Dissipation of Eutrophic Substances in Grass Carp Aquaculture Pond Water by Ozone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
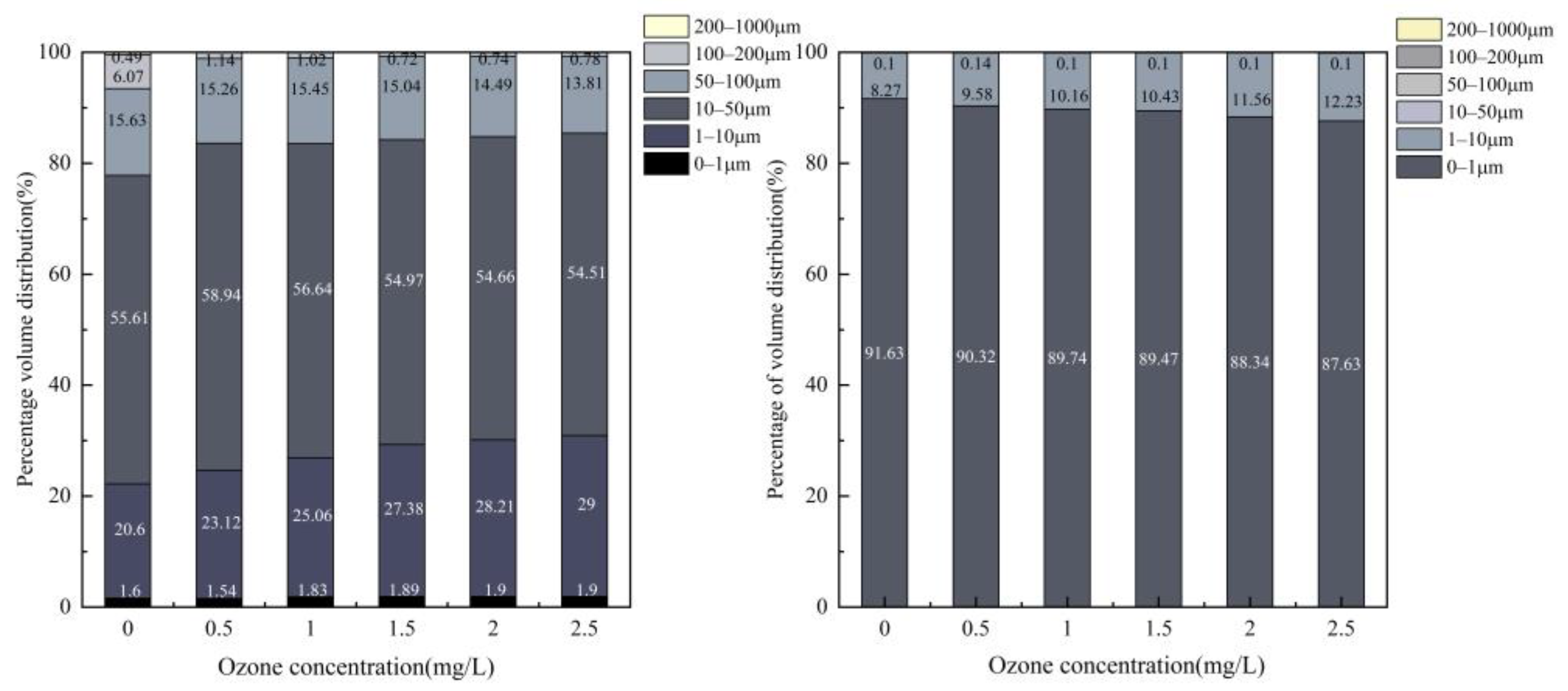
3.1. SS
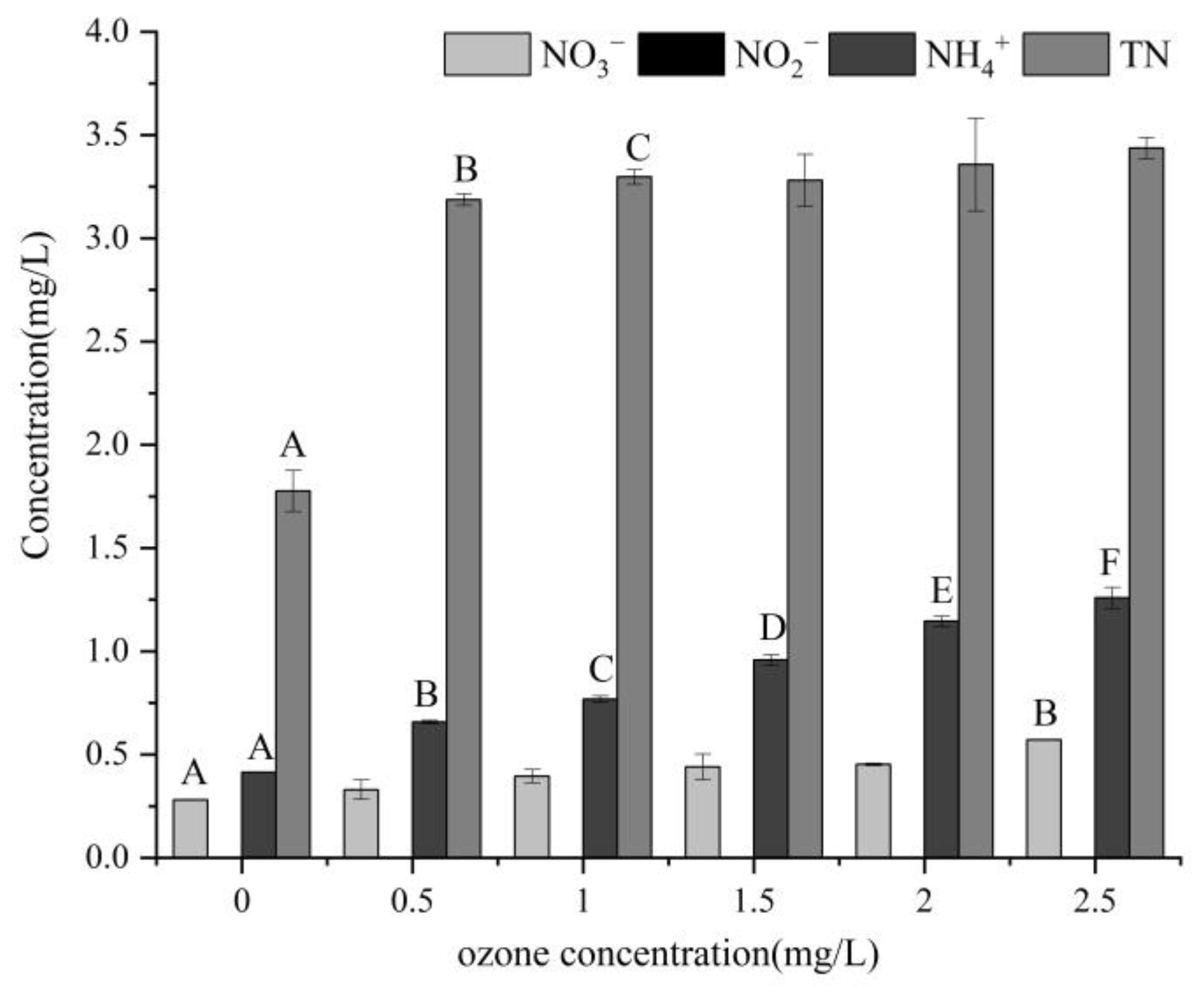
3.2. Water Quality Indicators
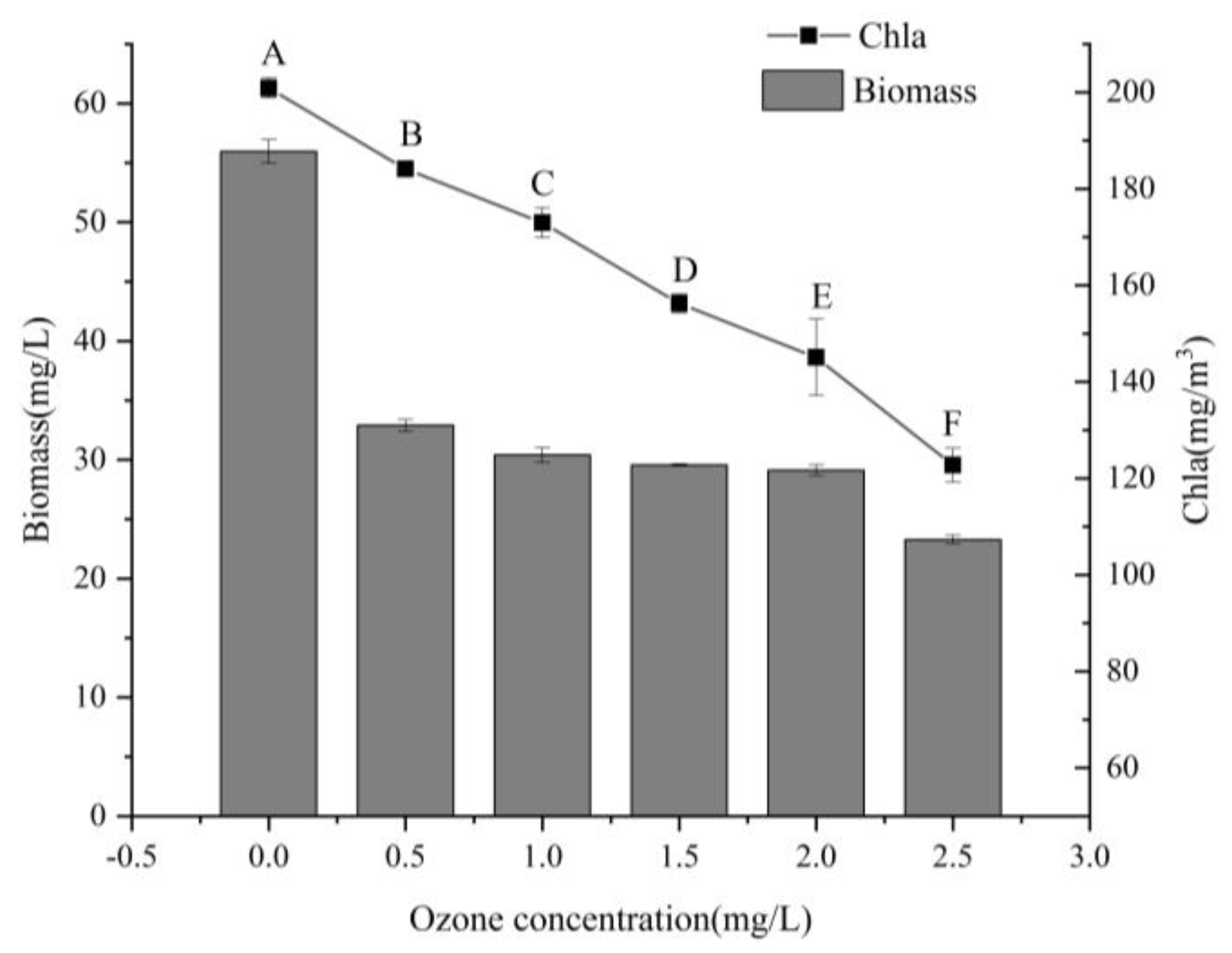
3.3. Algal Indicators
4. Discussion
4.1. Removal of Suspended Particulate Matter
4.2. Impact on Water Quality Indicators of Water Bodies
4.3. Effects on Algae
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.B.; Shi, Y.H.; Liu, Y.S. Nitrogen and phosphorus purification effect of aquaculture wastewater treatment system in freshwater culture ponds. J. Dalian Ocean Univ. 2022, 37, 104–112. [Google Scholar]
- Xu, J.B.; Liu, Y.S.; Shi, Y.H.; Yuan, X.C.; Wang, J.J.; Liu, J.Z. Comprehensive water quality evaluation of freshwater concentrated continuous ponds and aquaculture tail water treatment system. J. Shanghai Ocean Univ. 2022, 31, 170–180. [Google Scholar]
- Zhang, J.; Liu, A.S.; Li, L.; Cao, S. Hydrodynamic characteristics and separation efficiency of the series cyclone separator for pond aquaculture tail water treatment. Trans. Chin. Soc. Agric. Eng. 2022, 38, 49–58. [Google Scholar]
- Liu, X.; Shao, Z.; Cheng, G.; Lu, S.; Gu, Z.; Zhu, H.; Shen, H.; Wang, J.; Chen, X. Ecological engineering in pond aquaculture: A review from the whole-process perspective in China. Rev. Aquac. 2021, 13, 1060–1076. [Google Scholar] [CrossRef]
- Holan, A.B.; Wold, P.A.; Leiknes, T.O. Membrane performance and fouling behavior of membrane bioreactors installed in marine recirculating aquaculture systems. Aquac. Eng. 2014, 58, 45–51. [Google Scholar] [CrossRef]
