrification system. Bioresour. Technol. 2021, 322, 124508. [Google Scholar] [CrossRef] [PubMed]
- Radu, G.; Racoviteanu, G. Removing ammonium from water intended for human consumption. A review of existing technologies. IOP Conf. Ser. Earth Environ. Sci. 2021, 664, 012029. [Google Scholar] [CrossRef]
- Soler, P.; Faria, M.; Barata, C.; García-Galea, E.; Lorente, B.; Vinyoles, D. Improving water quality does not guarantee fish health: Effects of ammonia pollution on the behaviour of wild-caught pre-exposed fish. PLoS ONE 2021, 16, e0243404. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.; Zheng, S.; Kai, Z.; Jin, T.; Shi, R.; Huang, H.; Zheng, X. Effects of Wastewater Treatment and Manure Application on the Dissemination of Antimicrobial Resistance around Swine Feedlots. J. Clean. Prod. 2021, 280, 123794. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Chen, X.; Han, Y.; Cao, G. Transformation Kinetics and Pathways of Sulfamonomethoxine by UV/H2O2 in Swine Wastewater. Chemosphere 2021, 265, 129125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Liang, C.; Wei, D.; Zhang, S.; Ren, Q.; Shi, J.; Liu, L. Removal of Antibiotic Resistance Genes from Swine Wastewater by Membrane Filtration Treatment. Ecotoxicol. Environ. Saf. 2021, 210, 111885. [Google Scholar] [CrossRef]
- Sommer, M.O.; Dantas, G.; Church, G.M. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 2009, 325, 1128–1131. [Google Scholar] [CrossRef]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best Available Technologies and Treatment Trains to Address Current Challenges in Urban Wastewater Reuse for Irrigation of Crops in EU Countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef] [PubMed]
- WWF—World Wildlife. Water Scarcity. Available online: https://www.worldwildlife.org/threats/water-scarcity (accessed on 15 January 2024).
- Martins, R.C.; Quinta-Ferreira, R.M. Phenolic wastewaters depuration and biodegradability enhancement by ozone over active catalysts. Desalination 2011, 270, 90–97. [Google Scholar] [CrossRef]
- Domingues, E.; Gomes, J.; Quina, M.; Quinta-Ferreira, R.; Martins, R. Detoxification of Olive Mill Wastewaters by Fenton’s Process. Catalysts 2018, 8, 662. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Couras, C.; Karim, A.V.; Nadais, H. A Review of Integrated Advanced Oxidation Processes and Biological Processes for Organic Pollutant Removal. Chem. Eng. Commun. 2021, 209, 390–432. [Google Scholar] [CrossRef]
- M’Arimi, M.M.; Mecha, C.A.; Kiprop, A.K.; Ramkat, R. Recent Trends in Applications of Advanced Oxidation Processes (AOPs) in Bioenergy Production: Review. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater Treatment by Means of Advanced Oxidation Processes at Basic pH Conditions: A Review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, L.; Spasiano, D.; Oller, I.; Fernández-Calderero, I.; Agüera, A.; Malato, S. Solar Photo-Fenton Optimization for the Treatment of MWTP Effluents Containing Emerging Contaminants. Catal. Today 2013, 209, 188–194. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Saritha, P.; Aparna, C.; Himabindu, V.; Anjaneyulu, Y. Comparison of Various Advanced Oxidation Processes for the Degradation of 4-Chloro-2 Nitrophenol. J. Hazard. Mater. 2007, 149, 609–614. [Google Scholar] [CrossRef]
- Domingues, E.; Lincho, J.; Fernandes, M.J.; Gomes, J.; Martins, R.C. Low-Cost Materials for Swine Wastewater Treatment Using Adsorption and Fenton’s Process. Environ. Sci. Pollut. Res. 2023; ahead of print. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced Oxidation Process-Mediated Removal of Pharmaceuticals from Water: A Review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Litter, M.; Quici, N. Photochemical Advanced Oxidation Processes for Water and Wastewater Treatment. Recent Pat. Eng. 2010, 4, 217–241. [Google Scholar] [CrossRef]
- Chang, M.-C.; Shu, H.-Y.; Yu, H.-H. Olive mill wastewater degradation by Fenton oxidation with zero-valent iron and hydrogen peroxide. J. Hazard. Mater. 2006, B138, 574–581. [Google Scholar] [CrossRef]
- Kallel, M.; Belaid, C.; Boussahel, R.; Ksibi, M.; Montiel, A.; Elleuch, B. An integrated technique using zero-valent iron and UV/H2O2 sequential process for complete decolorization and mineralization of C.I. Acid Black 24 wastewater. J. Hazard. Mater. 2009, 163, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Bremner, D.H.; Burgess, A.E.; Houllemare, D.; Namkung, K.-C. Phenol degradation using hydroxyl radicals generated from zero-valent iron and hydrogen peroxide. Appl. Catal. B. 2006, 63, 15–19. [Google Scholar] [CrossRef]
