Removal of Cr and Organic Matter from Real Tannery Wastewater via Fenton-like Process Using Commercial Nano-Scale Zero-Valent Iron
Abstract
:1. Introduction
2. Materials and Methods
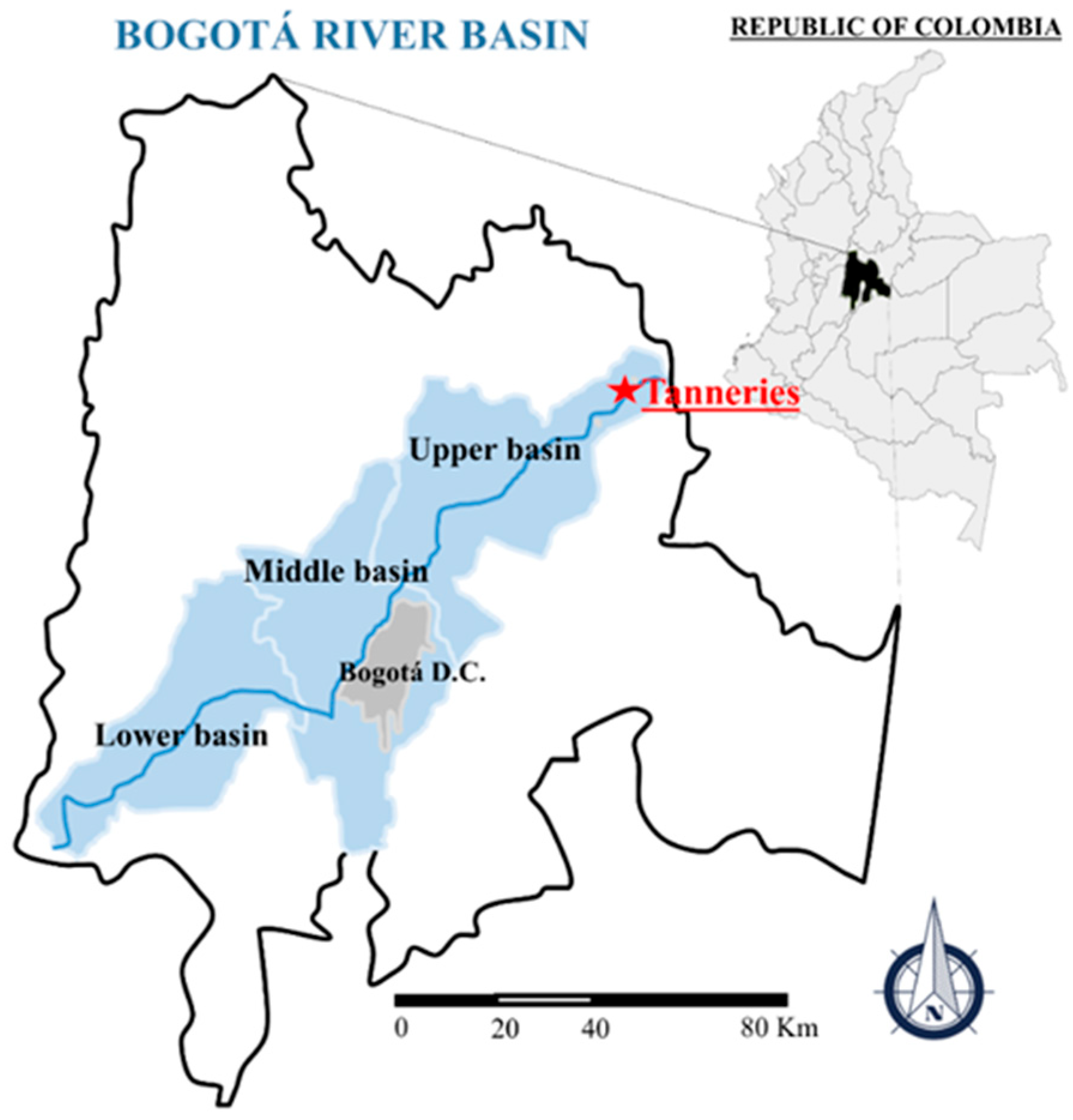
2.1. Site Description and TWT Characterization
2.2. Design of Experiments and Relevant Statistical Analysis
2.3. Experimentation
2.4. Validation the DOE Results
2.5. Identifying the Effect of the Leather-Related Co-Existing Substances
2.6. Recycled of Comercial nZVI
3. Results and Discussion
3.1. Analysis of Plackett–Burman Results to Identify Factors
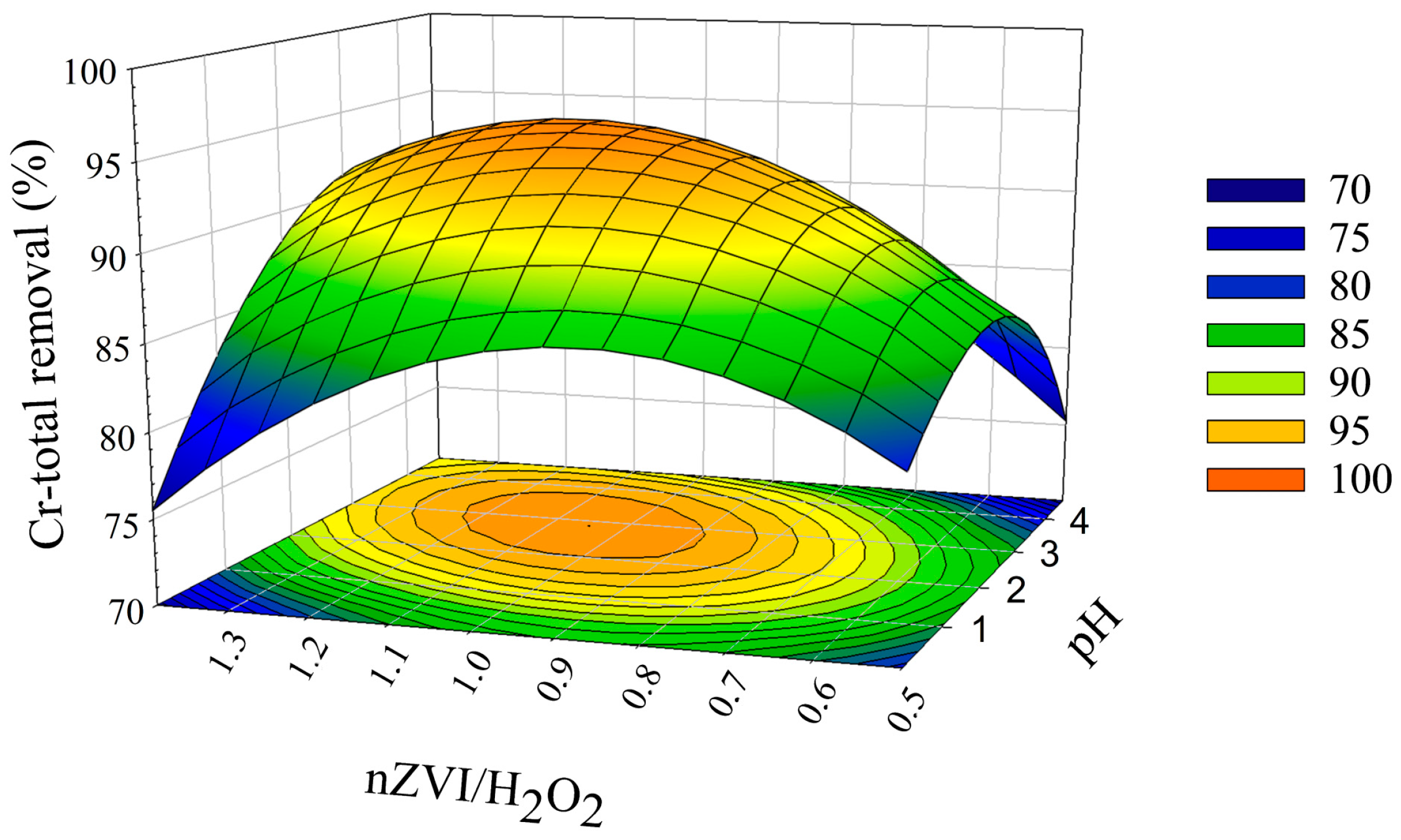
3.2. Optimization of the Process to Maximize Cr-Total Removal
3.3. Validation of the DOE Results
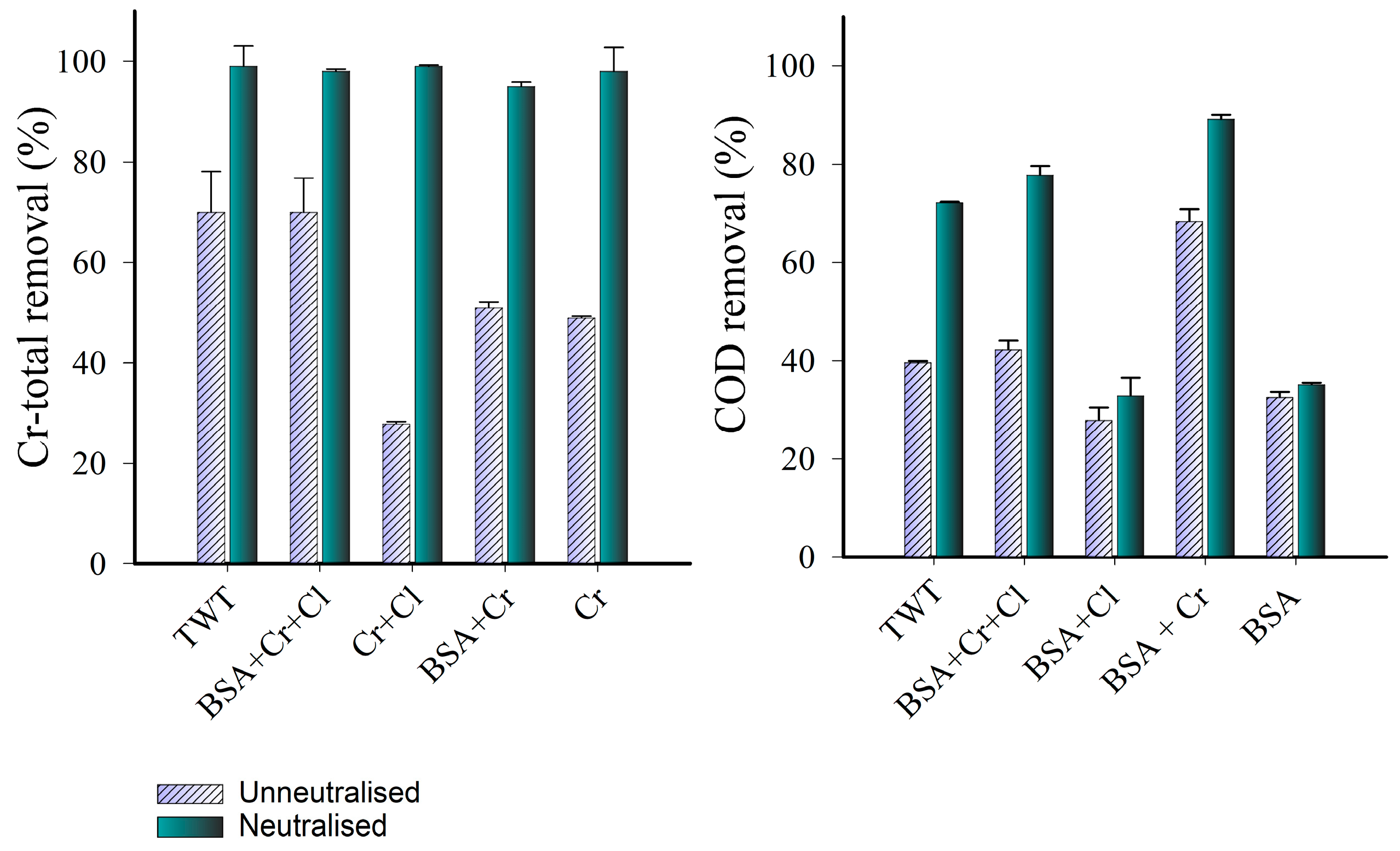
3.4. Identifying the Effect of the Leather-Related Co-Existing Substances
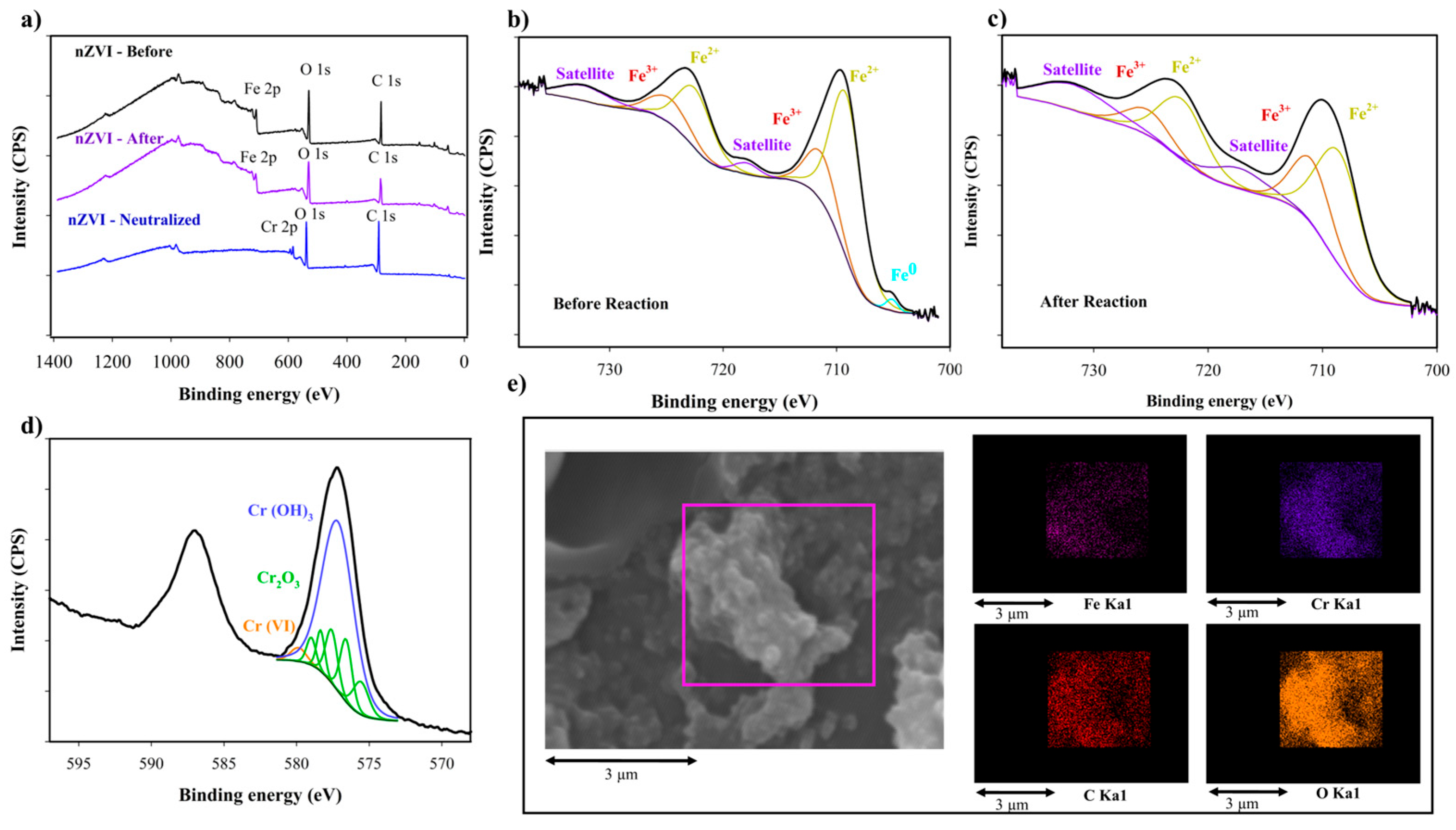
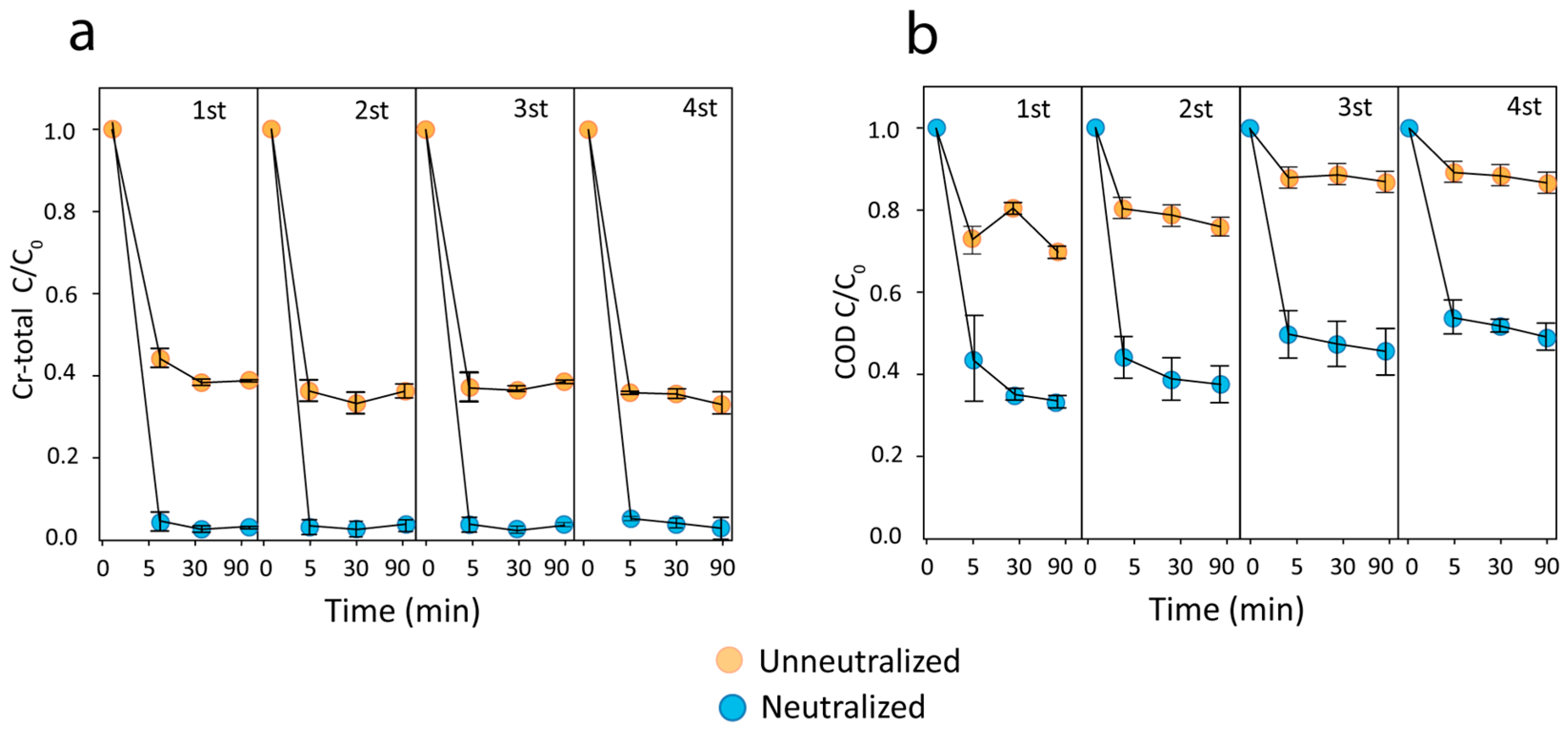
3.5. Recycled nZVI for Cr-Total and COD Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Wu, Q.; Tang, Y.; Zhou, J.; Guo, H. Tannery Wastewater Treatment: Conventional and Promising Processes, an Updated 20-Year Review. J. Leather Sci. Eng. 2022, 4, 4–10. [Google Scholar] [CrossRef]
- Urbina-suarez, N.A.; Machuca-martínez, F.; Barajas-solano, A.F. Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater. Molecules 2021, 26, 3222. [Google Scholar] [CrossRef]
- Reyes-Serrano, A.; López-Alejo, J.E.; Hernández-Cortázar, M.A.; Elizalde, I. Removing Contaminants from Tannery Wastewater by Chemical Precipitation Using CaO and Ca(OH)2. Chin. J. Chem. Eng. 2020, 28, 1107–1111. [Google Scholar] [CrossRef]