- Qu, J.; Bi, F.; Li, S.; Feng, Z.; Li, Y.; Zhang, G.; Wang, L.; Wang, Y.; Zhang, Y. Microwave-assisted synthesis of polyethylenimine-grafted nanocellulose with ultra-high adsorption capacity for lead and phosphate scavenging from water. Bioresour. Technol. 2022, 362, 127819. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.H.; Li, Y.J.; Wang, X.C.; Ratnaweera, H.; Xu, L.; Zhu, H. Research progress of aquaculture tail water treatment technology and equipment. J. Nanjing Agric. Univ. 2022, 46, 1–12. [Google Scholar]
- Zhang, J.X.; Liu, C.F.; Jie, H.E.; Deng, G.; Mo-Ran, L.I. Analysis of the Solid-liquid Separation Characteristics and Treating Process Choice of Industrial Recycled Aquaculture Wastewater. J. Agro-Environ. Sci. 2008, 27, 1173–1176. [Google Scholar]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Gesto, M.; De Jesus Gregersen, K.J.; Pedersen, L.-F. Effects of ozonation and foam fractionation on rainbow trout condition and physiology in a small-scale freshwater recirculation aquaculture system. Aquaculture 2022, 557, 738312. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.G.; Ni, Q.; Liu, Z.; Qu, J.; Wang, B. The application and purification effect of the arc screen in the factorized aquaculture systems. Prog. Fish. Sci. 2011, 32, 116–120. [Google Scholar]
- Wong, K.B.; Piedrahita, R.H. Settling velocity characterization of aquacultural solids. Aquac. Eng. 2000, 21, 233–246. [Google Scholar] [CrossRef]
