Removal of Arsenic from Water with Non-Thermal Plasma (NTP), Coagulation and Membrane Filtration
Abstract
:1. Introduction
2. Materials and Methods
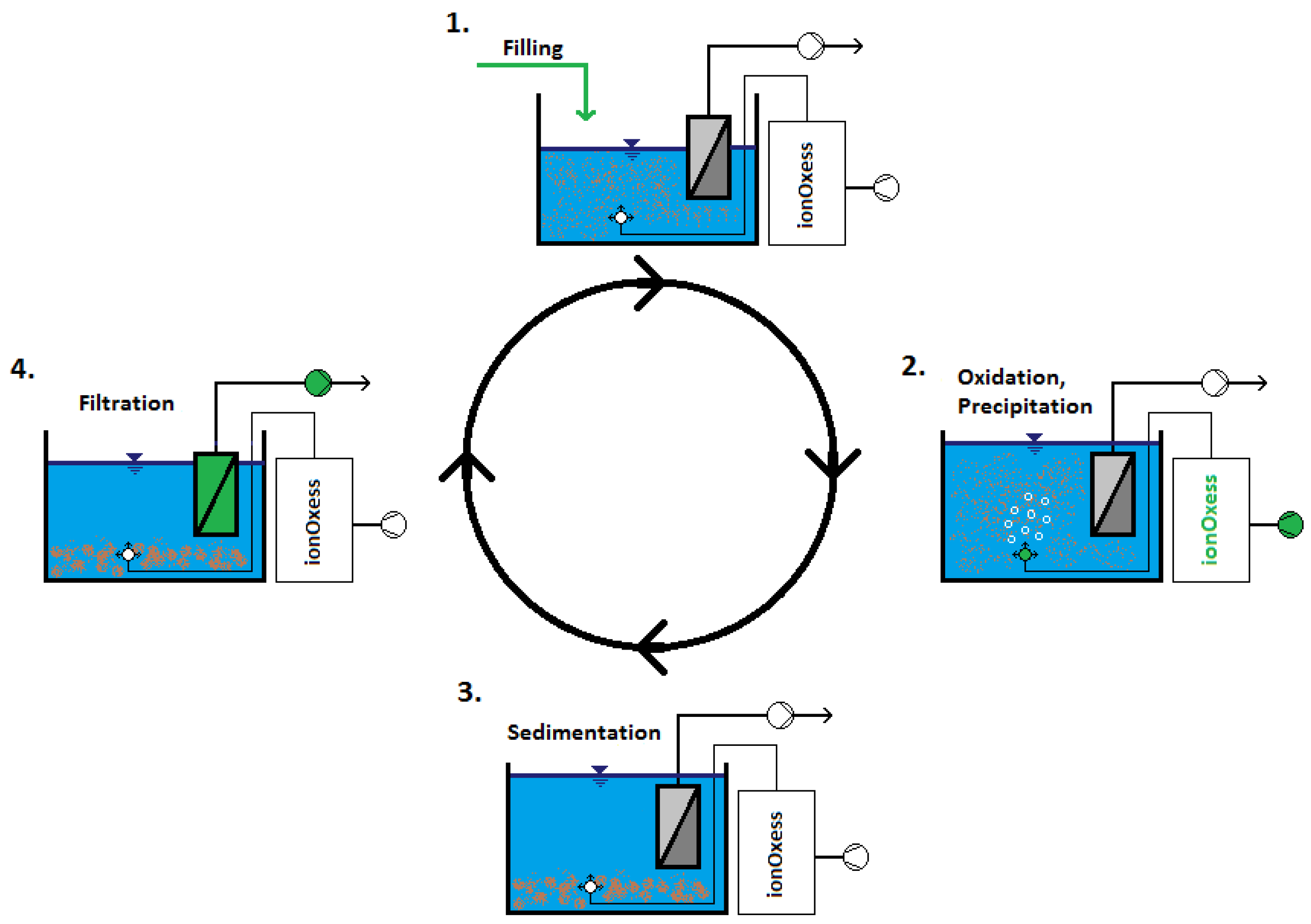
2.1. Pilot Plant Set-Up
2.2. Non-Thermal Plasma
2.3. Coagulation and Precipitation
2.4. Ultrafiltration
3. Results and Discussion
3.1. Influence of Different Coagulants (A1–A5)
3.2. Influence of NTP on Arsenic Removal (A6–A13)
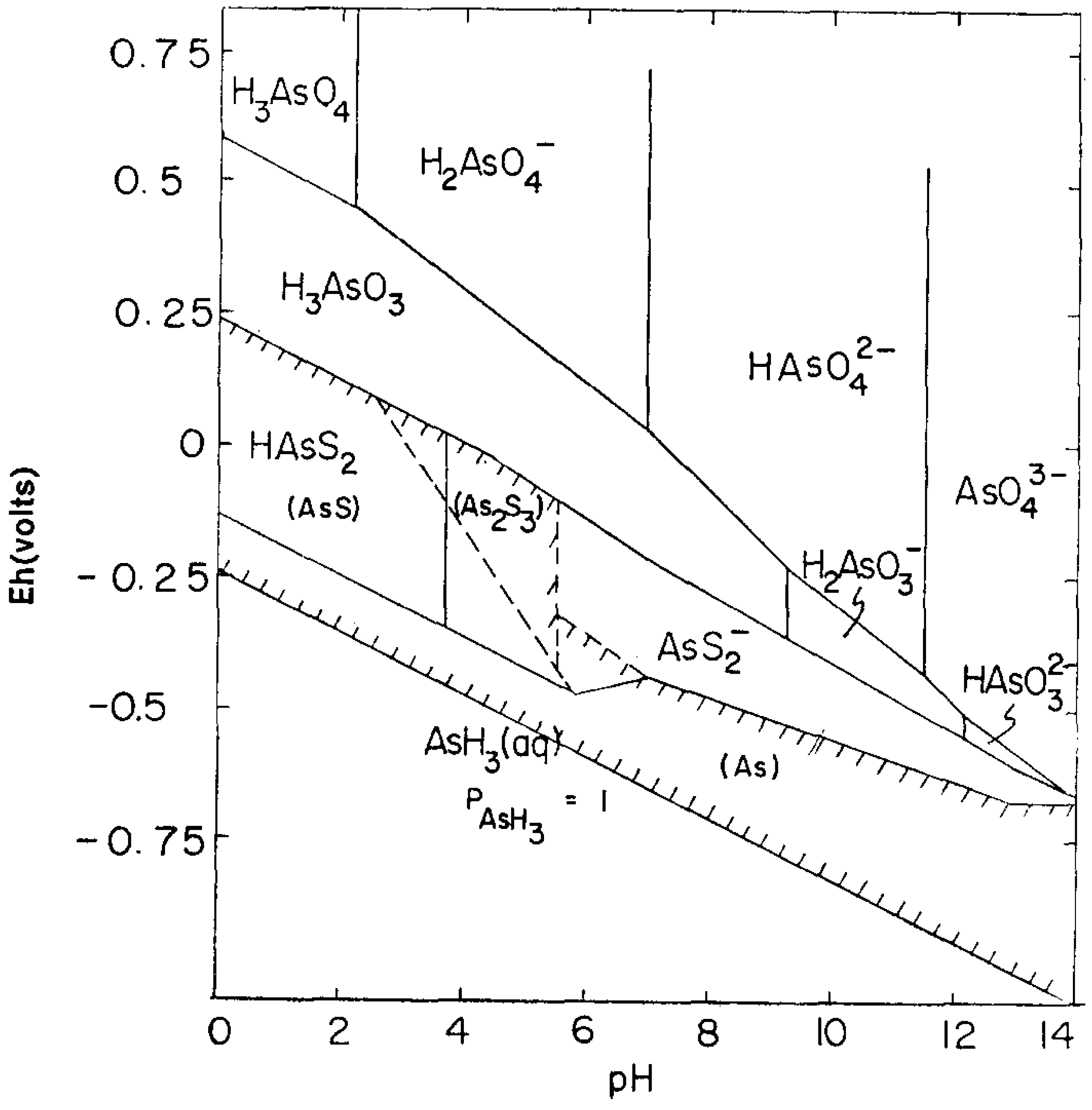
3.3. Thermodynamic Considerations
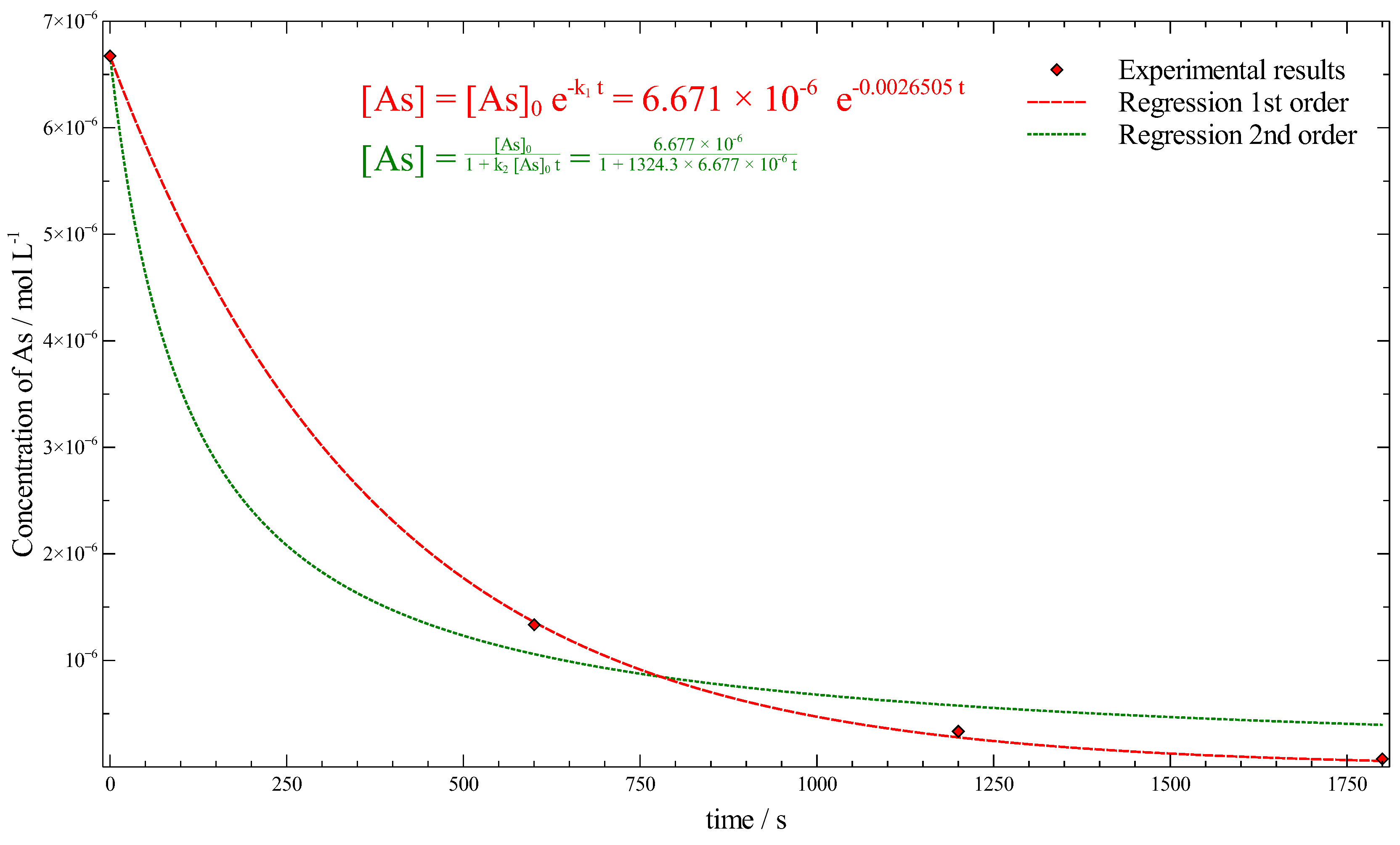
3.4. Kinetic Considerations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| NTP | Non-Thermal Plasma |
| ROS | Reactive Oxygen Species |
| UF | Ultrafiltration |
| WHO | World Health Organisation |
References
- World Health Organization (WHO); Public Health and Environment (Eds.) Exposure to Arsenic: A Major Public Health Concern; WHO Document Production Services: Geneva, Switzerland, 2010. [Google Scholar]
- Dekant, W.; Vamvakas, S. Toxikologie für Chemiker und Biologen, 1st ed.; Spektrum Lehrbuch, Spektrum Akad. Verl.: Heidelberg, Germany, 1995. [Google Scholar]
- Sen, M.; Manna, A.; Pal, P. Removal of arsenic from contaminated groundwater by membrane-integrated hybrid treatment system. J. Membr. Sci. 2010, 354, 108–113. [Google Scholar] [CrossRef]
