Sea Water Contamination in the Vicinity of the Italian Minor Islands Caused by Microplastic Pollution
Abstract
1. Introduction
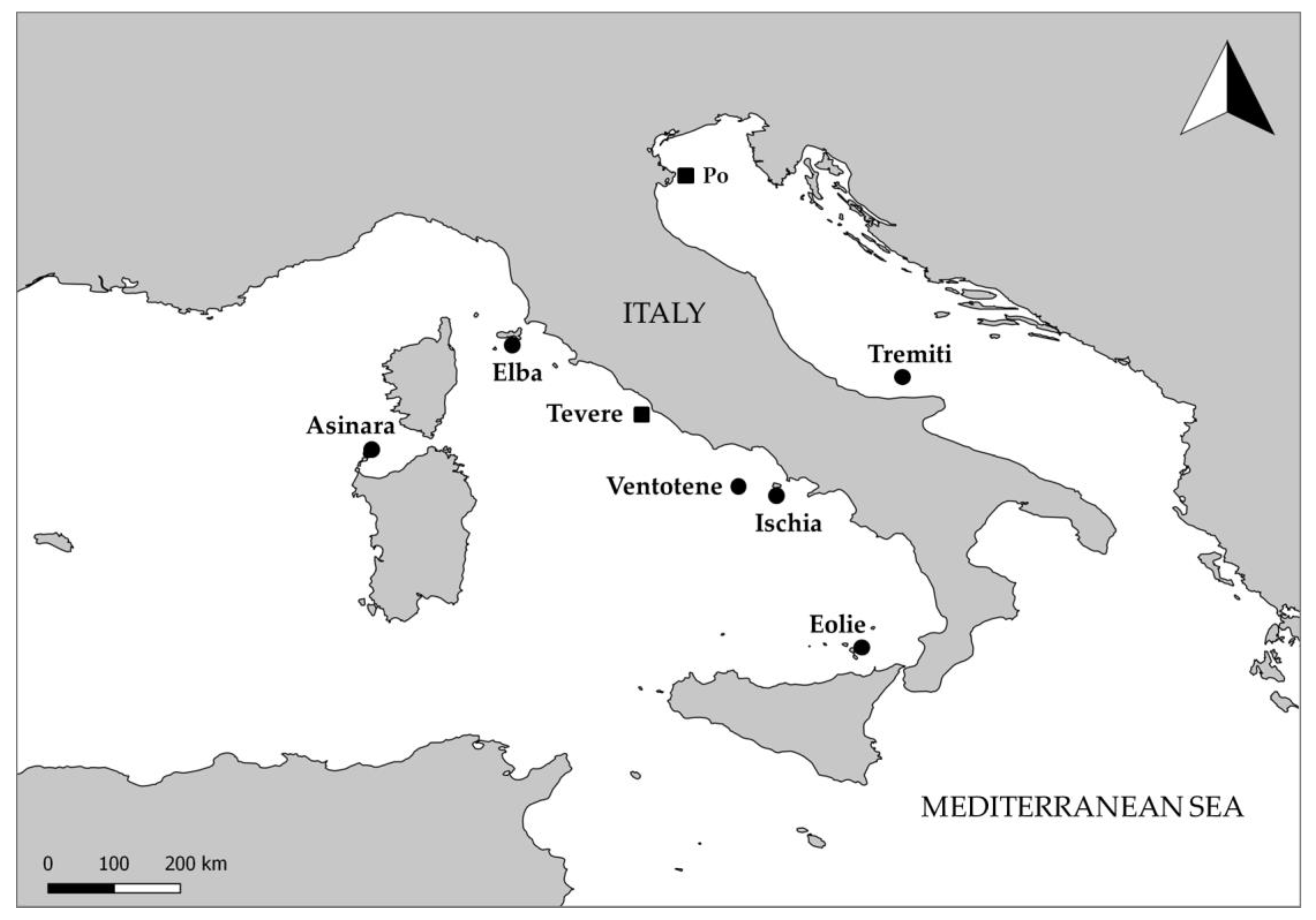
2. Materials and Methods
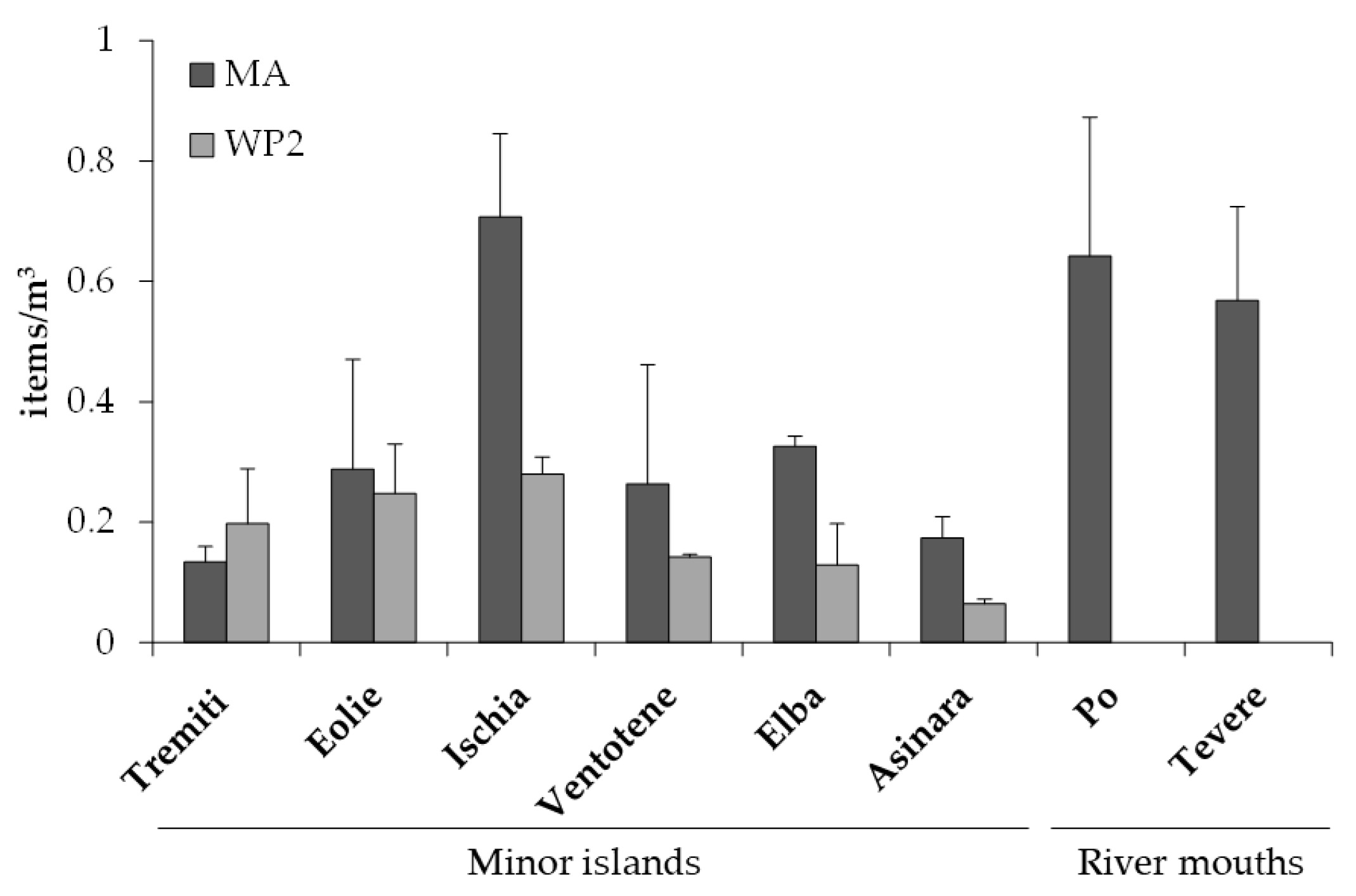
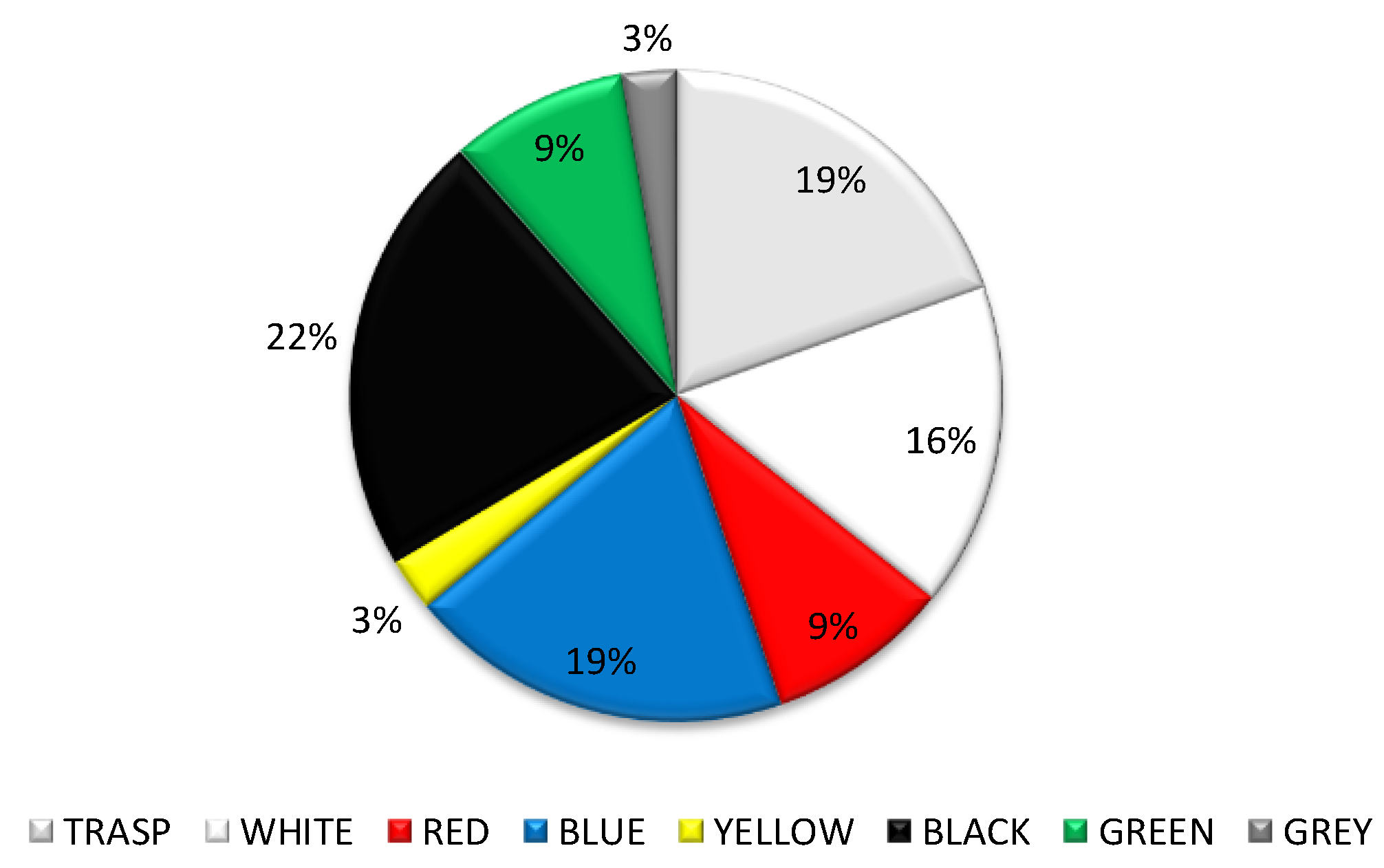
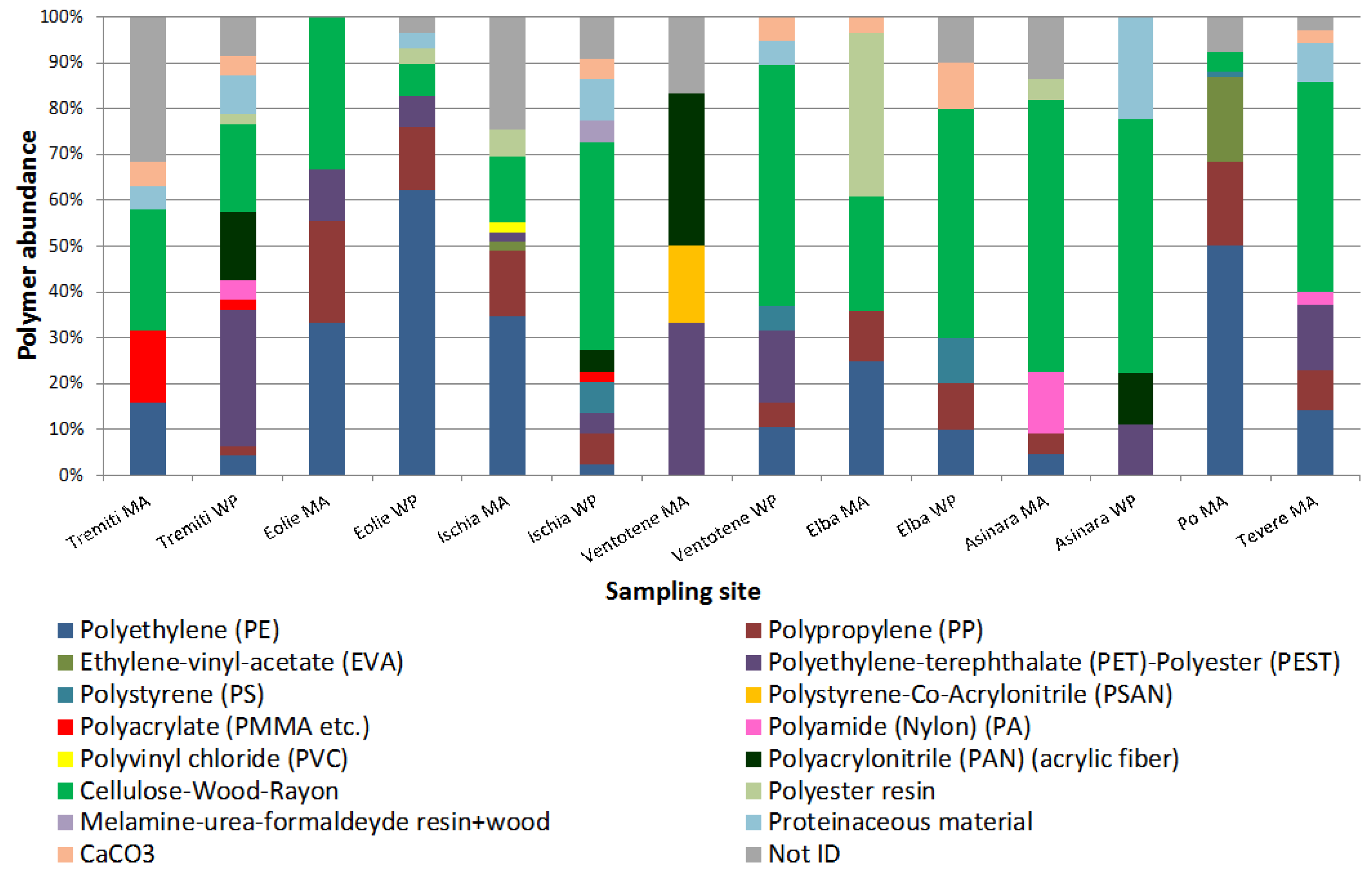
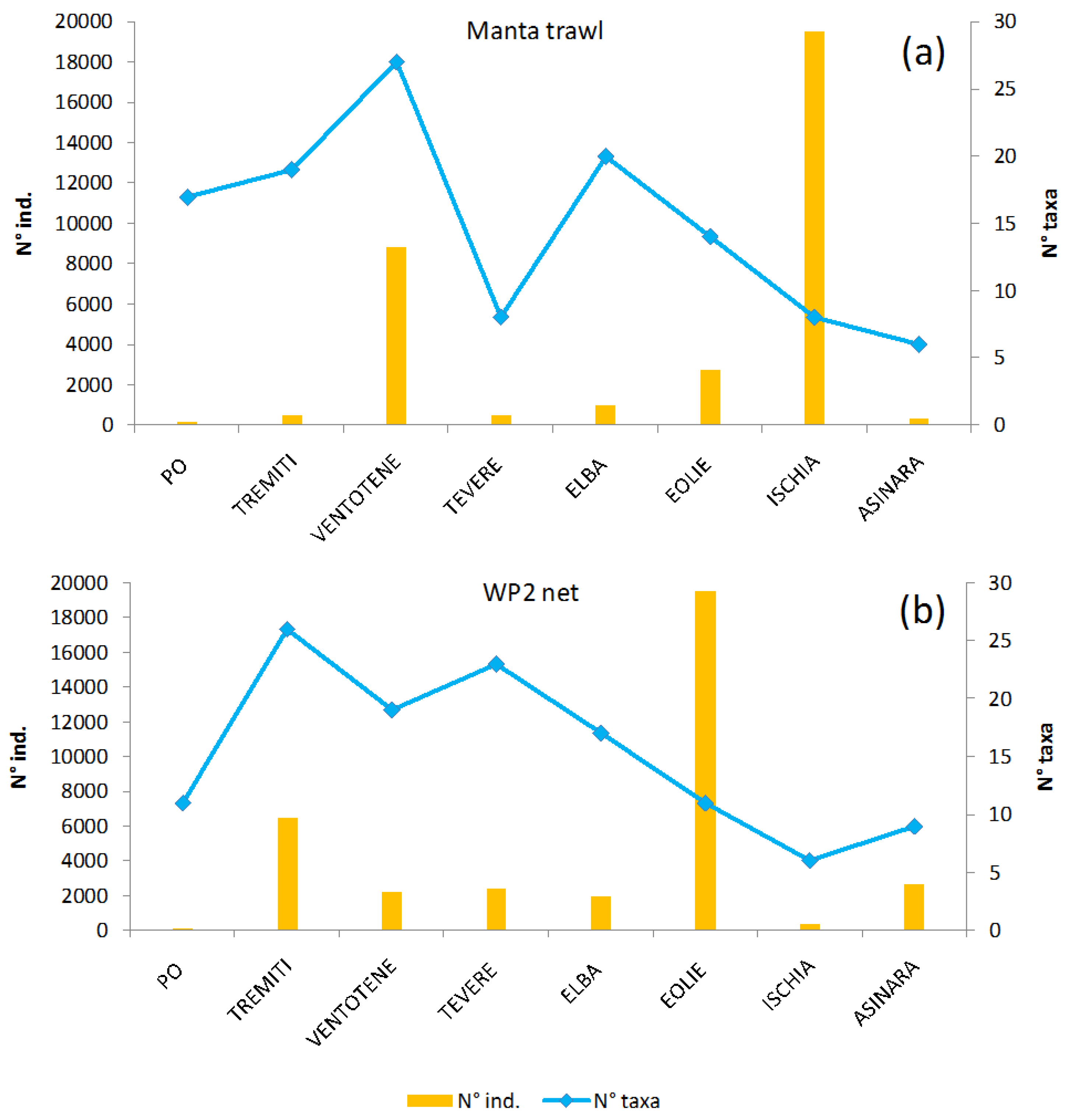
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C. Plastic debris in the marine environment: Consequences and solutions. In Marine Nature Conservation in Europe; Krause, J.C., Nordheim, H., Bräger, S., Eds.; Federal Agency for Nature Conservation: Stralsund, Germany, 2006; pp. 107–115. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; Anthony, W.G.J.; McGonigle, D.; Russell, A. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.K.; Fok, L.; Hung, P.L.; Cheung, L.T.O. Spatio-temporal comparison of neustonic microplastic density in Hong Kong waters under the influence of the Pearl River Estuary. Sci. Total Environ. 2018, 628, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.D.; Leads, R.; Wertz, H.; Weinstein, J.E. Microplastic in two South Carolina Estuaries: Occurrence, distribution, and composition. Mar. Pollut. Bull. 2018, 128, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings: Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial patterns of plastic debris along Estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.; Bamford, H. NOAA Technical Memorandum NOS-OR & R-30. In Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2009; NOAA: Silver Spring, MD, USA. 530p. [Google Scholar]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Meester, S.D.; Landuyt, L.V.; Clerck, K.D.