- Rueter, J.; Johnson, R. The use of ozone to improve solids removal during disinfection. Aquac. Eng. 1995, 14, 123–141. [Google Scholar] [CrossRef]
- Krumins, V.; Ebeling, J.M.; Wheaton, F. Ozone’s effects on power-law particle size distribution in recirculating aquaculture systems. Aquac. Eng. 2001, 25, 13–24. [Google Scholar] [CrossRef]
- Qu, J.; Liu, R.; Bi, X.; Li, Z.; Li, K.; Hu, Q.; Zhang, X.; Zhang, G.; Ma, S.; Zhang, Y. Remediation of atrazine contaminated soil by microwave activated persulfate system: Performance, mechanism and DFT calculation. J. Clean. Prod. 2023, 399, 136546. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Hankins, J.A.; Weber, A.L.; Durant, M.D. Ozonation of a recirculating rainbow trout culture system II. Effects on microscreen filtration and water quality. Aquaculture 1997, 158, 57–67. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S. The effects of ozone and water exchange rates on water quality and rainbow trout Oncorhynchus mykiss performance in replicated water recirculating systems. Aquac. Eng. 2011, 44, 80–96. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Sharrer, M.J.; Hollis, J.; Gleason, L.E.; Summerfelt, S.R. Dissolved ozone destruction using ultraviolet irradiation in a recirculating salmonid culture system. Aquac. Eng. 2004, 32, 209–223. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Sharrer, M.J.; Tsukuda, S.M.; Gearheart, M. Process requirements for achieving full-flow disinfection of recirculating water using ozonation and UV irradiation. Aquac. Eng. 2009, 40, 17–27. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Lu, C.C.; Wa, G.; Li, Q. Transformation of algae inclusions during ozonation of algae-laden water and the advanced treatment of ozonized effluent by resin adsorption. Chin. J. Environ. Eng. 2021, 15, 3536–3544. [Google Scholar]
- Aalto, S.L.; Syropoulou, E.; De Jesus Gregersen, K.J.; Tiirola, M.; Pedersen, P.B.; Pedersen, L.-F. Microbiome response to foam fractionation and ozonation in RAS. Aquaculture 2022, 550, 737846. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Li, S.; Ding, G.; Wang, K.; Zhuang, T.; Huang, X.; Wang, X. Synergistic effect of UV/chlorine in bacterial inactivation, resistance gene removal, and gene conjugative transfer blocking. Water Res. 2020, 185, 116290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Chen, H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017, 317, 309–316. [Google Scholar] [CrossRef]
- HJ 636-2012; Water Quality—Determination of Total Nitrogen-Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/method_standard2/201206/t20120618_231805.shtml (accessed on 30 August 2023).
- HJ 535-2009; Water Quality—Determination of Ammonia Nitrogen―Nessler’s Reagent Spectrophotometry. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/method_standard2/201010/t20101027_196755.shtml (accessed on 30 August 2023).
- GB 7493-87; Water Quality—Determination of Nitrogen (Nitrite)-Spectrophotometric Method. 1987. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/method_standard2/200807/t20080710_125478.shtml (accessed on 30 August 2023).
