Rhodococcus erythropolis and Its γ-Lactone Catabolic Pathway: An Unusual Biocontrol System That Disrupts Pathogen Quorum Sensing Communication
Abstract
:1. Introduction
2. Biocontrol Activity: Rhodococcal Quorum Quenching vs. Pectobacterial Quorum Sensing
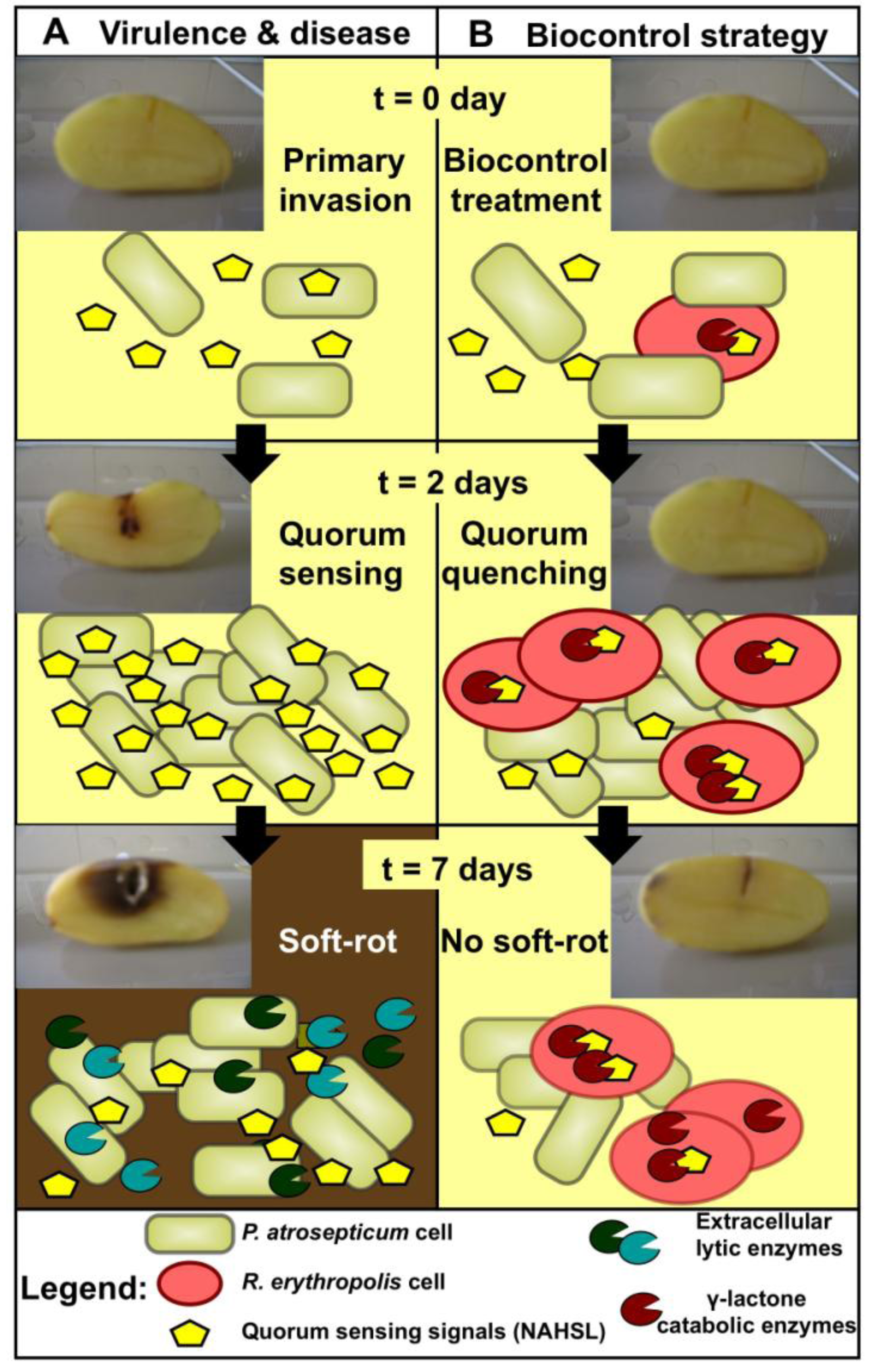
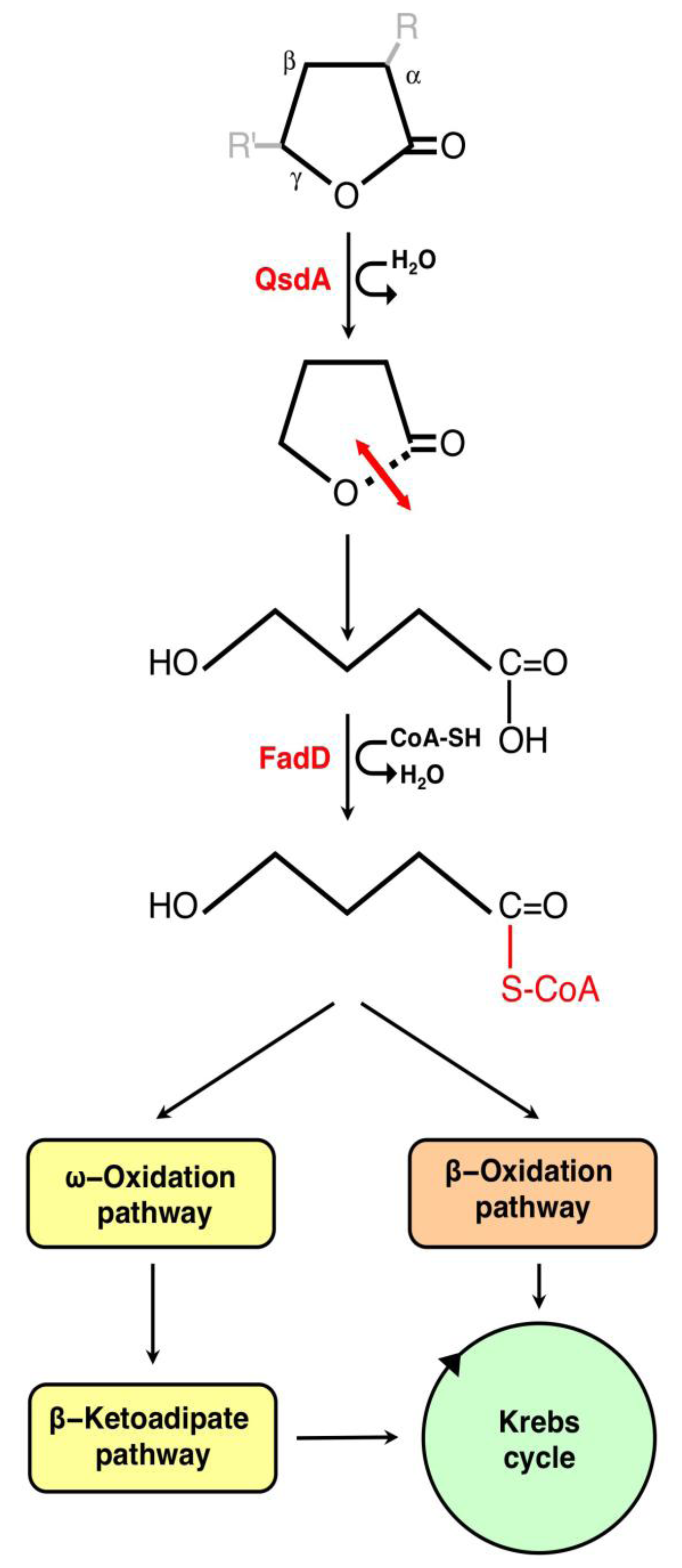
3. Catabolic Pathways of R. erythropolis Involved in Biocontrol
3.1. The γ-Lactone Catabolic Pathway
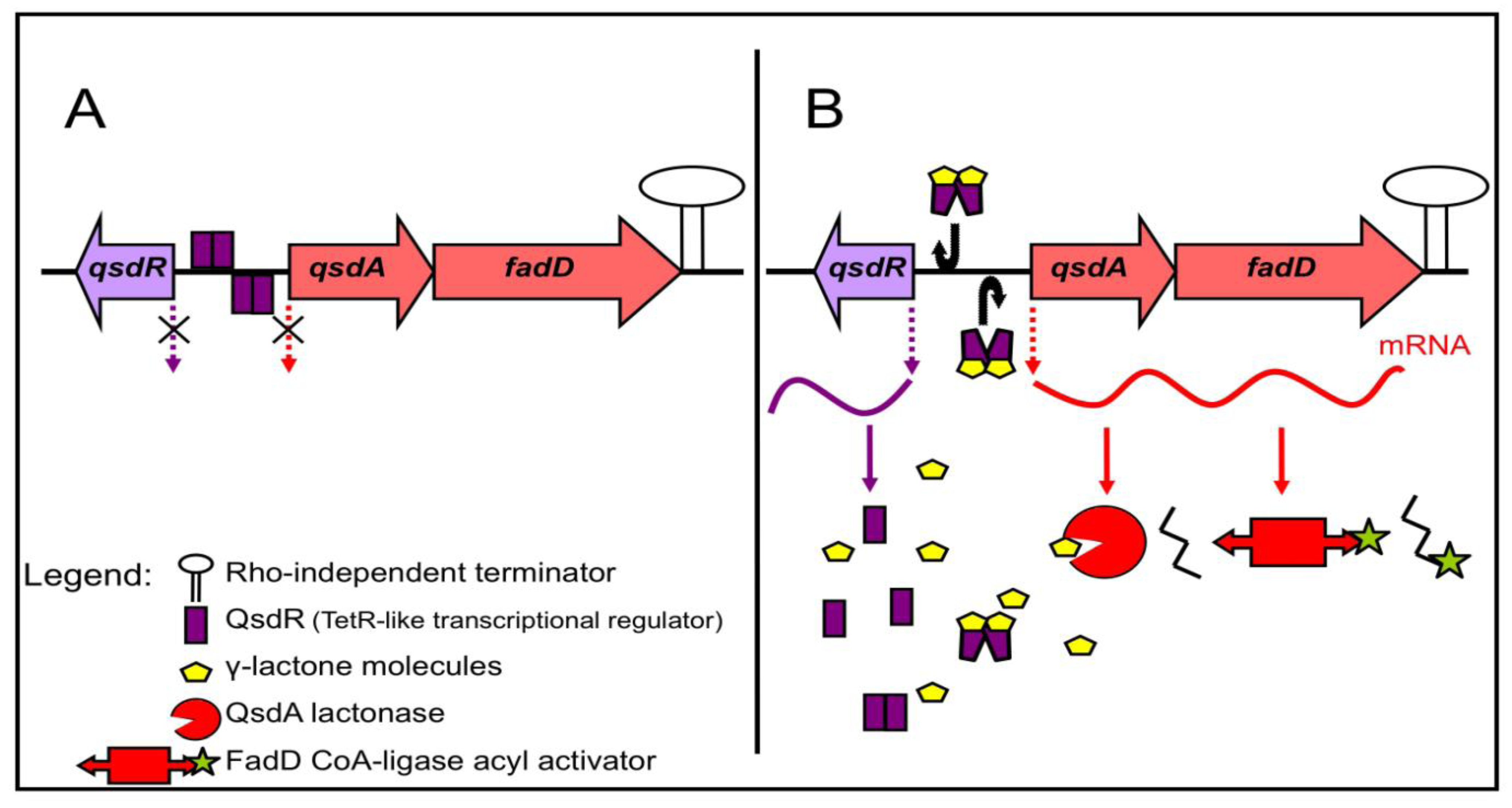
3.2. Regulation of the γ-Lactone Catabolic Pathway
3.3. Roles of the γ-Lactone Catabolic Pathway and Regulation of Rhodococcal Communication
3.4. Other Biocontrol Pathways in R. erythropolis
4. Improving Biocontrol Activity
4.1. Stimulation of Lactonase Activity
4.2. Stimulation of R. erythropolis Fitness
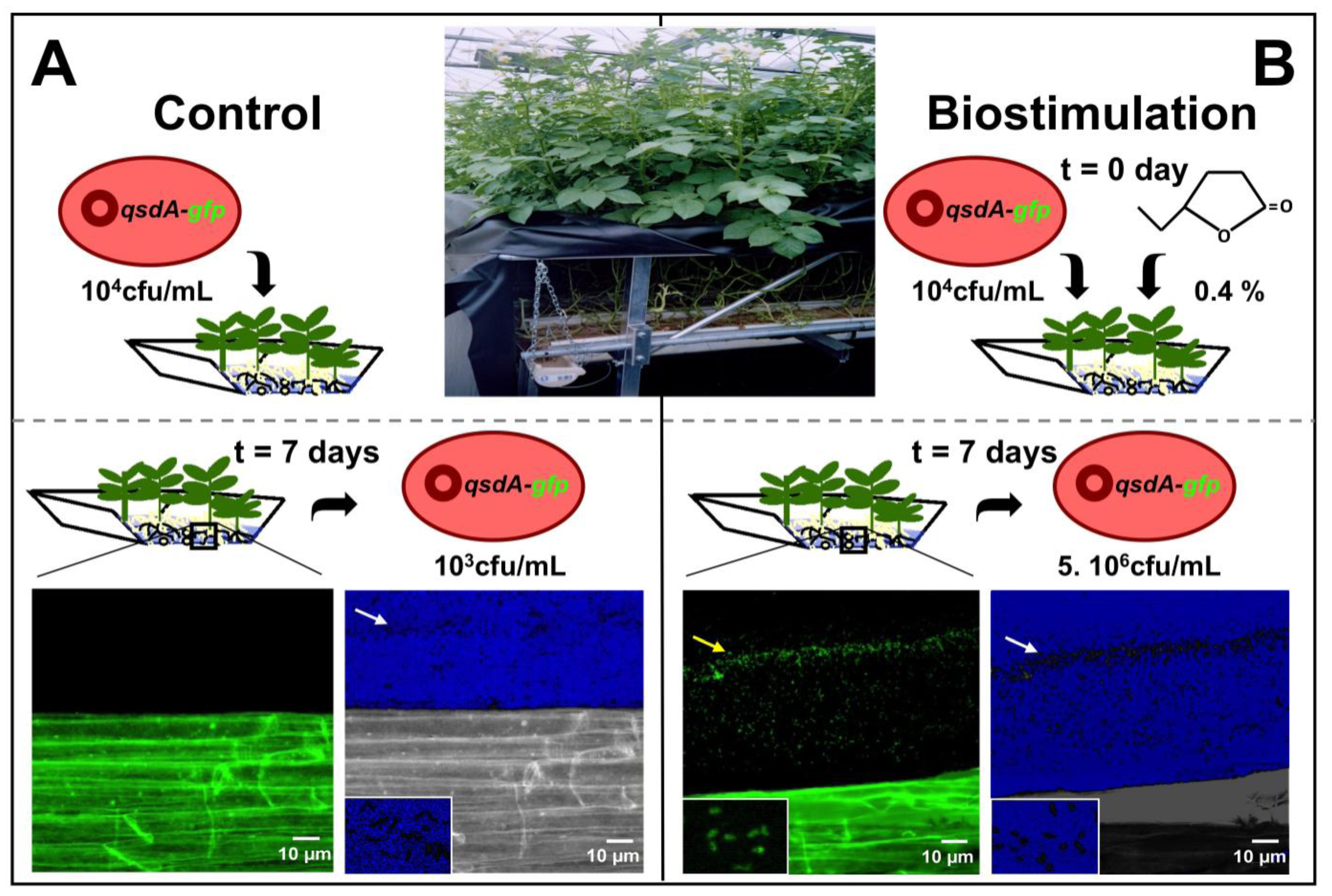
4.3. Formulations Incorporating R. erythropolis strains
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Von Bodman, S.B.; Bauer, W.D.; Coplin, D.L. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2003, 41, 455–482. [Google Scholar] [CrossRef]
- Cha, C.; Gao, P.; Chen, Y.C.; Shaw, P.; Farrand, S.K. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 1998, 11, 1119–1129. [Google Scholar] [CrossRef]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- Fray, R.G.; Throup, J.P.; Daykin, M.; Wallace, A.; Williams, P.; Stewart, G.S.; Grierson, D. Plants genetically modified to produce N-acylhomoserine lactones communicate with bacteria. Nat. Biotechnol. 1999, 17, 1017–1120. [Google Scholar] [CrossRef]
- Maë, A.; Montesano, M.; Koiv, V.; Palva, E.T. Transgenic plants producing the bacterial pheromone N-acylhomoserine lactone exhibit enhanced resistance to the bacterial phyto-pathogen Erwinia carotovora. Mol. Plant-Microbe Interact. 2001, 14, 1035–1042. [Google Scholar] [CrossRef]
- Barnard, A.M.; Salmond, G.P. Quorum sensing in Erwinia species. Anal. Bioanal. Chem. 2007, 387, 415–423. [Google Scholar] [CrossRef]
- Charkowsky, A.O. The Soft Rot Erwinia. In Plant-Associated Bacteria; Gnanamanickam, S., Ed.; Springer: Dordrecht, the Netherlands, 2006; pp. 423–505. [Google Scholar]
- Charkowski, A.; Blanco, C.; Condemine, G.; Expert, D.; Franza, T.; Hayes, C.; Hugouvieux-Cotte-Pattat, N.; López Solanilla, E.; Low, D.; Moleleki, L.; Pirhonen, M.; Pitman, A.; Perna, N.; Reverchon, S.; Rodríguez Palenzuela, P.; San Francisco, M.; Toth, I.; Tsuyumu, S.; van der Waals, J.; van der Wolf, J.; Van Gijsegem, F.; Yang, C.H.; Yedidia, I. The role of secretion systems and small molecules in soft-rot enterobacteriaceae pathogenicity. Annu. Rev. Phytopathol. 2012, 50, 425–449. [Google Scholar] [CrossRef]
