Utilization of Non-Living Microalgae Biomass from Two Different Strains for the Adsorptive Removal of Diclofenac from Water
Abstract
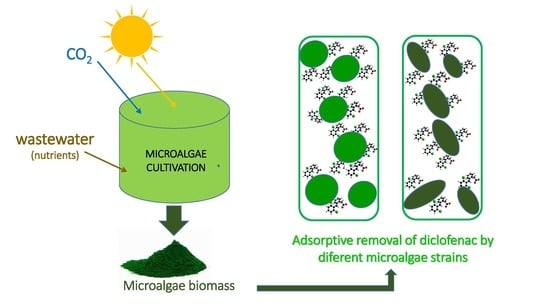
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Culture Conditions
2.2. Adsorbent Materials and Adsorption Experiments
2.2.1. Adsorption Kinetics
2.2.2. Adsorption Equilibrium
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheah, W.Y.; Show, P.L.; Chang, J.S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.A.; Meng, T.K. Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production. Sci. Total Environ. 2017, 584–585, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Acién, F.G.; Fernández, J.M.; Magan, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, H.-W. Photoautotrophic microalgae screening for tertiary treatment of livestock wastewater and bioresource recovery. Water 2017, 9, 192. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Khiewwijit, R.; Rijnaarts, H.; Temmink, H.; Keesman, K.J. Glocal assessment of integrated wastewater treatment and recovery concepts using partial nitritation/Anammox and microalgae for environmental impacts. Sci. Total Environ. 2018, 628, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Rugnini, L.; Costa, G.; Congestri, R.; Bruno, L. Testing of two different strains of green microalgae for Cu and Ni removal from aqueous media. Sci. Total Environ. 2017, 601–602, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Jaén-Gil, A.; Bello-Laserna, I.; Rodríguez-Mozaz, F.; Vicent, T.; Barceló, D.; Blánquez, P. Performance of a microalgal photobioreactor treating toilet wastewater: Pharmaceutically active compound removal and biomass harvesting. Sci. Total Environ. 2017, 592, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; García, A.I.; Otero, M. Comparative assessment of pharmaceutical removal from wastewater by the microalgae Chlorella sorokiniana, Chlorella vulgaris and Scenedesmus obliquus. In Biological Wastewater Treatment and Resource Recovery; IntechOpen: London, UK, 2017. [Google Scholar]
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; García, A.I.; Otero, M. Comparative assessment of diclofenac removal from water by different microalgae strains. Algal Res. 2016, 18, 127–134. [Google Scholar] [CrossRef]
- de Godos, I.; Muñoz, R.; Guieysse, B. Tetracycline removal during wastewater treatment in high-rate algal ponds. J. Hazard. Mater. 2012, 229, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Escapa, C.; Coimbra, R.N.; Nuevo, C.; Vega, S.; Paniagua, S.; García, A.I.; Calvo, L.F.; Otero, M. Valorization of microalgae biomass by its use for the removal of paracetamol from contaminated water. Water 2017, 9, 312. [Google Scholar] [CrossRef]
- Angulo, E.; Bula, L.; Mercado, I.; Montaño, A.; Cubillán, N. Bioremediation of Cephalexin with non-living Chlorella sp. biomass after lipid extraction. Bioresour. Technol. 2018, 257, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Brinza, L.; Dring, M.J.; Gavrilescu, M. Marine micro and macro algal species as biosorbents for heavy metals. Env. Eng. Manag. J. 2007, 6, 237–251. [Google Scholar] [CrossRef]