- Vilardi, G.; Ochando-Pulido, J.M.; Stoller, M.; Verdone, N.; Di Palma, L. Fenton Oxidation and Chromium Recovery from Tannery Wastewater by Means of Iron-Based Coated Biomass as Heterogeneous Catalyst in Fixed-Bed Columns. Chem. Eng. J. 2018, 351, 1–11. [Google Scholar] [CrossRef]
- DesMarias, T.L.; Costa, M. Mechanisms of Chromium-Induced Toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Montalvão, M.F.; de Souza, J.M.; Guimarães, A.T.B.; de Menezes, I.P.P.; da Silva Castro, A.L.; de Lima Rodrigues, A.S.; Malafaia, G. The Genotoxicity and Cytotoxicity of Tannery Effluent in Bullfrog (Lithobates catesbeianus). Chemosphere 2017, 183, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Gao, M.; Sun, B.; Wang, M.; Wang, S.; Wang, X. Simultaneous Removal of Cr and Organic Matters via Coupling Cr-Fenton-like Reaction with Cr Flocculation: The Key Role of Cr Flocs on Coupling Effect. Chemosphere 2022, 287, 131991. [Google Scholar] [CrossRef]
- Yahia, A.; Fersi, C.; Djebali, K.; Salah, I.B.; Touati, F. Modeling and Optimizing of Coagulation–Flocculation Process by Response Surface Methodology for Rehabilitation of Tannery Wastewater Treatment Plant. Desalination Water Treat. 2021, 225, 175–189. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Peleka, E.N.; Ntolia, A. Treatment of Tannery Wastewater with Vibratory Shear-Enhanced Processing Membrane Filtration. Separations 2019, 6, 20. [Google Scholar] [CrossRef]
- Yahya, M.D.; Obayomi, K.S.; Abdulkadir, M.B.; Iyaka, Y.A.; Olugbenga, A.G. Characterization of Cobalt Ferrite-Supported Activated Carbon for Removal of Chromium and Lead Ions from Tannery Wastewater via Adsorption Equilibrium. Water Sci. Eng. 2020, 13, 202–213. [Google Scholar] [CrossRef]