- Crépin, A.; Barbey, C.; Cirou, A.; Tannières, M.; Orange, N.; Feuilloley, M.; Dessaux, Y.; Burini, J.F.; Faure, D.; Latour, X. Biological control of pathogen communication in the rhizosphere: A novel approach applied to potato soft rot due to Pectobacterium atrosepticum. Plant Soil 2012, 358, 27–37. [Google Scholar] [CrossRef]
- Czajkowski, R.; Jafra, S. Quenching of acyl-homoserine lactone-dependent quorum-sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 2009, 56, 1–16. [Google Scholar]
- Czajkowski, R.; Pérombelon, M.C.M.; van Veen, J.A.; van der Wolf, J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Põllumaa, L.; Alamäe, T.; Mäe, A. Quorum sensing and expression of virulence in pectobacteria. Sensors 2012, 12, 3327–3349. [Google Scholar] [CrossRef]
- Smadja, B.; Latour, X.; Faure, D.; Chevalier, S.; Dessaux, Y.; Orange, N. Involvement of N-acylhomoserine lactones throughout the plant infection by Erwinia carotovora subsp. atroseptica (Pectobacterium atrosepticum). Mol. Plant-Microbe Interact. 2004, 17, 1269–1278. [Google Scholar] [CrossRef]
- Jafra, S.; Jalink, H.; van der Schoor, R.; van der Wolf, J.M. Pectobacterium carotovorum subsp. carotovorum strains show diversity in production of response to N-acyl homoserine lactones. J. Phytopathol. 2006, 154, 729–739. [Google Scholar] [CrossRef]
- Molina, L.; Constantinescu, F.; Michel, L.; Reimmann, C.; Duffy, B.; Défago, G. Degradation of pathogen quorum-sensing molecules by soil bacteria: A preventive and curative biological control mechanism. FEMS Microbiol. Ecol. 2003, 45, 71–81. [Google Scholar] [CrossRef]
- Uroz, S.; D’Angelo-Picard, C.; Carlier, A.; Elasri, M.; Sicot, C.; Petit, A.; Oger, P.; Faure, D.; Dessaux, Y. Novel bacteria degrading N-acylhomoserine lactones and their use as quenchers of quorum-sensing-regulated functions of plant-pathogenic bacteria. Microbiology 2003, 149, 1981–1989. [Google Scholar] [CrossRef]
- Crépin, A.; Beury-Cirou, A.; Barbey, C.; Farmer, C.; Helias, V.; Burini, J.F.; Faure, D.; Latour, X. N-acyl homoserine lactones in diverse Pectobacterium and Dickeya plant pathogens: Diversity, abundance, and involvement in virulence. Sensors 2012, 12, 3484–3497. [Google Scholar]