- Guan, X.; Du, J.; Meng, X.; Sun, Y.; Sun, B.; Hu, Q. Application of titanium dioxide in arsenic removal from water: A review. J. Hazard. Mater. 2012, 215–216, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Jang, M.; Ibrahim, S.; Park, B.; Cho, E.; Khim, J. Arsenite removal using a pilot system of ultrasound and ultraviolet followed by microfiltration. Ultrason. Sonochem. 2014, 21, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Jekel, M.; Amy, G.L. Arsenic removal during drinking water treatment. In Interface Science in Drinking Water Treatment—Theory and Application; Elsevier: London, UK; Amsterdam, The Netherlands, 2006; Volume 10, pp. 193–206. [Google Scholar] [CrossRef]
- Cheng, Z.; van Geen, A.; Louis, R.; Nikolaidis, N.; Bailey, R. Removal of Methylated Arsenic in Groundwater with Iron Filings. Environ. Sci. Technol. 2005, 39, 7662–7666. [Google Scholar] [CrossRef] [PubMed]
- Leupin, O.X.; Hug, S.J. Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 2005, 39, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.J.; McDonald, K.J.; King, H. A novel arsenic removal process for water using cupric oxide nanoparticles. J. Colloid Interface Sci. 2013, 397, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Dureja, V.; Bhattacharyya, G.; Maity, S.; Bhattacharjee, S. Removal of arsenic from groundwater using low cost ferruginous manganese ore. Water Res. 2002, 36, 625–632. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.K.; Bajpai, J.; Bajpai, A.K.; Shrivastava, R.B. Iron crosslinked alginate as novel nanosorbents for removal of arsenic ions and bacteriological contamination from water. J. Mater. Res. Technol. 2014, 3, 195–202. [Google Scholar] [CrossRef]