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.; Hecq, J.H.; Galgani, F.; Voisin, P.; Collard, F.; Goffart, A. Neustonic microplastic and zooplankton in the North Western Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.; Rios-Mendoza, L.; Takada, H.S.; Thompson, R.C. Policy: Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the World’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.M.; Morrison, L.; Croot, P.L.; Allcock, A.L.; MacLoughlin, E.; Savard, O.; Brownlow, H.; Doyle, T.K. Frequency of Microplastics in Mesopelagic Fishes from the Northwest Atlantic. Front. Mar. Sci. 2018, 5, 39. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.A.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [PubMed]
- Frias, J.P.G.L.; Sobral, P.; Ferreira, M. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Analyt. Chem. 2014, 65, 47–53. [Google Scholar] [CrossRef]
- Renner, G.; Schmidt, T.C.; Schram, J. Analytical methodologies for monitoring micro(nano)plastics: Which are fit for purpose? Curr. Opin. Environ. Sci. Health 2018, 1, 55–61. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Chapman, M.G.; Thompson, R.C.; Amaral Zettler, L.A.; Jambeck, J.; Mallos, N.J. Spatial and Temporal Patterns of Stranded Intertidal Marine Debris: Is There a Picture of Global Change? Environ. Sci. Technol. 2015, 49, 7082–7094. [Google Scholar] [CrossRef] [PubMed]