- Korpe, S.; Rao, P.V. Application of Advanced Oxidation Processes and Cavitation Techniques for Treatment of Tannery Wastewater—A Review. J. Environ. Chem. Eng. 2021, 9, 105234. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A Review on Fenton-like Processes for Organic Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Sivagami, K.; Sakthivel, K.P.; Nambi, I.M. Advanced Oxidation Processes for the Treatment of Tannery Wastewater. J. Environ. Chem. Eng. 2018, 6, 3656–3663. [Google Scholar] [CrossRef]
- Wang, D.; He, S.; Shan, C.; Ye, Y.; Ma, H.; Zhang, X.; Zhang, W.; Pan, B. Chromium Speciation in Tannery Effluent after Alkaline Precipitation: Isolation and Characterization. J. Hazard. Mater. 2016, 316, 169–177. [Google Scholar] [CrossRef] [PubMed]
- He, D.Q.; Wang, L.F.; Jiang, H.; Yu, H.Q. A Fenton-like Process for the Enhanced Activated Sludge Dewatering. Chem. Eng. J. 2015, 272, 128–134. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.G.; Theng, B.K.G.; Mora, M.L. Clays and Oxide Minerals as Catalysts and Nanocatalysts in Fenton-like Reactions—A Review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mohamed, N.Y.; Mostafa, M.K.; Mahmoud, M.S. Effective Chromium Adsorption from Aqueous Solutions and Tannery Wastewater Using Bimetallic Fe/Cu Nanoparticles: Response Surface Methodology and Artificial Neural Network. Air Soil Water Res. 2021, 14, 11786221211028162. [Google Scholar] [CrossRef]