- Cirou, A.; Diallo, S.; Kurt, C.; Latour, X.; Faure, D. Growth promotion of quorum-quenching bacteria in the rhizosphere of Solanum tuberosum. Environ. Microbiol. 2007, 9, 1511–1522. [Google Scholar] [CrossRef]
- D'Angelo-Picard, C.; Faure, D.; Penot, I.; Dessaux, Y. Diversity of N-acyl homoserine lactone-producing and -degrading bacteria in soil and tobacco rhizosphere. Environ. Microbiol. 2005, 7, 1796–808. [Google Scholar]
- Jafra, S.; Przysowa, J.; Czajkowski, R.; Michta, A.; Garbeva, P.; van der Wolf, J.M. Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can. J. Microbiol. 2006, 52, 1006–1015. [Google Scholar] [CrossRef]
- Ma, A.; Lv, D.; Zhuang, X.; Zhuang, G. Quorum quenching in culturable phyllosphere bacteria from tobacco. Int. J. Mol. Sci. 2013, 14, 14607–14619. [Google Scholar] [CrossRef]
- Bell, K.S.; Philp, J.C.; Aw, D.W.; Christofi, N. The genus Rhodococcus. J. Appl. Microbiol. 1998, 85, 195–210. [Google Scholar]
- de Carvalho, C.C.; da Fonseca, M.M. The remarkable Rhodococcus erythropolis. Appl. Microbiol. Biotechnol. 2005, 67, 715–726. [Google Scholar] [CrossRef]
- Ruberto, L.A.M.; Vasquez, S.; Lobalbo, A.; Mac Cormack, W.P. Psychrotolerant hydrocarbon-degrading Rhodococcus strains isolated from polluted Antarctic soils. Antarct. Sci. 2005, 17, 47–56. [Google Scholar] [CrossRef]
- Chang, W.N.; Liu, C.W.; Liu, H.S. Hydrophobic cell surface and bioflocculation behavior of Rhodococcus erythropolis RID B-4865-2009. Process. Biochem. 2009, 44, 955–962. [Google Scholar] [CrossRef]
- Schreiberová, O.; Hedbávná, P.; Cejková, A.; Jirků, V.; Masák, J. Effect of surfactants on the biofilm of Rhodococcus erythropolis, a potent degrader of aromatic pollutants. N. Biotechnol. 2012, 30, 62–68. [Google Scholar] [CrossRef]
- Larkin, M.J.; Kulakov, L.A.; Allen, C.C.R. Biodegradation and Rhodococcus—Masters of catabolic versatility. Curr. Opin. Microbiol. 2005, 16, 282–290. [Google Scholar]
- Larkin, M.J.; Kulakov, L.A.; Allen, C.C. Biodegradation by members of the genus Rhodococcus: Biochemistry, physiology, and genetic adaptation. Adv. Appl. Microbiol. 2006, 59, 1–29. [Google Scholar]
- Martinkova, L.; Uhnakova, B.; Patek, M.; Nesvera, J.; Kren, V. Biodegradation potential of the genus Rhodococcus. Environ. Int. 2009, 35, 162–177. [Google Scholar]
- van der Geize, R.; Dijkhuizen, L. Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications. Curr. Opin. Microbiol. 2004, 7, 255–261. [Google Scholar] [CrossRef]