- Dümichen, E.; Barthel, A.K.; Braun, U.; Bannick, C.G.; Brand, K.; Jekel, M.; Senz, R. Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res. 2015, 85, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis-Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Feet, D.; Van Franeker, J.; Katsanevakis, S.; Maes, T.; Oosterbaan, L.; Poito, I.; Hanke, G.; Thompson, R.; Amato, E.; et al. Marine Strategy Framework Directive; Task Group 10, Marine Litter Report; European Commission Joint Research Center, IFREMER & ICES: Nantes, France, 2010; p. 58. [Google Scholar]
- Käppler, A.; Windrich, F.; Löder, M.G.; Malanin, M.; Fischer, D.; Labrenz, M.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm−1 for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [PubMed]
- US. EPA. The Risk Assessment Guidelines of 1986; EPA/600/8-87/045; Office of Health and Environmental Assessment: Washington, DC, USA, 1987.
- De Lucia, G.A.; Caliani, I.; Marra, S.; Camedda, A.; Coppa, S.; Alcaro, L.; Campani, T.; Giannetti, M.; Coppola, D.; Cicero, A.M.; et al. Amount and distribution of neustonic micro-plastic off the western Sardinian coast (Central-Western Mediterranean Sea). Mar. Environ. Res. 2014, 100, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Kang, J.H.; Kwon, O.Y.; Han, G.M.; Shim, W.J. Large Accumulation of Micro-sized Synthetic Polymer Particles in the Sea Surface Microlayer. Environ. Sci. Technol. 2014, 48, 9014–9021. [Google Scholar] [CrossRef] [PubMed]