- Vilardi, G.; Sebastiani, D.; Miliziano, S.; Verdone, N.; Di Palma, L. Heterogeneous NZVI-Induced Fenton Oxidation Process to Enhance Biodegradability of Excavation by-Products. Chem. Eng. J. 2018, 335, 309–320. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Song, Y.; Zhang, X.; Liu, S.; Du, Q. Effect of Spatial Distribution of NZVI on the Corrosion of NZVI Composites and Its Subsequent Cr(VI) Removal FromWater. Nanomaterials 2022, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Plaza, J.; Arencibia, A.; López-Muñoz, M.J. Evaluation of NZVI for the Degradation of Atrazine in Heterogeneous Fenton-like Systems at Circumneutral PH. J. Environ. Chem. Eng. 2021, 9, 106641. [Google Scholar] [CrossRef]
- Vilardi, G.; Rodríguez-Rodríguez, J.; Ochando-Pulido, J.M.; Verdone, N.; Martinez-Ferez, A.; Di Palma, L. Large Laboratory-Plant Application for the Treatment of a Tannery Wastewater by Fenton Oxidation: Fe(II) and NZVI Catalysts Comparison and Kinetic Modelling. Process. Saf. Environ. Prot. 2018, 117, 629–638. [Google Scholar] [CrossRef]
- Yu, R.F.; Chen, H.W.; Cheng, W.P.; Lin, Y.J.; Huang, C.L. Monitoring of ORP, PH and DO in Heterogeneous Fenton Oxidation Using NZVI as a Catalyst for the Treatment of Azo-Dye Textile Wastewater. J. Taiwan Inst. Chem. Eng. 2014, 45, 947–954. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, X.; Dai, M.; Lopez-Valdivieso, A.; Raza, S.; Ali, I.; Peng, C.; Li, J.; Naz, I. The Sequestration of Aqueous Cr(VI) by Zero Valent Iron-Based Materials: From Synthesis to Practical Application. J. Clean. Prod. 2021, 312, 127678. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The Use of Zero-Valent Iron for Groundwater Remediation and Wastewater Treatment: A Review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Vilardi, G.; Di Palma, L.; Verdone, N. On the Critical Use of Zero Valent Iron Nanoparticles and Fenton Processes for the Treatment of Tannery Wastewater. J. Water Process Eng. 2018, 22, 109–122. [Google Scholar] [CrossRef]
- Ken, D.S.; Sinha, A. Recent Developments in Surface Modification of Nano Zero-Valent Iron (NZVI): Remediation, Toxicity and Environmental Impacts. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100344. [Google Scholar] [CrossRef]
- Oprčkal, P.; Mladenovič, A.; Vidmar, J.; Mauko Pranjić, A.; Milačič, R.; Ščančar, J. Critical Evaluation of the Use of Different Nanoscale Zero-Valent Iron Particles for the Treatment of Effluent Water from a Small Biological Wastewater Treatment Plant. Chem. Eng. J. 2017, 321, 20–30. [Google Scholar] [CrossRef]
- Kašlík, J.; Kolařík, J.; Filip, J.; Medřík, I.; Tomanec, O.; Petr, M.; Malina, O.; Zbořil, R.; Tratnyek, P.G. Nanoarchitecture of Advanced Core-Shell Zero-Valent Iron Particles with Controlled Reactivity for Contaminant Removal. Chem. Eng. J. 2018, 354, 335–345. [Google Scholar] [CrossRef]
- Pavelková, A.; Stejskal, V.; Pluhař, T.; Nosek, J. Advanced Remediation Using Nanosized Zero-Valent Iron and Electrical Current in Situ—A Comparison with Conventional Remediation Using Nanosized Zero-Valent Iron Alone. J. Environ. Chem. Eng. 2021, 9, 106124. [Google Scholar] [CrossRef]