- Mar, K.K.; Karnawati, D.; Sarto; Putra, D.; Igarashi, T.; Tabelin, C.B. Comparison of Arsenic Adsorption on Lignite, Bentonite, Shale, and Iron Sand from Indonesia. Procedia Earth Planet. Sci. 2013, 6, 242–250. [Google Scholar] [CrossRef]
- Mólgora, C.C.; Domínguez, A.M.; Avila, E.M.; Drogui, P.; Buelna, G. Removal of arsenic from drinking water: A comparative study between electrocoagulation-microfiltration and chemical coagulation-microfiltration processes. Sep. Purif. Technol. 2013, 118, 645–651. [Google Scholar] [CrossRef]
- Lohokare, H.R.; Muthu, M.R.; Agarwal, G.P.; Kharul, U.K. Effective arsenic removal using polyacrylonitrile-based ultrafiltration (UF) membrane. J. Membr. Sci. 2008, 320, 159–166. [Google Scholar] [CrossRef]
- Knoll, M.; Eichmeier, J.; Schön, R.; Hoegl, A.; Hock, A.; Schmeer, H. Properties, measurement, and bioclimatic action of “small” multimolecular atmospheric ions. In Advances in Electronics and Electron Physics; Elsevier: New York, NY, USA, 1964; Volume 19, pp. 177–254. [Google Scholar]
- Goldstein, N.I.; Goldstein, R.N.; Merzlyak, M.N. Negative air ions as a source of superoxide. Int. J. Biometeorol. 1992, 36, 118–122. [Google Scholar] [CrossRef]
- Challenger, O.; Braven, J.; Harwood, D.; Rosén, K.; Richardson, G. Negative air ionisation and the generation of hydrogen peroxide. Sci. Total Environ. 1996, 177, 215–219. [Google Scholar] [CrossRef]
