Can ELISPOT Be Applied to A Clinical Setting as A Diagnostic Utility for Neuroborreliosis?
Abstract
:1. Introduction
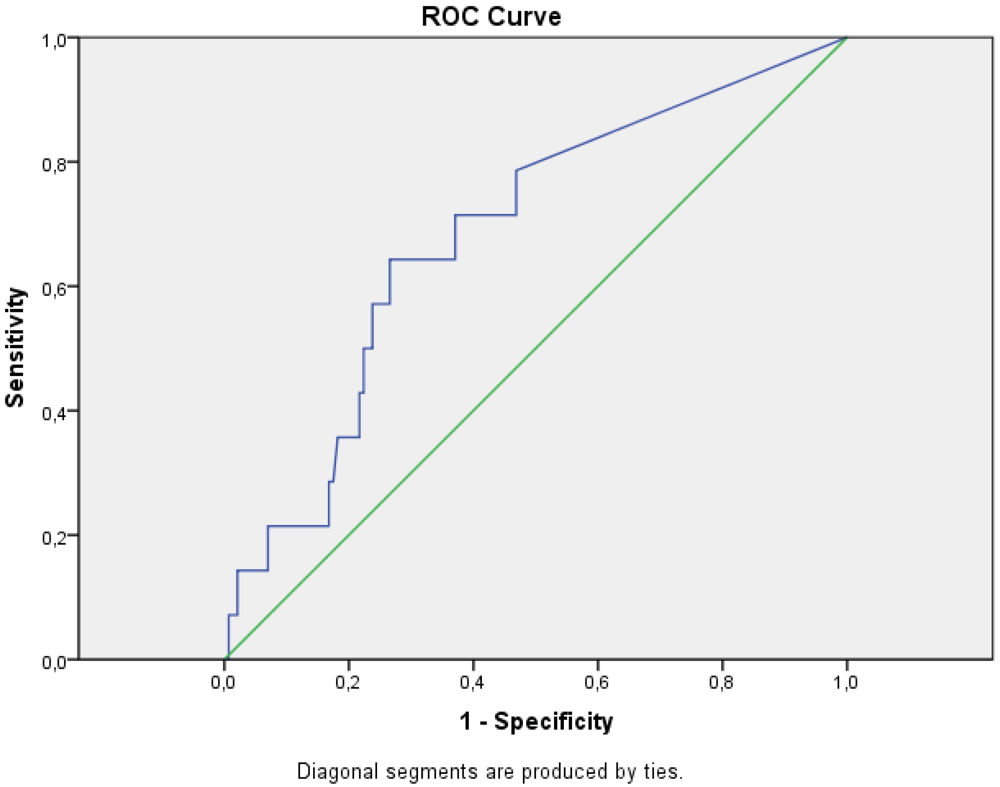
2. Results and Discussion
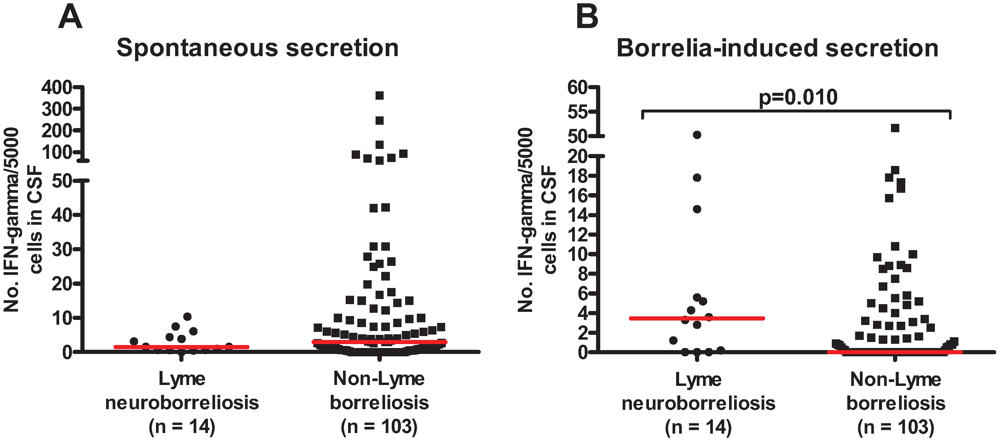
| Diagnostic groups | ELISPOT cut-off ≥5 spots | ELISPOT cut-off ≥10 spots | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Lyme neuroborreliosis (n = 14) | 5 (36%) | 9 (64%) | 3 (21%) | 11 (79%) |
| Non-Lyme borreliosis (n = 103) | 19 (18%) | 84 (82%) | 8 (8%) | 95 (92%) |
| Diagnostic groups Non-Lyme neuroborreliosis | Number (n = 19) |
| Pain/myalgia/arthralgia/fatigue | n = 6 |
| Other infection Pyelonephritis Unspecific infection Tendinitis Tooth infection | n = 4 |
| Other CNS disease Alzheimer’s disease Neuropathy Sensibility disorder | n = 3 |
| Autoimmune Collagenosis Rheumatoid arthritis | n = 2 |
| Inflammatory CNS disease Tick-borne encephalitis | n = 2 |
| Endocrine Hypothyroidism | n = 1 |
| Lymphoma | n = 1 |
| (A) | |
| Diagnostic groups Non-Lyme neuroborreliosis | Number (n = 23) |
| Pain/myalgia/arthralgia/fatigue | n = 8 |
| Autoimmune Mb Reiter Collagenosis Rheumatoid arthritis | n = 4 |
| Other CNS disease Epilepsy Neuropathy Sensibility disorder | n = 4 |
| Other infection Pyelonephritis Tooth infection Herpes keratitis | n = 3 |
| Inflammatory CNS disease Tick-borne encephalitis | n = 2 |
| Lymphoma | n = 1 |
| Unknown diagnosis | n = 1 |
| (B) | |
3. Experimental Section
3.1. Material
3.1.1. Patients
| Confirmed | Probable |
|---|---|
|
|
3.2. Methods
| No | Sex F/M | Age Years | Symptoms on admission | Seroconversion/increasing titers of serum Bb-antibodies | Intrathecal Bb-antibody production | CSF pleocytosis≥5 MNC/µL’ | BBB-damage In CSF | Duration of symptoms before treatment | Time to recovery after treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 76 | Facial nerve palsy, fatigue, | + | + | 7 | Pos | 1 day | <4 months |
| Pain (back, abdomen) | + | ||||||||
| 2 | M | 57 | Facial nerve palsy, radiculitis | + | + | 85 | Pos | 1 day | <4 months |
| 3 | F | 72 | Meningoradiculitis | + | + | 31 | Neg | Missing data | Missing data |
| 4 | M | 77 | Pain (leg, scapula), | + | + | 6 | Pos | 2 years | 1 year |
| Cognitive disturbances | + | ||||||||
| 5 | F | 69 | Facial nerve palsy, pain | + | + | 126 | Pos | 1 day | 2 weeks |
| 6 | M | 87 | Fatigue, radiculitis, pain (leg) | + | + | 19 | Neg | Missing data | 2 weeks |
| 7 | F | 59 | Headache, paresis, dysarthria | + | + | 31 | Neg | 2 weeks | 3 months |
