Effects of Platelet Count on Blood Pressure: Evidence from Observational and Genetic Investigations
Abstract
:1. Introduction
Plain Language Summary
2. Methods
2.1. Observational Analysis
2.2. Mendelian Randomization Analysis
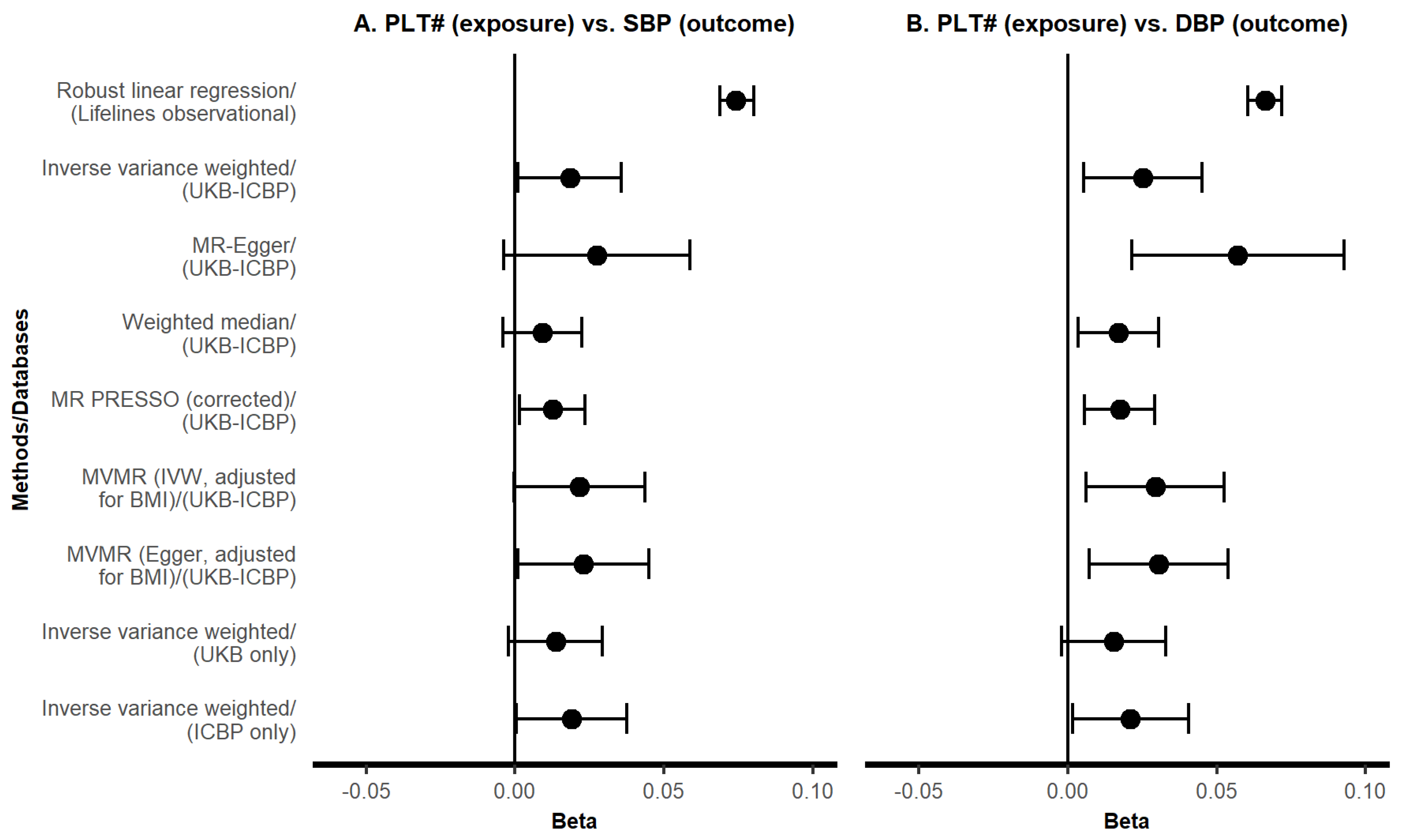
3. Results
3.1. Observational Results
3.2. MR Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. A Brief Description of the Lifelines Cohort
Appendix B. Description of the Variables used in the Lifelines Study
References
- Poulter, N.R.; Prabhakaran, D.; Caulfield, M. Hypertension. Lancet 2015, 386, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Zarbock, A.; Hidalgo, A. Platelets as autonomous drones for hemostatic and immune surveillance. J. Exp. Med. 2017, 214, 2193–2204. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Tao, L.; Mahara, G.; Yan, Y.; Cao, K.; Liu, X.; Chen, S.; Xu, Q.; Liu, L.; Wang, C.; et al. An association of platelet indices with blood pressure in Beijing adults: Applying quadratic inference function for a longitudinal study. Medicine 2016, 95, e4964. [Google Scholar] [CrossRef]
- Chiu, P.-C.; Chattopadhyay, A.; Wu, M.-C.; Hsiao, T.-H.; Lin, C.-H.; Lu, T.-P. Elucidation of a Causal Relationship Between Platelet Count and Hypertension: A Bi-Directional Mendelian Randomization Study. Front. Cardiovasc. Med. 2021, 8, 743075. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Burgess, S.; Wade, K.H.; Bowden, J.; Relton, C.; Smith, G.D. Best (but oft-forgotten) practices: The design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 2016, 103, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Tilling, K.; Davey Smith, G. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 2016, 45, 1866–1886. [Google Scholar] [CrossRef] [PubMed]
- UK Biobank GWAS Results. Available online: http://www.nealelab.is/uk-biobank (accessed on 1 December 2020).
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.L.; Dotinga, A.; Vonk, J.M.; Van Dijk, F.; Van Zon, S.K.R.; Wijmenga, C.; Wolffenbuttel, B.H.R.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015, 44, 1172–1180. [Google Scholar] [CrossRef]
- Sijtsma, A.; Rienks, J. Cohort Profile Update: Lifelines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2021, 51, e295–e302. [Google Scholar] [CrossRef]