- Guzmán, A.; Nava, J.L.; Coreño, O.; Rodríguez, I.; Gutiérrez, S. Arsenic and fluoride removal from groundwater by electrocoagulation using a continuous filter-press reactor. Chemosphere 2016, 144, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Hwang, Y.; Ji, M.; Jeon, B.H.; Kang, J.W. Ozone/membrane hybrid process for arsenic removal in iron-containing water. Desalin. Water Treat. 2011, 31, 138–143. [Google Scholar] [CrossRef]
- Back, J.O.; Obholzer, T.; Winkler, K.; Jabornig, S.; Rupprich, M. Combining ultrafiltration and non-thermal plasma for low energy degradation of pharmaceuticals from conventionally treated wastewater. J. Environ. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Yan, X.P.; Kerrich, R.; Hendry, M.J. Distribution of arsenic (III), arsenic (V) and total inorganic arsenic in porewaters from a thick till and clay-rich aquitard sequence, Saskatchewan, Canada. Geochim. Cosmochim. Acta 2000, 64, 2637–2648. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Gavis, J. A review of the arsenic cycle in natural waters. Water Res. 1972, 6, 1259–1274. [Google Scholar] [CrossRef]


| Set-Up | As(III) /g L | Coagulation |
|---|---|---|
| A1 | 100 | 10 ppm Fe3+ as FeCl3 |
| A2 | 500 | 10 ppm Fe3+ as FeCl3 |
| A3 | 100 | 10 ppm Al3+ as Al2(SO4)3 |
| A4 | 100 | 50 ppm Fe3+ as FeCl3 |
| A5 | 100 | 25 ppm Fe3+ as FeCl3 and 25 ppm Al3+ as Al2(SO4)3 |
| Set-Up (Oxidant) | Temp./C | /S cm | pH | As/g L | Turbidity/NTU | Color/Pt-Co | Step (2)/min | As Reduction/% | |
|---|---|---|---|---|---|---|---|---|---|