- Scarpato, A.; Romanelli, G.; Galgani, F.; Giovanardi, F.; Giordano, P.; Calvo, M.; Caixap, J.; BenBrahim, S.; Sammari, C.; Deudero, S.; et al. Western Mediterranean coastal waters-Monitoring PCBs and pesticides accumulation in Mytilus galloprovincialis by active mussel watching: The Mytilos project. J. Environ. Monit. 2010, 12, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J.; Moore, S.L.; Leecaster, M.K.; Weisberg, S.B. A comparison of plastic and plankton in the North Pacific Central Gyre. Mar. Pollut. Bull. 2001, 42, 1297–1300. [Google Scholar] [CrossRef]
- Lima, A.R.A.; Costa, M.F.; Barletta, M. Distribution patterns of microplastics within the plankton of a tropical estuary. Environ. Res. 2014, 132, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Frias, J.G.L.; Micaelo, A.C.; Sobral, P. Resin pellets from beaches of the Portuguese coast and adsorbed persistent organic pollutants. Estuar. Coast. Shelf Sci. 2013, 130, 62–69. [Google Scholar] [CrossRef]
- Wang, F.W.; Charle, C.; Da, L.; Xingwen, W.; Fei, Y.; Zeng, E. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Turci, R.; Businaro, J.; Minoia, C.; Sturchio, E.; Ficociello, B.; Signorini, S.; Colosio, C.; Imbriani, M. Interferenti endocrini, schede monografiche: DDT, DDE e DDD. G. Ital. Med. Lav. Erg. 2010, 32, 93–144. [Google Scholar]