- Warhurst, A.M.; Fewson, C.A. Biotransformations catalyzed by the genus Rhodococcus. Crit. Rev. Biotechnol. 1994, 14, 29–73. [Google Scholar] [CrossRef]
- Ciapina, E.M.; Melo, W.C.; Santa Anna, L.M.; Santos, A.S.; Freire, D.M.; Pereira, N., Jr. Biosurfactant production by Rhodococcus erythropolis grown on glycerol as sole carbon source. Appl. Biochem. Biotechnol. 2006, 129–132, 880–886. [Google Scholar]
- Pacheco, G.J.; Ciapina, E.M.; Gomes Ede, B.; Junior, N.P. Biosurfactant production by Rhodococcus erythropolis and its application to oil removal. Braz. J. Microbiol. 2010, 41, 685–693. [Google Scholar] [CrossRef]
- Pirog, T.; Sofilkanych, A.; Shevchuk, T.; Shulyakova, M. Biosurfactants of Rhodococcus erythropolis IMV Ас-5017: Synthesis intensification and practical application. Appl. Biochem. Biotechnol. 2013, 170, 880–894. [Google Scholar]
- Qi, Y.; Zhao, L.; Olusheyi, O.Z.; Tan, X. Isolation and preliminary characterization of a 3-chlorobenzoate degrading bacteria. J. Environ. Sci. 2007, 19, 332–337. (in Chinese). [Google Scholar] [CrossRef]
- Whyte, L.G.; Hawari, J.; Zhou, E.; Bourbonniere, L.; Inniss, W.E.; Greer, C.W. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl. Environ. Microbiol. 1998, 64, 2578–2584. [Google Scholar]
- Whyte, L.G.; Slagman, S.J.; Pietrantonio, F.; Bourbonniere, L.; Koval, S.F.; Lawrence, J.R.; Inniss, W.E.; Greer, C.W. Physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15. Appl. Environ. Microbiol. 1999, 65, 2961–2968. [Google Scholar]
- Yakimov, M.M.; Giuliano, L.; Bruni, V.; Scarfì, S.; Golyshin, P.N. Characterization of antarctic hydrocarbon-degrading bacteria capable of producing bioemulsifiers. New Microbiol. 1999, 22, 249–256. [Google Scholar]
- de Carvalho, C.C.; Fatal, V.; Alves, S.S.; da Fonseca, M.M. Adaptation of Rhodococcus erythropolis cells to high concentrations of toluene. Appl. Microbiol. Biotechnol. 2007, 76, 1423–1430. [Google Scholar] [CrossRef]
- de Carvalho, C.C. Adaptation of Rhodococcus erythropolis cells for growth and bioremediation under extreme conditions. Res. Microbiol. 2012, 163, 125–136. [Google Scholar] [CrossRef]
- De Schrijver, A.; De Mot, R. Degradation of pesticides by actinomycetes. Crit. Rev. Microbiol. 1999, 25, 85–119. [Google Scholar] [CrossRef]