| 8 | M | 58 | Pain (leg) | + | + | 228 | Pos | 3.5 months | 2 weeks |
| 9 | F | 51 | Pain (knees, hips) | + | + | 74 | Pos | 2 weeks | 3 months |
| 10 | M | 12 | Facial nerve palsy | + | + | 350 | Neg | 1 day | 2.5 months |
| 11 | F | 6 | Facial nerve palsy | + | + | 300 | Pos | 5 days | 2 weeks |
| 12 | F | 6 | Facial nerve palsy | + | + | 168 | Pos | 9 days | 2 weeks |
| 13 | F | 66 | Facial nerve palsy | + | + | 132 | Pos | 1 day | 11 days |
| 14 | F | 58 | Pain (abdomen) | + | + | 45 | Pos | Missing data | Missing data |
3.2.1. Enumeration of Borrelia-Induced IFN-Gamma Secreting Cells
3.2.2. Data handling and Statistics
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Stanek, G.; Fingerle, V.; Hunfeld, K.P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in europe. Clin. Microbiol. Infect. 2011, 17, 697–699. [Google Scholar] [CrossRef]
- Stanek, G.; Strle, F. Lyme borreliosis. Lancet 2003, 362, 1639–1647. [Google Scholar] [CrossRef]
- Mygland, A.; Ljostad, U.; Fingerle, V.; Rupprecht, T.; Schmutzhard, E.; Steiner, I. Efns guidelines on the diagnosis and management of european lyme neuroborreliosis. Eur. J. Neurol. 2010, 17, e11–e14. [Google Scholar] [CrossRef]
- Ljostad, U.; Skarpaas, T.; Mygland, A. Clinical usefulness of intrathecal antibody testing in acute lyme neuroborreliosis. Eur. J. Neurol. 2007, 14, 873–876. [Google Scholar] [CrossRef]
- Wilske, B. Epidemiology and diagnosis of lyme borreliosis. Ann. Med. 2005, 37, 568–579. [Google Scholar] [CrossRef]
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.J.; Bjoersdorff, A.; Blanco, J.R.; Caruso, G.; Cinco, M.; Fournier, P.E.; Francavilla, E.; et al. Guidelines for the diagnosis of tick-borne bacterial diseases in europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132. [Google Scholar] [CrossRef]
- Aguero-Rosenfeld, M.E.; Wang, G.; Schwartz, I.; Wormser, G.P. Diagnosis of lyme borreliosis. Clin. Microbiol. Rev. 2005, 18, 484–509. [Google Scholar] [CrossRef]
- Hammers-Berggren, S.; Hansen, K.; Lebech, A.M.; Karlsson, M. Borrelia burgdorferi-specific intrathecal antibody production in neuroborreliosis: A follow-up study. Neurology 1993, 43, 169–175. [Google Scholar] [CrossRef]
- Kruger, H.; Reuss, K.; Pulz, M.; Rohrbach, E.; Pflughaupt, K.W.; Martin, R.; Mertens, H.G. Meningoradiculitis and encephalomyelitis due to borrelia burgdorferi: A follow-up study of 72 patients over 27 years. J. Neurol. 1989, 236, 322–328. [Google Scholar] [CrossRef]