- Storelli, M.M.; Storelli, A.; Marcotrigiano, G.O. Polychlorinated biphenyls, hexachlorobenzene, hexachlorocyclohexane isomers, and pesticide organochlorine residues in cod-liver oil dietary supplements. J. Food Prot. 2004, 67, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Xinzhen, L.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Gálvez, J.Á.; Irigoien, X.; Duarte, C.M. Plastic Accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, e0121762. [Google Scholar] [CrossRef] [PubMed]
| PCB ng/g in Surface Water | PCB ng/g in Deep Water | |||||||||||
| Site | Asinara | Ischia | Ventotene | Tevere | Tremiti | Eolie | Po | Elba | Asinara | Ischia | Ventotene | Elba |
| PCB 31 | 10.87 | 1.28 | 4.45 | 1.4 | 0.45 | 7.23 | 5 | 14.24 | 8.01 | 0.41 | 3.39 | 7.12 |
| PCB 28 | 6.34 | 0.51 | 3.89 | 1.18 | 0.62 | 6.04 | 4.39 | 7.29 | 4.21 | 0.41 | 2.46 | 4.42 |
| PCB 52 | 286.11 | 21.08 | 116.51 | 40.72 | 18.09 | 160.13 | 81.78 | 251.9 | 168.15 | 8.51 | 80.04 | 123.03 |
| PCB 35 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| PCB 101 | 623.58 | 25.31 | 211.58 | 85.18 | 24.71 | 314.52 | 149.59 | 427.35 | 242.57 | 13.06 | 135.49 | 163.36 |
| PCB 81 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| PCB 110 | 654.58 | 20.34 | 194.9 | 79.1 | 25.63 | 293.95 | 143.9 | 361.22 | 198.54 | 12.96 | 119.58 | 135.27 |
| PCB 77 | 1.44 | 0.1 | <0.10 | <0.10 | <0.10 | <0.10 | 1.13 | 3.75 | 3.14 | 0.41 | 1.2 | <0.10 |