- Zafar, A.M.; Javed, M.A.; Hassan, A.A.; Mohamed, M.M. Groundwater Remediation Using Zero-Valent Iron Nanoparticles (NZVI). Groundw. Sustain. Dev. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like Processes with in-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Degradation of Emerging Contaminants: Advances and Prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Akhtar, A.; Aslam, Z.; Asghar, A.; Bello, M.M.; Raman, A.A.A. Electrocoagulation of Congo Red Dye-Containing Wastewater: Optimization of Operational Parameters and Process Mechanism. J. Environ. Chem. Eng. 2020, 8, 104055. [Google Scholar] [CrossRef]
- Khalid, R.; Aslam, Z.; Abbas, A.; Ahmad, W.; Ramzan, N.; Shawabkeh, R. Adsorptive Potential of Acacia Nilotica Based Adsorbent for Chromium(VI) from an Aqueous Phase. Chin. J. Chem. Eng. 2018, 26, 614–622. [Google Scholar] [CrossRef]
- Djimtoingar, S.S.; Derkyi, N.S.A.; Kuranchie, F.A.; Yankyera, J.K. A Review of Response Surface Methodology for Biogas Process Optimization. Cogent. Eng. 2022, 9, 2115283. [Google Scholar] [CrossRef]
- Saeed, M.O.; Azizli, K.; Isa, M.H.; Bashir, M.J.K. Application of CCD in RSM to Obtain Optimize Treatment of POME Using Fenton Oxidation Process. J. Water Process Eng. 2015, 8, 7–16. [Google Scholar] [CrossRef]
- Martinez Buitrago, S.Y.; Romero Coca, J.A. Current State Review of the Industry of Tanneries in Its Processes and Products: A Competitiveness Analysis. Rev. Fac. Cienc. Económ. 2017, 26, 113–124. [Google Scholar]
- Ministerio de Ambiente y Desarrollo Sostenible “Resolución 0631 de 2015”. 2015, 73. Available online: https://www.minambiente.gov.co/documento-normativa/resolucion-631-de-2015/ (accessed on 20 January 2024).