- Cerar, T.; Ogrinc, K.; Strle, F.; Ruzic-Sabljic, E. Humoral immune responses in patients with lyme neuroborreliosis. Clin. Vaccine Immunol. 2010, 17, 645–650. [Google Scholar] [CrossRef]
- Coyle, P.K.; Schutzer, S.E.; Deng, Z.; Krupp, L.B.; Belman, A.L.; Benach, J.L.; Luft, B.J. Detection of borrelia burgdorferi-specific antigen in antibody-negative cerebrospinal fluid in neurologic lyme disease. Neurology 1995, 45, 2010–2015. [Google Scholar]
- Lawrence, C.; Lipton, R.B.; Lowy, F.D.; Coyle, P.K. Seronegative chronic relapsing neuroborreliosis. Eur. Neurol. 1995, 35, 113–117. [Google Scholar] [CrossRef]
- Oksi, J.; Uksila, J.; Marjamaki, M.; Nikoskelainen, J.; Viljanen, M.K. Antibodies against whole sonicated borrelia burgdorferi spirochetes, 41-kilodalton flagellin, and p39 protein in patients with pcr- or culture-proven late lyme borreliosis. J. Clin. Microbiol. 1995, 33, 2260–2264. [Google Scholar]
- Schutzer, S.E.; Coyle, P.K.; Belman, A.L.; Golightly, M.G.; Drulle, J. Sequestration of antibody to borrelia burgdorferi in immune complexes in seronegative lyme disease. Lancet 1990, 335, 312–315. [Google Scholar]
- Dattwyler, R.J.; Volkman, D.J.; Luft, B.J.; Halperin, J.J.; Thomas, J.; Golightly, M.G. Seronegative lyme disease. Dissociation of specific t- and b-lymphocyte responses to borrelia burgdorferi. New Engl. J. Med. 1988, 319, 1441–1446. [Google Scholar] [CrossRef]
- Wilske, B.; Fingerle, V.; Schulte-Spechtel, U. Microbiological and serological diagnosis of lyme borreliosis. FEMS Immunol. Med. Microbiol. 2007, 49, 13–21. [Google Scholar] [CrossRef]
- Seriburi, V.; Ndukwe, N.; Chang, Z.; Cox, M.E.; Wormser, G.P. High frequency of false positive igm immunoblots for borrelia burgdorferi in clinical practic. Clin. Microbiol. Infect. 2011. [Google Scholar] [CrossRef]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the infectious diseases society of america. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar]
- Wilske, B. Diagnosis of lyme borreliosis in europe. Vector Borne Zoonotic Dis. 2003, 3, 215–227. [Google Scholar] [CrossRef]
- Blanc, F.; Jaulhac, B.; Fleury, M.; de Seze, J.; de Martino, S.J.; Remy, V.; Blaison, G.; Hansmann, Y.; Christmann, D.; Tranchant, C. Relevance of the antibody index to diagnose lyme neuroborreliosis among seropositive patients. Neurology 2007, 69, 953–958. [Google Scholar] [CrossRef]