- Gamarra-Güere, C.D.; Dionisio, D.; Santos, G.O.S.; Vasconcelos Lanza, M.R.; de Jesus Motheo, A. Application of Fenton, Photo-Fenton and Electro-Fenton Processes for the Methylparaben Degradation: A Comparative Study. J. Environ. Chem. Eng. 2022, 10, 106992. [Google Scholar] [CrossRef]
- Riga, A.; Soutsas, K.; Ntampegliotis, K.; Karyannis, V.; Papapolymerou, G. Effect of system parameters and of inorganic salts on the decolorization and degradation of Procion H-exl dyes. Comparison of H2O2/UV, Fenton, UV/Fenton, TiO2/UV and TiO2/UV/H2O2 processes. Desalination 2007, 211, 72–86. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Esplugas, S.; Giménez, J. Degradation of the emerging contaminant ibuprofen in water by photo-Fenton. Water Res. 2010, 44, 589–595. [Google Scholar] [CrossRef]
- Lopez, N.; Plaza, S.; Afkhami, A.; Marco, P.; Giménez, J.; Esplugas, S. Treatment of Diphenhydramine with different AOPs including photo-Fenton at circumneutral pH. J. Chem. Eng. 2017, 318, 112–120. [Google Scholar] [CrossRef]
- Funai, D.H.; Didier, F.; Giménez, J.; Esplugas, S.; Marco, P.; Machulek, A. Photo-Fenton Treatment of Valproate under UVC, UVA and Simulated Solar Radiation. J. Hazard. Mater. 2017, 323, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Carra, I.; Santos-Juanes, L.; Fernández, G.A.; Malato, S.; Pérez, J.A.S. New approach to solar photo-Fenton operation. Raceway ponds as tertiary treatment technology. J. Hazard. Mater. 2014, 279, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Malato, S.; Pulgarin, C.; Contreras, S.; Curcó, D.; Giménez, J.; Esplugas, S. Optimizing the solar photo-Fenton process in the treatmen of contaminated water. Determination of intrinsic kinetic constants for scale-up. Sol. Energy 2005, 79, 360–368. [Google Scholar] [CrossRef]
- Sánchez-Pérez, J.A.; Soriano-Molina, P.; Rivas, G.; García Sánchez, J.L.; Casas López, J.L.; Fernández Sevilla, J.M. Effect of temperature and photon absorption on the kinetics of micropollutant removal by solar photo-Fenton in raceway pond reactors. J. Chem. Eng. 2017, 310, 464–472. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Cao, H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere 2015, 121, 1–17. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Mecha, A.C.; Chollom, N.N. Photocatalytic ozonation of wastewater: A review. Environ. Chem. Lett. 2020, 18, 1491–1507. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Lee, H.; Shoda, M. Removal of COD and Color from Livestock Wastewater by the Fenton Method. J. Hazard. Mater. 2008, 153, 1314–1319. [Google Scholar] [CrossRef]
- Riaño, B.; Coca, M.; García-González, M.C. Evaluation of Fenton Method and Ozone-Based Processes for Colour and Organic Matter Removal from Biologically Pre-Treated Swine Manure. Chemosphere 2014, 117, 193–199. [Google Scholar] [CrossRef]
- Gomes, J.; Domingues, E.; Fernandes, E.; Castro, L.; Martins, R.C.; Quinta-Ferreira, R.M. Coagulation and Biofiltration by Corbicula Fluminea for COD and Toxicity Reduction of Swine Wastewater. J. Water Process Eng. 2021, 42, 102145. [Google Scholar] [CrossRef]
- Domingues, E.; Assunção, N.; Gomes, J.; Lopes, D.V.; Frade, J.R.; Quina, M.J.; Quinta-Ferreira, R.M.; Martins, R.C. Catalytic Efficiency of Red Mud for the Degradation of Olive Mill Wastewater through Heterogeneous Fenton’s Process. Water 2019, 11, 1183. [Google Scholar] [CrossRef]
- Martins, R.C.; Henriques, L.R.; Quinta-Ferreira, R.M. Catalytic Activity of Low Cost Materials for Pollutants Abatement by Fenton’s Process. Chem. Eng. Sci. 2013, 100, 225–233. [Google Scholar] [CrossRef]
- Trautmann, N.M.; Krasny, M.E. Composting in the Classroom: Scientific Inquiry for High School Students; Nature Science Foundation, Cornell Waste Management Institute and Cornell Center for the Environment: New York, NY, USA, 1997. [Google Scholar]
- Greenberg, A.; Clesceri, I.; Eaton, A. Standard Methods for the Examination of Water and Wastewater, 16th ed.; American Public Health Association (APHA): Washington, DC, USA, 1985. [Google Scholar]
- DIN EN 13342:2001-01; Characterization of Sludges—Determination of Kjeldahl Nitrogen. Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 2001.