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for Examination of Water and Wastewater 22nd Ed; American Public Health Association: Washington, DC, USA, 2012; Volume 5, ISBN 978-087553-013-0. [Google Scholar]
- USEPA. Flame Atomic Absorption Spectrophotometry. In Method 7000B; USEPA: Washington, DC, USA, 2007; Volume 30, pp. 1–23. [Google Scholar]
- Hodaifa, G.; Ochando-Pulido, J.M.; Rodriguez-Vives, S.; Martinez-Ferez, A. Optimization of Continuous Reactor at Pilot Scale for Olive-Oil Mill Wastewater Treatment by Fenton-like Process. Chem. Eng. J. 2013, 220, 117–124. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic Activity of Metals in Heterogeneous Fenton-like Oxidation of Wastewater Contaminants: A Review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Hidaka, T.; Nishimura, F. Linear Relationship between Temperature and the Apparent Reaction Rate Constant of Hydroxyl Radical with 4-Chlorobenzoic Acid. Ozone Sci. Eng. 2022, 44, 274–280. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S.; Inglese, M.; Nikolau, A.; Belgiorno, V. Fenton Oxidation Treatment of Tannery Wastewater and Tanning Agents: Synthetic Tannin and Nonylphenol Ethoxylate Based Degreasing Agent. Desalination Water Treat. 2010, 23, 173–180. [Google Scholar] [CrossRef]
- Sharma, M.; Mohapatra, T.; Ghosh, P. Hydrodynamics, Mass and Heat Transfer Study for Emerging Heterogeneous Fenton Process in Multiphase Fluidized-Bed Reactor System for Wastewater Treatment—A Review. Chem. Eng. Res. Des. 2021, 171, 48–62. [Google Scholar] [CrossRef]
- Mahtab, M.S.; Farooqi, I.H.; Khursheed, A. Sustainable Approaches to the Fenton Process for Wastewater Treatment: A Review. Mater Today Proc. 2021, 47, 1480–1484. [Google Scholar] [CrossRef]
- Ling, H.; Zhu, X.; Zhou, T.; Su, F.; Du, J.; Bao, J. Hydrogen Peroxide Activation with Sulfidated Zero-Valent Iron for Synchronous Removal of Cr(VI) and BPA. Catalysts 2022, 12, 252. [Google Scholar] [CrossRef]
- Rusevova, K.; Kopinke, F.D.; Georgi, A. Nano-Sized Magnetic Iron Oxides as Catalysts for Heterogeneous Fenton-like Reactions-Influence of Fe(II)/Fe(III) Ratio on Catalytic Performance. J. Hazard. Mater. 2012, 241–242, 433–440. [Google Scholar] [CrossRef]
- Yan, Y.; Mao, Y.; Dong, Y.; Zhang, K.; Sun, X.; Ma, C. Exothermic Laws Applicable to the Degradation of: O-Phenylenediamine in Wastewater via a Fe3+/H2O2 Homogeneous Quasi-Fenton System. RSC Adv. 2019, 9, 26283–26290. [Google Scholar] [CrossRef]
- Brumovský, M.; Oborná, J.; Lacina, P.; Hegedüs, M.; Sracek, O.; Kolařík, J.; Petr, M.; Kašlík, J.; Hofmann, T.; Filip, J. Sulfidated Nano-Scale Zerovalent Iron Is Able to Effectively Reduce in Situ Hexavalent Chromium in a Contaminated Aquifer. J. Hazard. Mater. 2021, 405, 124665. [Google Scholar] [CrossRef]
- Singh, R.; Misra, V.; Singh, R.P. Synthesis, Characterization and Role of Zero-Valent Iron Nanoparticle in Removal of Hexavalent Chromium from Chromium-Spiked Soil. J. Nanoparticle Res. 2011, 13, 4063–4073. [Google Scholar] [CrossRef]
- Bui, X.-T.; Chiemchaisri, C.; Fujioka, T.; Varjani, S. (Eds.) Water and Wastewater Treatment Technologies; Springer: Singapore, 2019; ISBN 978-981-13-3259-3. [Google Scholar]
- Qiu, Y.; Zhang, Q.; Gao, B.; Li, M.; Fan, Z.; Sang, W.; Hao, H.; Wei, X. Removal Mechanisms of Cr(VI) and Cr(III) by Biochar Supported Nanosized Zero-Valent Iron: Synergy of Adsorption, Reduction and Transformation. Environ. Pollut. 2020, 265, 115018. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Ma, J.; Xie, L.; Tang, B.; Han, W.; Lin, S. Chromium Removal Using Resin Supported Nanoscale Zero-Valent Iron. J. Environ. Manag. 2013, 128, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Zhang, G.; Gao, X. Research on the Performance of Modified Blue Coke in Adsorbing Hexavalent Chromium. Sci. Rep. 2023, 13, 7223. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, W.; Wang, J.; Xia, P.; Duan, X.; He, Q.; Sirés, I.; Ye, Z. Accelerating Fe(III)/Fe(II) Redox Cycling in Heterogeneous Electro-Fenton Process via S/Cu-Mediated Electron Donor-Shuttle Regime. Appl. Catal. B 2024, 342, 7223. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-Ray Photoelectron Spectroscopy Studies of Chromium Compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]