| PCB 118 | 348.24 | 9.19 | 86.71 | 34.33 | 8.43 | 129.49 | 66.08 | 158.11 | 86.42 | 7.15 | 52.51 | 57.01 |
| PCB 153 | 200.24 | 6.16 | 45.28 | 17.57 | 5.11 | 62.09 | 45.33 | 81.99 | 47.6 | 4.78 | 25.65 | 30.63 |
| PCB 105 | 115.58 | 2.75 | 28.36 | 9.6 | 4.48 | 37.59 | 20.42 | 47.2 | 25.62 | 2.72 | 15.03 | 16.63 |
| PCB 138 | 172.18 | 4.44 | 38.49 | 14.19 | 3.13 | 51.18 | 34.06 | 64.17 | 36.73 | 5.23 | 25.89 | 24.93 |
| PCB 126 | 0.12 | <0.10 | 0.85 | <0.10 | <0.10 | 0.38 | <0.10 | 0.31 | 3.11 | 0.49 | <0.10 | <0.10 |
| PCB 128 | 47.19 | 1.13 | 7.53 | 2.17 | 2.24 | 11.77 | 7.63 | 13.49 | 7.99 | 1.51 | 4.19 | 5.11 |
| PCB 156 | 14.06 | 0.43 | 3.85 | 0.57 | 1.06 | 5.02 | 2.29 | 3.11 | 2.23 | 0.55 | 1.01 | 1.37 |
| PCB 180 | 23.95 | 1.11 | 4.43 | 1.86 | 2.1 | 1.96 | 8.18 | 5.82 | 3.39 | 1.09 | 1.08 | 2.68 |
| PCB 169 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | 1.54 | <0.10 | <0.10 | <0.10 |
| ∑ PCBs ng/g | 2504.09 | 93.8 | 748.56 | 287.87 | 96.05 | 1081.32 | 569.77 | 1439.95 | 849.35 | 59.29 | 467.51 | 571.56 |
| OC pesticides ng/g in surface water | OC pesticides ng/g in deep water | |||||||||||
| a-HCH | 5.21 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | 1.81 | <0.10 | <0.10 | <0.10 | <0.10 |
| b-HCH | <0.10 | 0.31 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | 0.14 | <0.10 | <0.10 |
| g-HCH | 8.8 | 0.25 | 1.99 | 1.19 | <0.10 | 2.7 | 1.23 | 3.22 | 2.08 | <0.10 | 1.36 | 1.23 |