- Hansen, K.; Cruz, M.; Link, H. Oligoclonal borrelia burgdorferi-specific igg antibodies in cerebrospinal fluid in lyme neuroborreliosis. J. Infect. Dis. 1990, 161, 1194–1202. [Google Scholar] [CrossRef]
- Steere, A.C.; Berardi, V.P.; Weeks, K.E.; Logigian, E.L.; Ackermann, R. Evaluation of the intrathecal antibody response to borrelia burgdorferi as a diagnostic test for lyme neuroborreliosis. J. Infect. Dis. 1990, 161, 1203–1209. [Google Scholar] [CrossRef]
- Stiernstedt, G.T.; Granstrom, M.; Hederstedt, B.; Skoldenberg, B. Diagnosis of spirochetal meningitis by enzyme-linked immunosorbent assay and indirect immunofluorescence assay in serum and cerebrospinal fluid. J. Clin. Microbiol. 1985, 21, 819–825. [Google Scholar]
- Picha, D.; Moravcova, L.; Zdarsky, E.; Benes, J. Clinical comparison of immunoblot and antibody index for detection of intrathecal synthesis of specific antibodies in lyme neuroborreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 805–806. [Google Scholar] [CrossRef]
- Tumani, H.; Nolker, G.; Reiber, H. Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology 1995, 45, 1663–1670. [Google Scholar]
- Aguero-Rosenfeld, M.E. Lyme disease: Laboratory issues. Infect. Dis. Clin. North Am. 2008, 22, 301–313. [Google Scholar] [CrossRef]
- Tjernberg, I.; Kruger, G.; Eliasson, I. C6 peptide elisa test in the serodiagnosis of lyme borreliosis in sweden. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 37–42. [Google Scholar] [CrossRef]
- Dressler, F.; Yoshinari, N.H.; Steere, A.C. The t-cell proliferative assay in the diagnosis of lyme disease. Ann. Intern. Med. 1991, 115, 533–539. [Google Scholar]
- Ekerfelt, C.; Ernerudh, J.; Forsberg, P.; Bergström, S. Augmented intrathecal secretion of interferon-γ in response to borrelia garinii in neuroborreliosis. J. Neuroimmunol. 1998, 89, 177–181. [Google Scholar] [CrossRef]
- Forsberg, P.; Ernerudh, J.; Ekerfelt, C.; Roberg, M.; Vrethem, M.; Bergstrom, S. The outer surface proteins of lyme disease borrelia spirochetes stimulate t cells to secrete interferon-gamma (ifn-gamma): Diagnostic and pathogenic implications. Clin. Exp. Immunol. 1995, 101, 453–460. [Google Scholar]
- Oksi, J.; Savolainen, J.; Pène, J.; Bòusquet, J.; Laippala, P.; Viljanen, M.K. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with lyme borreliosis. Infect. Immun. 1996, 64, 3620–3623. [Google Scholar]
- Widhe, M.; Ekerfelt, C.; Forsberg, P.; Bergström, S.; Ernerudh, J. Igg subclasses in lyme borreliosis: A study of specific igg subclass distribution in an ifn-γ predominated disease. Scand. J. Immunol. 1998, 47, 575–581. [Google Scholar]