| A6 | B | 19.6 | 330 | 7.8 | 500 | 0.33 | 26 | 10 | |
| (air) | A | 20.6 | 371 | 7.1 | 100 | 0.45 | 16 | 80 | |
| B | 17.9 | 333 | 7.8 | 500 | 0.32 | 30 | 30 | ||
| A7 | A | 16.8 | 375 | 7.1 | 100 | 0.38 | 35 | 80 | |
| (air) | B | 15.9 | 329 | 8 | 500 | 0.26 | 23 | 30 | |
| A | 16.1 | 375 | 6.9 | 100 | 0.26 | 25 | 80 | ||
| B | 16.8 | 334 | 7.9 | 500 | 0.26 | 24 | 60 | ||
| A8 | A | 18.8 | 375 | 7.1 | 100 | 0.24 | 31 | 80 | |
| (air) | B | 18.0 | 328 | 7.8 | 500 | 0.29 | 35 | 60 | |
| A | 20.0 | 373 | 7.0 | 100 | 0.33 | 19 | 80 | ||
| B | 18.5 | 340 | 7.9 | 100 | 0.2 | 31 | 30 | ||
| A | 18.2 | 367 | 7.0 | 10 (11) | 0.38 | 28 | 89 | ||
| A9 | B | 19.2 | 339 | 7.9 | 100 | 0.26 | 28 | 30 | |
| (NTP) | A | 17.7 | 380 | 7.1 | <10 (12) | 0.26 | 28 | 88 | |
| B | 16.5 | 338 | 7.9 | 100 | 0.21 | 34 | 30 | ||
| A | 16.6 | 374 | 6.8 | <10 (10) | 0.2 | 41 | 90 | ||
| B | 16.4 | 324 | 7.7 | 500 | 0.51 | 31 | 10 | ||
| A | 17.5 | 367 | 7.0 | 50-100 | 0.18 | 34 | >80 | ||
| A10 | B | 16.8 | 324 | 7.9 | 500 | 0.4 | 20 | 10 | |
| (NTP) | A | 18.0 | 364 | 7.1 | 50–100 | 0.34 | 29 | >80 | |
| B | 17.2 | 322 | 7.9 | 500 | 0.24 | 35 | 10 | ||
| A | 17.3 | 363 | 7.0 | 50–100 | 0.28 | 22 | >80 | ||
| B | 18.8 | 331 | 7.9 | 500 | 0.65 | 22 | 20 | ||
| A | 17.9 | 370 | 7.0 | 10–25 | 0.48 | 30 | >95 | ||
| A11 | B | 17.4 | 374 | 7.8 | 500 | 0.77 | 39 | 20 | |
| (NTP) | A | 17.5 | 372 | 7.0 | 10–25 | 0.25 | 31 | >95 | |
| B | 17.5 | 339 | 7.9 | 500 | 0.45 | 30 | 20 | ||
| A | 17.5 | 365 | 6.8 | 25 | 0.74 | 43 | 95 | ||