- Pettine, M.; Gennari, F.; Campanella, L.; Millero, F.J. The Effect of Organic Compounds in the Oxidation Kinetics of Cr(III) by H2O2. Geochim. Cosmochim. Acta 2008, 72, 5692–5707. [Google Scholar] [CrossRef]

| Factors | Unit | High Level (+1) | Low Level (−1) |
|---|---|---|---|
| H2O2/COD | w/w | 0.75 | 0.5 |
| nZVI/H2O2 | w/w | 1.0 | 0.75 |
| pH | 5.0 | 3.0 | |
| Reaction time | h | 1 | 0.5 |
| Agitation | rpm | 200 | 100 |
| Temperature | °C | 30 | 17 |
| Sedimentation time | h | 4 | 2 |
| A | B | C | D | E | F | G | COD (% R) | Cr−Total (% R) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nZVI | FeSO4•7H2O | nZVI | FeSO4•7H2O | ||||||||
| 1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 73.9 ± 9.1 | 39.2 ± 5.3 | 91.2 ± 2.5 | 48.7 ± 14.8 |
| 2 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 67.0 ± 12.4 | 34.8 ± 10.9 | 97.3 ±5.7 | 55.6 ± 10.0 |
| 3 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 68.2 ± 11.3 | 30.8 ± 23.6 | 91.5 ± 0.9 | 49.0 ± 3.7 |
| 4 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 63.8 ± 8.2 | 22.9 ± 4.7 | 91.7 ± 0.6 | 57.4 ± 8.3 |
| 5 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | 64.1 ± 8.6 | 42.0 ± 19.1 | 90.2 ± 1.2 | 56.4 ± 6.7 |
| 6 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | 66.3 ± 6.3 | 29.8 ± 9.1 | 91.7 ± 1.1 | 48.7 ± 8.1 |
| 7 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 65.9 ± 5.0 | 35.4 ± 8.9 | 89.2 ± 4.1 | 52.1 ± 8.2 |
| 8 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 71.9 ± 5.3 | 35.0 ± 20.3 | 90.5 ± 0.7 | 48.0 ± 1.5 |
| pH | nZVI/H2O2 | Reaction Time | Cr-Total (% R) | |||
|---|---|---|---|---|---|---|
| Experimental | Prediction | |||||
| 1 | −1 | +1 | +1 | 93.36 | ±0.19 | 93.01 |
| 2 | 0 | 0 | 0 | 95.83 | ±0.13 | 95.90 |
| 3 | 0 | −1.68 | 0 | 86.85 | ±1.50 | 84.57 |
| 4 | 0 | 1.68 | 0 | 96.41 | ±3.31 | 96.87 |
| 5 | −1 | −1 | −1 | 97.01 | ±0.27 | 97.20 |
| 6 | +1 | −1 | +1 | 92.24 | ±2.01 | 90.96 |
| 7 | 0 | 0 | 0 | 93.95 | ±0.46 | 93.34 |
| 8 | +1.68 | 0 | 0 | 96.36 | ±0.28 | 96.72 |
| 9 | −1.68 | 0 | 0 | 88.00 | ±0.04 | 88.20 |
| 10 | 0 | +1 | 0 | 97.74 | ±1.38 | 97.53 |
| 11 | 0 | 0 | 0 | 95.59 | ±5.42 | 95.50 |
| 12 | +1 | −1 | −1 | 94.04 | ±0.25 | 94.87 |
| 13 | −1 | −1 | +1 | 96.68 | ±1.14 | 96.70 |
| 14 | −1 | +1 | −1 | 96.05 | ±0.18 | 95.96 |
| 15 | 0 | 0 | 0 | 87.84 | ±0.60 | 87.90 |
| 16 | +1 | +1 | −1 | 75.52 | ±0.22 | 76.89 |
| 17 | 0 | 0 | −1.68 | 88.05 | ±0.34 | 85.22 |
| 18 | 0 | 0 | 1.68 | 91.05 | ±1.49 | 90.88 |
| 19 | +1 | +1 | +1 | 95.01 | ±0.59 | 94.87 |
| 20 | 0 | 0 | 0 | 90.10 | ±0.34 | 89.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasquez, Y.; Franco, J.; Vasquez, M.; Agudelo, F.; Petala, E.; Filip, J.; Galvis, J.; Herrera, O. Removal of Cr and Organic Matter from Real Tannery Wastewater via Fenton-like Process Using Commercial Nano-Scale Zero-Valent Iron. Water 2024, 16, 754. https://doi.org/10.3390/w16050754
Vasquez Y, Franco J, Vasquez M, Agudelo F, Petala E, Filip J, Galvis J, Herrera O. Removal of Cr and Organic Matter from Real Tannery Wastewater via Fenton-like Process Using Commercial Nano-Scale Zero-Valent Iron. Water. 2024; 16(5):754. https://doi.org/10.3390/w16050754
Chicago/Turabian StyleVasquez, Yaneth, Jair Franco, Mario Vasquez, Felipe Agudelo, Eleni Petala, Jan Filip, Jose Galvis, and Oscar Herrera. 2024. "Removal of Cr and Organic Matter from Real Tannery Wastewater via Fenton-like Process Using Commercial Nano-Scale Zero-Valent Iron" Water 16, no. 5: 754. https://doi.org/10.3390/w16050754