- Sutkowy, M.; Kłosowski, G. Use of the coenobial green algae Pseudopediastrum boryanum (Chlorophyceae) to remove hexavalent chromium from contaminated aquatic ecosystems and industrial wastewaters. Water 2018, 10, 712. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Chen, H.-R. Removal of malachite green from aqueous solution using low-cost chlorella-based biomass. J. Hazard. Mater. 2010, 175, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ying, G.; Yang, B.; Liu, S.; Lai, H.; Liu, Y.; Chen, Z.; Zhou, G. Biotransformation of progesterone and norgestrel by two freshwater microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): transformation kinetics and products identification. Chemosphere 2014, 95, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Escapa, C.; Torres, T.; Neuparth, T.; Coimbra, R.N.; García, A.I.; Santos, M.M.; Otero, M. Zebrafish embryo bioassays for a comprehensive evaluation of microalgal efficiency in the removal of diclofenac from water. Sci. Total Environ. 2018, 640, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Otero, M.; Morán, A.; García, A.I. Selection of native freshwater microalgae and cyanobacteria for CO2 biofixation. Environ. Technol. 2013, 34, 3137–31435. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Myers, J. On pigments growth and photosynthesis of Phaeodactylum Tricornutum. J. Phycol. 1968, 4, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 1808, 24, 1–39. [Google Scholar]
- Ho, I.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 2011, 34, 451–465. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Otero, M.; Zabkova, M.; Grande, C.A.; Rodrigues, A.E. Fixed-bed adsorption of salicylic acid onto polymeric adsorbents and activated charcoal. Ind. Eng. Chem. Res. 2005, 44, 927–936. [Google Scholar] [CrossRef]
- Jelínek, L.; Procházková, G.; Quintelas, C.; Beldíková, E.; Brányik, T. Chlorella vulgaris biomass enriched by biosorption of polyphenols. Algal Res. 2015, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Abbas, G.; Javed, I.; Iqbal, M.; Haider, R.; Hussain, F.; Qureshi, N. Adsorption of non-steroidal anti-inflammatory drugs (diclofenac and ibuprofen) from aqueous medium onto activated onion skin. Desalin. Water Treat. 2017, 95, 274–285. [Google Scholar] [CrossRef]
- An, H.J.; Bhadra, B.N.; Khan, N.A.; Jhung, S.H. Adsorptive removal of wide range of pharmaceutical and personal care products from water by using metal azolate framework-6-derived porous carbon. Chem. Eng. J. 2018, 343, 447–454. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Calisto, V.; Ferreira, C.I.A.; Esteves, V.I.; Otero, M. Removal of pharmaceuticals from municipal wastewater by adsorption onto pyrolyzed pulp mill sludge. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- de Franco, M.A.E.; de Carvalho, C.B.; Bonetto, M.M.; de Pelegrini Soares, R.; Féris, L.A. Diclofenac removal from water by adsorption using activated carbon in batch mode and fixed-bed column: Isotherms, thermodynamic study and breakthrough curves modelling. J. Clean. Prod. 2018, 181, 145–154. [Google Scholar] [CrossRef]
- Larous, S.; Meniai, A.-H. Adsorption of diclofenac from aqueous solution using activated carbon prepared from olive stones. Int. J. Hydrogen Energy 2016, 41, 10380–10390. [Google Scholar] [CrossRef]
- Lawal, I.A.; Moodley, B. Sorption mechanism of pharmaceuticals from aqueous medium on ionic liquid modified biomass. J. Chem. Tech. Biotech. 2017, 92, 808–818. [Google Scholar] [CrossRef]