| d-HCH | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| ∑ HCHs ng/g | 14.01 | 0.41 | 1.99 | 1.19 | <0.10 | 2.7 | 1.23 | 5.03 | 2.08 | 0.14 | 1.36 | 1.23 |
| 2,4 DDE | 16.62 | 0.77 | 5.41 | 2.23 | <0.10 | 6.26 | 5.97 | 9.74 | 5.85 | 0.43 | 3.52 | 3.94 |
| 4,4 DDE | 237.22 | 8.46 | 71.01 | 30.56 | 10.26 | 109.67 | 57.36 | 134.9 | 78.07 | 4.7 | 44.47 | 52.58 |
| 2,4 DDD | 6.37 | 0.53 | 3.33 | <0.10 | <0.10 | <0.10 | <0.10 | 2.18 | 4.84 | 0.33 | 1.22 | 2.41 |
| 4,4 DDD | 10.83 | 0.39 | 6.36 | 1.34 | <0.10 | 2.31 | 3 | 5.52 | 2.73 | 0.32 | 2.72 | 1.21 |
| 2,4 DDT | <0.10 | <0.10 | 2.48 | <0.10 | <0.10 | <0.10 | 0.63 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| 4,4 DDT | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| ∑ DDs ng/g | 271.05 | 9.89 | 82.51 | 34.14 | 10.26 | 118.24 | 66.95 | 152.34 | 91.49 | 5.78 | 51.94 | 60.14 |
| HCB | 5.73 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | 0.11 | <0.10 | <0.10 |
| Aldrin | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Dieldrin | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | 0.96 | <0.10 | <0.10 | <0.10 |
| PCB and OC pesticides ng/g in surface water | PCB and OC pesticides ng/g in deep water | |||||||||||
| Asinara | Ischia | Ventotene | Tevere | Tremiti | Eolie | Po | Elba | Asinara | Ischia | Ventotene | Elba | |
| ∑ PCBs ng/m³ | 0.36 | 0.54 | 0.39 | 0.25 | 0.026 | 0.43 | 0.5 | 0.83 | 0.47 | 0.32 | 0.77 | 0.36 |
| ∑ HCHs ng/m³ | 0.002 | 0.002 | 0.001 | 0.001 | - | 0.001 | 0.001 | 0.003 | 0.001 | 0.001 | 0.002 | 0.001 |