- Widhe, M.; Jarefors, S.; Ekerfelt, C.; Vrethem, M.; Bergström, S.; Forsberg, P.; Ernerudh, J. Borrelia specific ifn-g and il-4 secretion in blood and csf during the course of human lyme borreliosis: Relation to clinical outcome. J. Infect. Dis. 2004, 189, 1881–1891. [Google Scholar] [CrossRef]
- Yin, Z.; Braun, J.; Neure, L.; Wu, P.; Eggens, U.; Krause, A.; Kamradt, T.; Sieper, J. T cell cytokine pattern in the joints of patients with lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997, 40, 69–79. [Google Scholar] [CrossRef]
- Ekerfelt, C.; Ernerudh, J.; Bunikis, J.; Vrethem, M.; Aagesen, J.; Roberg, M.; Bergstrom, S.; Forsberg, P. Compartmentalization of antigen specific cytokine responses to the central nervous system in cns borreliosis: Secretion of ifn-gamma predominates over il-4 secretion in response to outer surface proteins of lyme disease borrelia spirochetes. J. Neuroimmunol. 1997, 79, 155–162. [Google Scholar] [CrossRef]
- Granström, M. Borrelios/lyme disease—Serologi/diagnostik. Inf. från Läkemedelsverket 1998, 9, 98. [Google Scholar]
- Gross, D.M.; Steere, A.C.; Huber, B.T. T helper 1 response is dominant and localized to the synovial fluid in patients with lyme arthritis. J. Immunol. 1998, 160, 1022–1028. [Google Scholar]
- Grusell, M.; Widhe, M.; Ekerfelt, C. Increased expression of the th1-inducing cytokines interleukin-12 and interleukin-18 in cerebrospinal fluid but not in sera from patients with lyme neuroborreliosis. J. Neuroimmunol. 2002, 131, 173–178. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Fredriksson, S.; Sun, J.-B.; Link, H. Lyme neuroborreliosis: Evidence for persistent up-regulation of borrelia burgdorferi-reactive cells secreting interferon-γ. Scand. J. Immunol. 1995, 42, 694–700. [Google Scholar] [CrossRef]
- Lange, C.; Mori, T. Advances in the diagnosis of tuberculosis. Respirology 2010, 15, 220–240. [Google Scholar] [CrossRef]
- Diel, R.; Goletti, D.; Ferrara, G.; Bothamley, G.; Cirillo, D.; Kampmann, B.; Lange, C.; Losi, M.; Markova, R.; Migliori, G.B.; et al. Interferon-gamma release assays for the diagnosis of latent mycobacterium tuberculosis infection: A systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 88–99. [Google Scholar] [CrossRef]
- Finland’s National institute for health and welfare, R.o.D., Statistical Database. Available online: http://www3.ktl.fi/stat/ (accessed on 9 May 2012).