- Lu, X.; Shao, Y.; Gao, N.; Chen, J.; Zhang, Y.; Wang, Q.; Lu, Y. Adsorption and removal of clofibric acid and diclofenac from water with MIEX resin. Chemosphere 2016, 161, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Samah, N.A.; Sánchez-Martín, M.-J.; Sebastián, R.M.; Valiente, M.; López-Mesas, M. Molecularly imprinted polymer for the removal of diclofenac from water: Synthesis and characterization. Sci. Total Environ. 2018, 631, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, R.N.; Escapa, C.; Paniagua, S.; Otero, M. Adsorptive removal of diclofenac from ultrapure and wastewater: a comparative assessment on the performance of a polymeric resin and activated carbons. Desalin. Water Treat. 2016, 57, 27914–27923. [Google Scholar] [CrossRef]
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Chen, C.-Y.; Guo, W.-Q.; Chang, H.-W.; Chen, W.-M.; Lee, D.-J.; Huang, C.-C.; Ren, N.-Q.; Chang, J.-S. Fixed-bed biosorption of cadmium using immobilized Scenedesmus obliquus CNW-N cells on loofa (Luffa cylindrica) sponge. Bioresource Technol. 2014, 160, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Iqbal, M. Immobilization of blue green microalgae on loofa sponge to biosorb cadmium in repeated shake flask batch and continuous flow fixed bed column reactor system. World J. Microbiol. Biotechnol. 2016, 22, 775–782. [Google Scholar] [CrossRef]



| Model | Parameter | Scenedesmus sp. | Synechocystis sp. | Activated Carbon |
|---|---|---|---|---|
| Kinetic Equations | ||||
| Pseudo-first order | k1 (min−1) | 0.0388 ± 0.0041 | 0.0393 ± 0.0024 | 0.0375 ± 0.0021 |
| qe (mg∙g−1) | 20.19 ± 0.54 | 17.55 ± 0.30 | 184.90 ± 2.98 | |
| r2 | 0.981 | 0.9944 | 0.9951 | |
| Sy.x | 1.05 | 0.52 | 5.16 | |
| Pseudo-second order | k2 (g∙m−1∙min−1) | 0.0023 ± 0.0002 | 0.0025 ± 0.0002 | 0.00022 ± 0.00002 |
| qe (mg∙g−1) | 22.64 ± 0.35 | 19.90 ± 0.34 | 210.80 ± 4.50 | |
| r2 | 0.9964 | 0.9968 | 0.9953 | |
| Sy.x | 0.45 | 0.40 | 5.09 | |
| Equilibrium Isotherms | ||||
| Freundlich | KF (mg∙g−1 (mg∙L−1)−N) | 3.48 ± 0.17 | 5.40 ± 1.01 | 43.55 ± 7.48 |
| N | 2.36 ± 0.07 | 3.42 ± 0.61 | 2.80 ± 0.36 | |
| r2 | 0.9989 | 0.9424 | 0.9579 | |
| Sy.x | 0.26 | 1.78 | 15.23 | |
| Langmuir | Qmax (mg∙g−1) | 28.34 ± 1.19 | 19.76 ± 0.57 | 232.20 ± 7.41 |
| KL (L∙mg−1) | 0.039 ± 0.005 | 0.143 ± 0.018 | 0.076 ± 0.007 | |
| r2 | 0.9941 | 0.9919 | 0.9932 | |
| Sy.x | 0.57 | 0.66 | 6.12 | |
| Adsorbent | Qmax (mg∙g−1) | Reference |
|---|---|---|
| Activated onion skin | 134 | [30] |
| Metal azolate framework-6 | 503 | [31] |
| Activated cork | 79 | [31] |
| Pyrolyzed pulp mill sludge | 27 | [32] |
| Granular activated carbon | 36 | [33] |
| Activated carbon from olive stones | 11 | [34] |
| Ionic liquid modified biomass | 197 | [35] |
| MIEX® resin | 52 | [36] |
| Molecular imprinted polymer | 160 | [37] |
| Powder activated carbon | 301 | [38] |
| Polymeric resin | 39 | [38] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coimbra, R.N.; Escapa, C.; Vázquez, N.C.; Noriega-Hevia, G.; Otero, M. Utilization of Non-Living Microalgae Biomass from Two Different Strains for the Adsorptive Removal of Diclofenac from Water. Water 2018, 10, 1401. https://doi.org/10.3390/w10101401
Coimbra RN, Escapa C, Vázquez NC, Noriega-Hevia G, Otero M. Utilization of Non-Living Microalgae Biomass from Two Different Strains for the Adsorptive Removal of Diclofenac from Water. Water. 2018; 10(10):1401. https://doi.org/10.3390/w10101401
Chicago/Turabian StyleCoimbra, Ricardo N., Carla Escapa, Nadyr C. Vázquez, Guillermo Noriega-Hevia, and Marta Otero. 2018. "Utilization of Non-Living Microalgae Biomass from Two Different Strains for the Adsorptive Removal of Diclofenac from Water" Water 10, no. 10: 1401. https://doi.org/10.3390/w10101401