| B | 18.9 | 335 | 7.9 | 500 | 0.51 | 26 | 30 | ||
| A | 15.7 | 413 | 7.1 | <10 (<5) | 0.31 | 36 | >98 | ||
| A12 | B | 17.0 | 329 | 7.9 | 500 | 0.69 | 36 | 30 | |
| (NTP) | A | 18.2 | 366 | 7.1 | <10 (6.8) | 0.56 | 50 | >98 | |
| B | 16.3 | 332 | 8.0 | 500 | 0.23 | 34 | 30 | ||
| A | 16.7 | 365 | 7.0 | <10 (<5) | 0.5 | 35 | >98 | ||
| B | 15.6 | 350 | 7.9 | 500 | 0.29 | 35 | 30 | ||
| A | 15.9 | 367 | 7.8 | <10 (11) | 0.39 | 38 | 97.5 | ||
| A13 | B | 19.2 | 338 | 7.7 | 500 | 0.23 | 36 | 30 | |
| (NTP) | A | 19.7 | 364 | 7.6 | 10 (11) | 0.25 | 40 | 97.5 | |
| B | 19.3 | 350 | 7.8 | 500 | 0.38 | 44 | 30 | ||
| A | 19.8 | 372 | 7.9 | <10 (11) | 0.22 | 41 | 97.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Back, J.O.; Stadlmayr, W.; Jabornig, S.; Winkler, F.; Winkler, K.; Rupprich, M. Removal of Arsenic from Water with Non-Thermal Plasma (NTP), Coagulation and Membrane Filtration. Water 2018, 10, 1385. https://doi.org/10.3390/w10101385
Back JO, Stadlmayr W, Jabornig S, Winkler F, Winkler K, Rupprich M. Removal of Arsenic from Water with Non-Thermal Plasma (NTP), Coagulation and Membrane Filtration. Water. 2018; 10(10):1385. https://doi.org/10.3390/w10101385
Chicago/Turabian StyleBack, Jan O., Werner Stadlmayr, Simon Jabornig, Florian Winkler, Karl Winkler, and Marco Rupprich. 2018. "Removal of Arsenic from Water with Non-Thermal Plasma (NTP), Coagulation and Membrane Filtration" Water 10, no. 10: 1385. https://doi.org/10.3390/w10101385