- Shrier, I.; Platt, R.W. Reducing bias through directed acyclic graphs. BMC Med. Res. Methodol. 2008, 8, 70. [Google Scholar] [CrossRef]
- Pearl, J. An introduction to causal inference. Int. J. Biostat. 2010, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Smith, G.D. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Bell, S.C. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann. Am. Thorac. Soc. 2019, 16, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Gilbody, J.; Borges, M.C.; Smith, G.D.; Sanderson, E. Multivariable MR can mitigate bias in two-sample MR using covariable-adjusted summary associations. medRxiv 2022. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, Y. Platelet indices and blood pressure: A multivariable mendelian randomization study. Thromb. J. 2023, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- El Haouari, M.; Rosado, J.A. Platelet function in hypertension. Blood Cells Mol. Dis. 2009, 42, 38–43. [Google Scholar] [CrossRef]
- Krötz, F.; Sohn, H.Y.; Pohl, U. Reactive oxygen species: Players in the platelet game. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1988–1996. [Google Scholar] [CrossRef]
- Förstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflug. Arch. Eur. J. Physiol. 2010, 459, 923–939. [Google Scholar] [CrossRef]
- Hermann, M.; Flammer, A.; Lüscher, T.F. Nitric oxide in hypertension. J. Clin. Hypertens. 2006, 8, 17–29. [Google Scholar] [CrossRef]

| Level | Adult Data (n = 152, 728) | Final Sample with Complete Data (n = 110, 117) | ||
|---|---|---|---|---|
| Sociodemographic | Age, y | 44.00 [36.00, 52.00] | 45.00 [36.00, 52.00] | |
| Sex, n (%) | Female | 89,340 (58.5) | 65,200 (59.2) | |
| Male | 63,388 (41.5) | 44,917 (40.8) | ||
| Marital status, n (%) | In a relationship | 113,784 (85.1) | 81,834 (85.8) | |
| Not in a relationship | 19,918 (14.9) | 13,538 (14.2) | ||
| Education, n (%) | No college degree | 102,533 (68.6) | 72,426 (67.0) | |
| College degree or higher | 46,863 (31.4) | 35,642 (33.0) | ||
| Ethnicity, n (%) | European | 120,486 (98.0) | 91,322 (98.1) | |
| Non-European | 2481 (2.0) | 1805 (1.9) | ||
| Anthropometrics | Weight, kg | 78.00 [68.50, 89.00] | 78.00 [68.50, 89.00] | |
| Height, cm | 174.79 ± 9.43 | 174.85 ± 9.37 | ||
| BMI, kg/m2 | 25.40 [23.10, 28.30] | 25.40 [23.10, 28.20] | ||
| Lifestyle | Non-occupational MVPA, minutes/week | 185.00 [60.00, 365.00] | 186.00 [60.00, 370.00] | |