- Widhe, M.; Skogman, B.H.; Jarefors, S.; Eknefelt, M.; Enestrom, G.; Nordwall, M.; Ekerfelt, C.; Croner, S.; Bergstrom, S.; Forsberg, P.; et al. Up-regulation of borrelia-specific il-4- and ifn-gamma-secreting cells in cerebrospinal fluid from children with lyme neuroborreliosis. Int. Immunol. 2005, 17, 1283–1291. [Google Scholar] [CrossRef]
- Ekerfelt, C.; Masreliez, C.; Svenvik, M.; Ernerudh, J.; Roberg, M.; Forsberg, P. Antibodies and t-cell reactivity to borrelia burgdorferi in an asymptomatic population: A study of healthy blood donors in an inland town district in the south-east of sweden. Scand. J. Infect. Dis. 2001, 33, 806–808. [Google Scholar]
- Berglund, J.; Eitrem, R.; Ornstein, K.; Lindberg, A.; Ringer, A.; Elmrud, H.; Carlsson, M.; Runehagen, A.; Svanborg, C.; Norrby, R. An epidemiologic study of lyme disease in southern sweden. New Engl. J. Med. 1995, 333, 1319–1327. [Google Scholar] [CrossRef]
- Kalish, R.A.; McHugh, G.; Granquist, J.; Shea, B.; Ruthazer, R.; Steere, A.C. Persistence of immunoglobulin m or immunoglobulin g antibody responses to borrelia burgdorferi 10–20 years after active lyme disease. Clin. Infect. Dis. 2001, 33, 780–785. [Google Scholar] [CrossRef]
- Bergstrom, S.; Sjostedt, A.; Dotevall, L.; Kaijser, B.; Ekstrand-Hammarstrom, B.; Wallberg, C.; Skogman, G.; Barbour, A.G. Diagnosis of lyme borreliosis by an enzyme immunoassay detecting immunoglobulin g reactive to purified borrelia burgdorferi cell components. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 422–427. [Google Scholar] [CrossRef]
- Ekerfelt, C.; Ernerudh, J.; Forsberg, P.; Bergstrom, S. Augmented intrathecal secretion of interferon-gamma in response to borrelia garinii in neuroborreliosis. J. Neuroimmunol. 1998, 89, 177–181. [Google Scholar] [CrossRef]
- Widhe, M.; Jarefors, S.; Ekerfelt, C.; Vrethem, M.; Bergstrom, S.; Forsberg, P.; Ernerudh, J. Borrelia-specific interferon-gamma and interleukin-4 secretion in cerebrospinal fluid and blood during lyme borreliosis in humans: Association with clinical outcome. J. Infect. Dis. 2004, 189, 1881–1891. [Google Scholar]
- Widhe, M.; Ekerfelt, C.; Jarefors, S.; Skogman, B.H.; Peterson, E.M.; Bergstrom, S.; Forsberg, P.; Ernerudh, J. T-cell epitope mapping of the borrelia garinii outer surface protein a in lyme neuroborreliosis. Scand. J. Immunol. 2009, 70, 141–148. [Google Scholar] [CrossRef]
- Reiber, H. Flow rate of cerebrospinal fluid (csf)—A concept common to normal blood-csf barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 1994, 122, 189–203. [Google Scholar] [CrossRef]
- Nyman, D.; Wahlberg, P.; Carlsson, S.-A.; Granlund, H.; Jansson, C.; Johansson, C.; Nyberg, C. Lyme borreliosis -praktiska riktlinjer för diagnos och behandling. Finl. Läkartidning 2006, 61, 3219. [Google Scholar]
- Griner, P.F.; Mayewski, R.J.; Mushlin, A.I.; Greenland, P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann. Intern. Med. 1981, 94, 557–592. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nordberg, M.; Forsberg, P.; Nyman, D.; Skogman, B.H.; Nyberg, C.; Ernerudh, J.; Eliasson, I.; Ekerfelt, C. Can ELISPOT Be Applied to A Clinical Setting as A Diagnostic Utility for Neuroborreliosis? Cells 2012, 1, 153-167. https://doi.org/10.3390/cells1020153
Nordberg M, Forsberg P, Nyman D, Skogman BH, Nyberg C, Ernerudh J, Eliasson I, Ekerfelt C. Can ELISPOT Be Applied to A Clinical Setting as A Diagnostic Utility for Neuroborreliosis? Cells. 2012; 1(2):153-167. https://doi.org/10.3390/cells1020153
Chicago/Turabian StyleNordberg, Marika, Pia Forsberg, Dag Nyman, Barbro H. Skogman, Clara Nyberg, Jan Ernerudh, Ingvar Eliasson, and Christina Ekerfelt. 2012. "Can ELISPOT Be Applied to A Clinical Setting as A Diagnostic Utility for Neuroborreliosis?" Cells 1, no. 2: 153-167. https://doi.org/10.3390/cells1020153
APA StyleNordberg, M., Forsberg, P., Nyman, D., Skogman, B. H., Nyberg, C., Ernerudh, J., Eliasson, I., & Ekerfelt, C. (2012). Can ELISPOT Be Applied to A Clinical Setting as A Diagnostic Utility for Neuroborreliosis? Cells, 1(2), 153-167. https://doi.org/10.3390/cells1020153