| ∑ DDs ng/m³ | 0.039 | 0.056 | 0.043 | 0.03 | 0.003 | 0.047 | 0.058 | 0.088 | 0.051 | 0.032 | 0.084 | 0.038 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lucia, G.A.; Vianello, A.; Camedda, A.; Vani, D.; Tomassetti, P.; Coppa, S.; Palazzo, L.; Amici, M.; Romanelli, G.; Zampetti, G.; et al. Sea Water Contamination in the Vicinity of the Italian Minor Islands Caused by Microplastic Pollution. Water 2018, 10, 1108. https://doi.org/10.3390/w10081108
De Lucia GA, Vianello A, Camedda A, Vani D, Tomassetti P, Coppa S, Palazzo L, Amici M, Romanelli G, Zampetti G, et al. Sea Water Contamination in the Vicinity of the Italian Minor Islands Caused by Microplastic Pollution. Water. 2018; 10(8):1108. https://doi.org/10.3390/w10081108
Chicago/Turabian StyleDe Lucia, Giuseppe Andrea, Alvise Vianello, Andrea Camedda, Danilo Vani, Paolo Tomassetti, Stefania Coppa, Luca Palazzo, Marina Amici, Giulia Romanelli, Giorgio Zampetti, and et al. 2018. "Sea Water Contamination in the Vicinity of the Italian Minor Islands Caused by Microplastic Pollution" Water 10, no. 8: 1108. https://doi.org/10.3390/w10081108
APA StyleDe Lucia, G. A., Vianello, A., Camedda, A., Vani, D., Tomassetti, P., Coppa, S., Palazzo, L., Amici, M., Romanelli, G., Zampetti, G., Cicero, A. M., Carpentieri, S., Di Vito, S., & Matiddi, M. (2018). Sea Water Contamination in the Vicinity of the Italian Minor Islands Caused by Microplastic Pollution. Water, 10(8), 1108. https://doi.org/10.3390/w10081108