- Huang, L.; Ma, T.; Li, D.; Liang, F.L.; Liu, R.L.; Li, G.Q. Optimization of nutrient component for diesel oil degradation by Rhodococcus erythropolis. Mar. Pollut. Bull. 2008, 56, 1714–1718. [Google Scholar]
- Yu, B.; Xu, P.; Shi, Q.; Ma, C. Deep desulfurization of diesel oil and crude oils by a newly isolated Rhodococcus erythropolis strain. Appl. Environ. Microbiol. 2006, 72, 54–58. [Google Scholar] [CrossRef]
- Fanget, N.V.; Foley, S. Starvation/stationary-phase survival of Rhodococcus erythropolis SQ1: A physiological and genetic analysis. Arch. Microbiol. 2011, 193, 1–13. [Google Scholar] [CrossRef]
- Christofi, N.; Ivshina, I.B. Microbial surfactants and their use in field studies of soil remediation. J. Appl. Microbiol. 2002, 93, 915–929. [Google Scholar] [CrossRef]
- de Carvalho, C.C.; da Fonseca, M.M. Degradation of hydrocarbons and alcohols at different temperatures and salinities by Rhodococcus erythropolis DCL14. FEMS Microbiol. Ecol. 2005, 51, 389–399. [Google Scholar] [CrossRef]
- Leigh, M.B.; Prouzová, P.; Macková, M.; Macek, T.; Nagle, D.P.; Fletcher, J.S. Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Appl. Environ. Microbiol. 2006, 72, 2331–2342. [Google Scholar] [CrossRef]
- Leilei, Z.; Mingxin, H.; Suiyi, Z. Enzymatic remediation of the polluted crude oil by Rhodococcus. Afr. J. Microbiol. Res. 2012, 6, 1540–1547. [Google Scholar]
- Lofgren, J.; Haddad, S.; Kendall, K. Emerging Technologies in Hazardous Waste Management; Tedder, W., Pohland, F.G., Eds.; ACS Symposium Series; ACS Publication: Columbus, OH, USA, 1995; Volume 607, pp. 252–263. [Google Scholar]
- Rappert, S.; Li, R.; Kokova, M.; Antholz, M.; Nagorny, S.; Francke, W.; Müller, R. Degradation of 2,5-dimethylpyrazine by Rhodococcus erythropolis strain DP-45 isolated from a waste gas treatment plant of a fishmeal processing company. Biodegradation 2007, 18, 585–596. [Google Scholar] [CrossRef]
- Solyanikova, I.; Golovleva, L. Biochemical features of the degradation of pollutants by Rhodococcus as a basis for contaminated wastewater and soil cleanup. Mikrobiologiia 2011, 80, 579–594. [Google Scholar]
- Diallo, S.; Crepin, A.; Barbey, C.; Orange, N.; Burini, J.F.; Latour, X. Mechanisms and recent advances in biological control mediated through the potato rhizosphere. FEMS Microbiol. Ecol. 2011, 75, 351–364. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). International Year of the Potato 2008, New Light on A Hidden Treasure; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; p. 144.