| Smoking, n (%) | Never smoker | 67,586 (46.2) | 51,469 (46.7) | |
| Ex-smoker | 48,319 (33.1) | 36,357 (33.0) | ||
| Current smoker | 30,264 (20.7) | 22,291 (20.2) | ||
| Blood biomarkers | Platelet count, 109/L | 245.00 [211.00, 282.00] | 244.00 [211.00, 282.00] | |
| eGFR, mL/min/1.73 m2 | 96.17 ± 15.07 | 95.94 ± 15.13 | ||
| HbA1c, mmol/mol | 37.00 [35.00, 39.00] | 37.00 [34.00,39.00] | ||
| Outcomes | SBP, mm Hg | 125.00 [115.00, 137.00] | 124.00 [115.00, 136.00] | |
| DBP, mm Hg | 74.00 [67.00, 81.00] | 73.00 [67.00, 81.00] | ||
| Hypertension, n (%) | No | 111,701 (73.8) | 82,259 (74.7) | |
| Yes | 39,646 (26.2) | 27,858 (25.3) |
| Logistic Regression (HTN as Outcome) | Robust Linear Regression (SBP as Outcome) | Robust Linear Regression (DBP as Outcome) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | ||||||||||
| Adjustments | OR * | Lower | Upper | p | β # | Lower | Upper | p | β # | Lower | Upper | p |
| PLT# as exposure (n = 110, 117) | ||||||||||||
| Crude | 0.977 | 0.963 | 0.990 | 0.001 | −0.019 | −0.025 | −0.013 | <0.001 | −0.020 | −0.026 | −0.014 | <0.001 |
| Age + gender | 1.147 | 1.129 | 1.165 | <0.001 | 0.085 | 0.079 | 0.091 | <0.001 | 0.073 | 0.067 | 0.078 | <0.001 |
| MSAS | 1.117 | 1.099 | 1.135 | <0.001 | 0.074 | 0.069 | 0.080 | <0.001 | 0.066 | 0.060 | 0.072 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Chen, Z.; de Borst, M.H.; Zhang, Q.; Snieder, H.; Thio, C.H.L.; on behalf of the International Consortium of Blood Pressure. Effects of Platelet Count on Blood Pressure: Evidence from Observational and Genetic Investigations. Genes 2023, 14, 2233. https://doi.org/10.3390/genes14122233
He Z, Chen Z, de Borst MH, Zhang Q, Snieder H, Thio CHL, on behalf of the International Consortium of Blood Pressure. Effects of Platelet Count on Blood Pressure: Evidence from Observational and Genetic Investigations. Genes. 2023; 14(12):2233. https://doi.org/10.3390/genes14122233
Chicago/Turabian StyleHe, Zhen, Zekai Chen, Martin H. de Borst, Qingying Zhang, Harold Snieder, Chris H. L. Thio, and on behalf of the International Consortium of Blood Pressure. 2023. "Effects of Platelet Count on Blood Pressure: Evidence from Observational and Genetic Investigations" Genes 14, no. 12: 2233. https://doi.org/10.3390/genes14122233