- U.S. Environmental Protection Agency. Method 365.3: Phosphorous, All Forms (Colorimetric, Ascorbic Acid, Two Reagent); U.S. Environmental Protection Agency: Washington, DC, USA, 1978. [Google Scholar]
- Domingues, E.; Fernandes, E.; Gomes, J.; Martins, R.C. Swine Wastewater Treatment by Fenton’s Process and Integrated Methodologies Involving Coagulation and Biofiltration. J. Clean. Prod. 2021, 293, 126105. [Google Scholar] [CrossRef]
- Domınguez, J.R.; Palo, P.; Gonzalez, T.; Peres, J.A.; Cuerda-Correa, E.M. Fenton Advanced Oxidation of Emerging Pollutants: Parabens. Int. J. Energy. Environ. Eng. 2014, 5, 89. [Google Scholar] [CrossRef]
- Nieto, L.M.; Hodaifa, G.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Degradation of Organic Matter in Olive-Oil Mill Wastewater through Homogeneous Fenton-like Reaction. Chem. Eng. J. 2011, 173, 503–510. [Google Scholar] [CrossRef]
- Rezaei, F.; Vione, D. Effect of pH on zero valent iron performance in heterogeneous Fenton and Fenton-like processes: A review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef] [PubMed]
- Le, S.-T.; Israpanich, A.; Phenrat, T. Using Sequential H2O2 Addition to Sustain 1,2-Dichloroethane Detoxification by a Nanoscale Zerovalent Iron-Induced Fenton’s System at a Natural pH. Chemosphere 2022, 305, 135376. [Google Scholar] [CrossRef]
- Decree of Law No. 236/98 (Portugal). Republic Diary No. 176, Series 1, 1998-08-01. pp. 3676–3722. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/236-430457 (accessed on 15 January 2024).
- Decree of Law No. 119/2019 (Portugal). Republic Diary No. 159, Series 1, 2019-08-21. pp. 21–44. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/119-2019-124097549 (accessed on 15 January 2024).
- Gomes, J.; Pereira, J.L.; Rosa, I.C.; Saraiva, P.M.; Gonçalves, F.; Costa, R. Evaluation of Candidate Biocides to Control the Biofouling Asian Clam in the Drinking Water Treatment Industry: An Environmentally Friendly Approach. J. Gt. Lakes Res. 2014, 40, 421–428. [Google Scholar] [CrossRef]


| Parameter | Initial Value | Fenton after PDADMAC Coagulation | Fenton after RM Adsorption |
|---|---|---|---|
| COD (mgO2/L) | 1700 | 49–264 (850) 1 | 10–171 (240) 1 |
| BOD5 (mgO2/L) | 219 | 4.4 | 30.9 |
| TKN (mg/L) | 1706 | 87.6 | 175.1 |
| Phosphorous (mg/L) | 245 | 0.0 | 0.3 |
| Total Iron (mg/L) | 12 | 58.5 | 55.3 |
| L. sativum GI at 48 h | 54.9/Strong inhibition | 92/No inhibition (47.5/Strong inhibition) 2 | 88.7/No inhibition (95/No inhibition) 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lincho, J.; Gomes, J.; Martins, R.C.; Domingues, E. Continuous Heterogeneous Fenton for Swine Wastewater Treatment: Converting an Industry Waste into a Wastewater Treatment Material. Water 2024, 16, 781. https://doi.org/10.3390/w16050781
Lincho J, Gomes J, Martins RC, Domingues E. Continuous Heterogeneous Fenton for Swine Wastewater Treatment: Converting an Industry Waste into a Wastewater Treatment Material. Water. 2024; 16(5):781. https://doi.org/10.3390/w16050781
Chicago/Turabian StyleLincho, João, João Gomes, Rui C. Martins, and Eva Domingues. 2024. "Continuous Heterogeneous Fenton for Swine Wastewater Treatment: Converting an Industry Waste into a Wastewater Treatment Material" Water 16, no. 5: 781. https://doi.org/10.3390/w16050781