- Toth, I.K.; van der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Hélias, V.; Pirhonen, M.; Tsror (Lahkim), L.; Elphinstone, J.G. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Crépin, A.; Barbey, C.; Beury-Cirou, A.; Helias, V.; Taupin, L.; Reverchon, S.; Nasser, W.; Faure, D.; Dufour, A.; Orange, N.; Feuilloley, M.; Heurlier, K.; Burini, J.F.; Latour, X. Quorum sensing signaling molecules produced by reference and emerging soft-rot bacteria (Dickeya and Pectobacterium spp.). PLoS One 2012, 7, e35176. [Google Scholar] [CrossRef]
- Smadja, B.; Latour, X.; Trigui, S.; Burini, J-F.; Chevalier, S.; Orange, N. Thermodependence of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.). Can. J. Microbiol. 2004, 50, 19–27. [Google Scholar] [CrossRef]
- Liu, H.; Coulthurst, S.J.; Pritchard, L.; Hedley, P.E.; Ravensdale, M.; Humphris, S.; Burr, T.; Takle, G.; Brurberg, M.B.; Birch, P.R.; Salmond, G.P.; Toth, I.K. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 2008, 4, e1000093. [Google Scholar] [CrossRef]
- Monson, R.; Burr, T.; Liu, H.; Hedley, P.; Toth, I.; Salmond, G.P. Identification of genes in the VirR regulon of Pectobacterium atrosepticum and characterization of their roles in quorum sensing-dependent virulence. Environ. Microbiol. 2012, 15, 687–701. [Google Scholar]
- Latour, X.; Diallo, S.; Chevalier, S.; Morin, D.; Smadja, B.; Burini, J.F.; Haras, D.; Orange, N. Thermoregulation of N-acyl homoserine lactones-based quorum sensing in the soft rot bacterium Pectobacterium atrosepticum. Appl. Environ. Microbiol. 2007, 73, 4078–4081. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.J.; Shin, M.H.; Kim, J.A.; Kim, H.K.; Lee, J.K. N-acylhomoserine lactonase producing Rhodococcus spp. with different AHL-degrading activities. FEMS Microbiol. Lett. 2006, 261, 102–108. [Google Scholar] [CrossRef]
- Uroz, S.; Chhabra, S.R.; Camara, M.; Williams, P.; Oger, P.; Dessaux, Y. N-acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 2005, 151, 3313–3322. [Google Scholar] [CrossRef]
- Uroz, S.; Oger, P.M.; Chapelle, E.; Adeline, M.T.; Faure, D.; Dessaux, Y. A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl. Environ. Microbiol. 2008, 74, 1357–1366. [Google Scholar] [CrossRef]
- Barbey, C.; Crépin, A.; Bergeau, D.; Ouchiha, A.; Mijouin, L.; Taupin, L.; Orange, N.; Feuilloley, M.; Dufour, A.; Burini, J.F.; Latour, X. In planta biocontrol of Pectobacterium atrosepticum by Rhodococcus erythropolis involves silencing of pathogen communication by the rhodococcal gamma-lactone catabolic pathway. PLoS One 2013, 8, e66642. [Google Scholar] [CrossRef]
- Barbey, C.; Crépin, A.; Cirou, A.; Budin-Verneuil, A.; Orange, N.; Feuilloley, M.; Faure, D.; Dessaux, Y.; Burini, J.F.; Latour, X. Catabolic pathway of gamma-caprolactone in the biocontrol agent Rhodococcus erythropolis. J. Proteome Res. 2012, 11, 206–216. [Google Scholar] [CrossRef]
- Afriat, L.; Roodveldt, C.; Manco, G.; Tawfik, D.S. The latent promiscuity of newly identified microbial lactonases is linked to a recently diverged phosphotriesterase. Biochemistry 2006, 45, 13677–13686. [Google Scholar] [CrossRef]
- Curragh, H.; Flynn, O.; Larkin, M.J.; Stafford, T.M.; Hamilton, J.T.; Harper, D.B. Haloalkane degradation and assimilation by Rhodococcus rhodochrous NCIMB 13064. Microbiology 1994, 140, 1433–1442. [Google Scholar] [CrossRef]
- Singh, B.K. Organophosphorus-degrading bacteria: Ecology and industrial applications. Nat. Rev. Microbiol. 2009, 7, 156–164. [Google Scholar] [CrossRef]
- Sekine, M.; Tanikawa, S.; Omata, S.; Saito, M.; Fujisawa, T.; Tsukatani, N.; Tajima, T.; Sekigawa, T.; Kosugi, H.; Matsuo, Y.; et al. Sequence analysis of three plasmids harboured in Rhodococcus erythropolis strain PR4. Environ. Microbiol. 2006, 8, 334–346. [Google Scholar] [CrossRef]
- Ramos, J.L.; Martinez-Bueno, M.; Molina-Henares, A.J.; Teran, W.; Watanabe, K.; Zhang, X.; Gallegos, M.T.; Brennan, R.; Tobes, R. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 2005, 69, 326–356. [Google Scholar]
- Cuthbertson, L.; Nodwell, J.R. The TetR family of regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. [Google Scholar] [CrossRef]
- Si, D.; Urano, N.; Shimizu, S.; Kataoka, M. LplR, a repressor belonging to the TetR family, regulates expression of the L-pantoyl lactone dehydrogenase gene in Rhodococcus erythropolis. Appl. Environ. Microbiol. 2012, 78, 7923–7930. [Google Scholar] [CrossRef]