- HJ/T 346-2007; Water Quality—Determination of Nitrate-Nitrogen—Ultraviolet Spectrophotometry. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/method_standard2/200807/t20080704_125017.shtml (accessed on 30 August 2023).
- HJ 897-2017; Water Quality—Determination of Chlorophyll a—Spectrophotometric Method. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/method_standard2/201801/t20180105_429208.shtml (accessed on 30 August 2023).
- De Jesus Gregersen, K.J.; Pedersen, L.-F.; Pedersen, P.B.; Syropoulou, E.; Dalsgaard, J. Foam fractionation and ozonation in freshwater recirculation aquaculture systems. Aquac. Eng. 2021, 95, 102195. [Google Scholar] [CrossRef]
- Ji, M.D. A Study on Removal Mechanisms of Suspended Solids and Its Size Distribution Characteristics in Marine Recirculating Aquaculture Systems. Ph.D. Thesis, Zhe Jiang University, Hangzhou, China, 2020. [Google Scholar]
- Yang, F.; Ma, Y.W.; Zhang, D.S.; Sun, P.-H.; Li, Q.-H.; Liu, T. Control of Haliotis discus hannai Ino culture water quality with Ulva pertusa and ozone. J. Dalian Fish. Univ. 2003, 2, 79–83. [Google Scholar]
- Guo, E.Y.; Tan, H.X.; Luo, G.Z.; Sun, D.C.; Lai, C.S. Advanced treatment of recirculating aquaculture wastewater by O3/BAC. Environ. Pollut. Control 2009, 31, 6–9. [Google Scholar]
- De Vera, G.A.; Gernjak, W.; Weinberg, H.; Farré, M.J.; Keller, J.; von Gunten, U. Kinetics and mechanisms of nitrate and ammonium formation during ozonation of dissolved organic nitrogen. Water Res. 2016, 108, 451. [Google Scholar] [CrossRef]
- Song, B.B.; Ni, Q.; Zhang, Y.L.; Guan, C.W. Effect of ozone on the purification of water in partial-reused aquaculture system for turbot (Scophthalmus maximus). Fish. Mod. 2011, 38, 11–15. [Google Scholar]
- Guan, C.W.; Zhang, Y.L.; Song, H.Q.; Zhang, H.G.; Wang, J. Study on the effect and mechanism of ozone on purification of recirculating aquaculture water. Fish. Mod. 2018, 45, 14–18. [Google Scholar]
- Zeng, T.; Zhao, W.T.; Li, T.; He, C.; Wan, Y. Research Progress of Algae Removal Process Based on Ozonation. Environ. Sci. Technol. 2023, 36, 71–76. [Google Scholar]
- Sun, W.T. Research on the Efficacy and Mechanism of Ozonation Microbubble Enhanced Air Flotation for Algae Removal. Master’s Thesis, Shangdong Jianzhu University, Jinan, China, 2022. [Google Scholar]


| Ozone Concentration (mg/L) | Microcystis (107 cells/L) | Melosira (106 cells/L) | Scenedesmus (106 cells/L) | Anabaena (106 cells/L) | Euglena (105 cells/L) |
|---|---|---|---|---|---|
| 0 | 3.52 | 6.04 | 6.90 | 5.44 | 4.41 |
| 0.5 | 2.11 | 3.69 | 4.73 | 2.53 | 2.21 |
| 1.0 | 2.04 | 3.43 | 4.15 | 2.09 | 1.60 |
| 1.5 | 1.93 | 3.39 | 3.89 | 1.81 | 1.79 |
| 2.0 | 1.86 | 3.37 | 3.75 | 1.79 | 1.81 |
| 2.5 | 1.30 | 2.91 | 3.69 | 1.82 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Liu, X.; Cheng, X.; Guo, Z. Dissipation of Eutrophic Substances in Grass Carp Aquaculture Pond Water by Ozone. Water 2023, 15, 3167. https://doi.org/10.3390/w15183167
Chen Z, Liu X, Cheng X, Guo Z. Dissipation of Eutrophic Substances in Grass Carp Aquaculture Pond Water by Ozone. Water. 2023; 15(18):3167. https://doi.org/10.3390/w15183167
Chicago/Turabian StyleChen, Zhe, Xingguo Liu, Xiangyu Cheng, and Zeyu Guo. 2023. "Dissipation of Eutrophic Substances in Grass Carp Aquaculture Pond Water by Ozone" Water 15, no. 18: 3167. https://doi.org/10.3390/w15183167