- Cha, C.J.; Cain, R.B.; Bruce, N.C. The modified beta-ketoadipate pathway in Rhodococcus rhodochrous N75: Enzymology of 3-methylmuconolactone metabolism. J. Bacteriol. 1998, 180, 6668–6673. [Google Scholar]
- Alvarez, H.M. Relationship between β-oxidation pathway and the hydrocarbon-degrading profile in actinomycetes bacteria. Intern. Biodeterior. Biodegrad. 2003, 52, 35–42. [Google Scholar] [CrossRef]
- Van der Vlugt-Bergmans, C.J.; van der Werf, M.J. Genetic and biochemical characterization of a novel monoterpene epsilon-lactone hydrolase from Rhodococcus erythropolis DCL14. Appl. Environ. Microbiol. 2001, 67, 733–741. [Google Scholar] [CrossRef]
- Kaufmann, G.F.; Sartorio, R.; Lee, S.H.; Rogers, C.J.; Meijler, M.M.; Moss, J.A.; Clapham, B.; Brogan, A.P.; Dickerson, T.J.; Janda, K.D. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA. 2005, 102, 309–314. [Google Scholar] [CrossRef]
- Roche, D.M.; Byers, J.T.; Smith, D.S.; Glansdorp, F.G.; Spring, D.R.; Welch, M. Communications blackout? Do N-acylhomoserine-lactone-degrading enzymes have any role in quorum sensing? Microbiology 2004, 150, 2023–2028. [Google Scholar] [CrossRef]
- Reading, N.C.; Sperandio, V. Quorum sensing: the many language of bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11. [Google Scholar] [CrossRef]
- Takano, E. Gamma-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr. Opin. Microbiol. 2006, 9, 287–294. [Google Scholar] [CrossRef]
- Nishida, H.; Ohnishi, Y.; Beppu, T.; Horinouchi, S. Evolution of γ-butyrolactone synthases and receptors in Streptomyces. Environ. Microbiol. 2007, 9, 1986–1994. [Google Scholar] [CrossRef]
- Carlier, A.; Uroz, S.; Smadja, B.; Fray, R.; Latour, X.; Dessaux, Y.; Faure, D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-Acyl homoserine lactonase activity. Appl. Environ. Microbiol. 2003, 69, 4989–4993. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zhang, L.H. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar]
- Barbey, C.; Kwasiborski, A.; Burini, J-F.; Faure, D.; Latour, X. Identification of a wide range of catabolic enzymes involved in the assimilation of various N-acyl homoserine lactones by Rhodococcus erythropolis. 2013; unpublished. [Google Scholar]
- Reimmann, C.; Ginet, N.; Michel, L.; Keel, C.; Michaux, P.; Krishnapillai, V.; Zala, M.; Heurlier, K.; Triandafillu, K.; Harms, H.; Défago, G.; Haas, D. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 2002, 148, 923–932. [Google Scholar]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Appl. Environ. Microbiol. 2008, 74, 1357–1366. [Google Scholar] [CrossRef]
- Cirou, A.; Raffoux, A.; Diallo, S.; Latour, X.; Dessaux, Y.; Faure, D. Gamma-caprolactone stimulates the growth of quorum-quenching Rhodococcus populations in a large-scale hydroponic system for culturing Solanum tuberosum. Res. Microbiol. 2011, 162, 945–950. [Google Scholar] [CrossRef]
- Maga, J.A. Lactones in foods. CRC Crit. Rev. Food Sci. Nutr. 1976, 8, 1–56. [Google Scholar]
- Murib, J.H.; Kahn, J.H. Process for Preparing Gamma-Caprolactone by Isomerization of Epsilon-Caprolactone. U.S. Patent 4,611,069, 9 September 1986. [Google Scholar]
- Nuñez, M.T.; Martin, V.S. Efficient oxidation of phenyl group to carboxylic acids with ruthenium tetraoxide. A simple synthesis of (R)-γ-caprolactone, the pheromone of Trogoderma granarium. J. Org. Chem. 1990, 55, 1928–1932. [Google Scholar] [CrossRef]
- Cirou, A.; Mondy, S.; An, S.; Charrier, A.; Sarrazin, A.; Thoison, O.; DuBow, M.; Faure, D. Efficient biostimulation of the native and introduced quorum-quenching Rhodococcus erythropolis is revealed by a combination of analytical chemistry, microbiology and pyrosequencing. Appl. Environ. Microbiol. 2012, 78, 481–492. [Google Scholar] [CrossRef]
- Latour, X.; Faure, D.; Diallo, S.; Cirou, A.; Smadja, B.; Dessaux, Y.; Orange, N. Control of bacterial diseases of potato caused by Pectobacterium spp. (Erwinia carotovora). Cah. Agric. 2008, 17, 355–359. [Google Scholar]
- Van Peer, R.; Schippers, B. Plant growth responses to bacterization with selected Pseudomonas spp. strains and rhizosphere microbial development in hydroponic cultures. Revue canadienne de microbiologie 1989, 35, 456–463. [Google Scholar] [CrossRef]
- Cirou, A.; Uroz, S.; Chapelle, E.; Latour, X.; Orange, N.; Faure, D.; Dessaux, Y. Quorum sensing as a target for novel biocontrol strategies. In Plant Pathology in the 21st Century; Gisi, U., Chet, I., Gullino, M.L., Eds.; Springer: Berlin, Germany, 2009; pp. 121–132. [Google Scholar]
- Vancov, T.; Jury, K.; Van Zwieten, L. Atrazine degradation by encapsulated Rhodococcus erythropolis NI86/21. J. Appl. Microbiol. 2005, 99, 767–775. [Google Scholar] [CrossRef]
- Vancov, T.; Jury, K.; Rice, N.; van Zwieten, L.; Morris, S. Enhancing cell survival of atrazine degrading Rhodococcus erythropolis NI86/21 cells encapsulated in alginate beads. J. Appl. Microbiol. 2007, 102, 212–220. [Google Scholar] [CrossRef]
- Mobed-Miremadi, M.; Darbha, S. Immobilization of R. erythropolis in alginate-based artificial cells for simulated plaque degradation in aqueous media. J. Microencapsul. 2013. [Google Scholar] [CrossRef]
- Guo, X.L.; Deng, G.; Xu, J.; Wang, M.X. Immobilization of Rhodococcus sp. AJ270 in alginate capsules and its application in enantioselective biotransformation of trans-2-methyl-3-phenyl-oxiranecarbonitrile and amide. Enzyme Microb. Tech. 2006, 39, 1–5. [Google Scholar] [CrossRef]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Alabouvette, C.; Olivain, C.; Steinberg, C. Biological control of plant pathogens: The European situation. Eur. J. Plant Pathol. 2006, 114, 329–341. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Montesinos, E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef]
- Latour, X.; Delorme, S.; Mirleau, P.; Lemanceau, P. Identification of traits implicated in the rhizosphere competence of fluorescent pseudomonads: Description of a strategy based on population and model strain studies. Agron. Sustain. Dev. 2003, 23, 397–405. [Google Scholar]
- Mark, G.L.; Morrissey, J.P.; Higgins, P.; O’Gara, F. Molecular-based strategies to exploit Pseudomonas biocontrol strains for environmental biotechnology applications. FEMS Microbiol. Ecol. 2006, 56, 161–177. [Google Scholar]
- Brazelton, J.N.; Pfeufer, E.E.; Sweat, T.A.; McSpadden-Gardener, B.B.; Coenen, C. 2,4-Diacetylphloroglucinol alters plant root development. Mol. Plant-Microbe Interact. 2008, 21, 1349–1358. [Google Scholar] [CrossRef]
- Schippers, B.; Bakker, A.W.; Bakker, P.A.H.M. Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Ann. Rev. Phytopathol. 1987, 25, 339–358. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; Toth, I.; Salmond, G.; Foster, G.D. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Flavier, A.B.; Ganova-Raeva, L.M.; Schell, M.A.; Denny, T.P. Hierarchical autoinduction in Ralstonia solanacearum: Control of acyl-homoserine lactone production by a novel autoregulatory system responsive to 3-hydroxypalmitic acid methyl ester. J. Bacteriol. 1997, 179, 7089–7097. [Google Scholar]
- Quiñones, B.; Dulla, G.; Lindow, S.E. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol. Plant Microbe Interact. 2005, 18, 682–693. [Google Scholar] [CrossRef]
- Venturi, V.; Venuti, C.; Devescovi, G.; Lucchese, C.; Friscina, A.; Degrassi, G.; Aguilar, C.; Mazzucchi, U. The plant pathogen Erwinia amylovora produces acyl-homoserine lactone signal molecules in vitro and in planta. FEMS Microbiol. Lett. 2004, 241, 179–183. [Google Scholar] [CrossRef]
- Becker, R.; Behrendt, U.; Hommel, B.; Kropf, S.; Ulrich, A. Effect of transgenic fructan producing potatoes on the community structure of rhizosphere and phyllosphere bacteria. FEMS Microbiol. Ecol. 2008, 66, 411–425. [Google Scholar] [CrossRef]
- Heuer, H.; Kroppenstedt, R.M.; Lottmann, J.; Berg, G.; Smalla, K. Effect of T4 lysozyme release from transgenic potato roots on bacterial rhizosphere communities are negligible relative to natural factors. Appl. Environ. Microbiol. 2002, 68, 1325–1335. [Google Scholar] [CrossRef]
- Lottmann, J.; Heuer, H.; Smalla, K.; Berg, G. Influence of transgenic T4-lysozyme-producing potato plants on potentially beneficial plant-associated bacteria. FEMS Microbiol. Ecol. 1999, 29, 365–377. [Google Scholar] [CrossRef]
- Van Overbeek, L.; van Elsas, J.D. Effects of plant genotype and growth stage on the structure of bacterial communities associated with potato (Solanum tuberosum L.). FEMS Microbiol. Ecol. 2008, 64, 283–296. [Google Scholar] [CrossRef]
- Zarkani, A.A.; Stein, E.; Röhrich, C.R.; Schikora, M.; Evguenieva-Hackenberg, E.; Degenkolb, T.; Vilcinskas, A.; Klug, G.; Kogel, K.H.; Schikora, A. Homoserine lactones influence the reaction of plants to rhizobia. Int. J. Mol. Sci. 2013, 14, 17122–17146. [Google Scholar] [CrossRef]
- Bais, H.P. Shoot the messages not the messengers. Plant Soil 2012, 358, 7–10. [Google Scholar] [CrossRef]
- Delalande, L.; Faure, D.; Raffoux, A.; Uroz, S.; D’Angelo-Picard, C.; Elasri, M.; Carlier, A.; Berruyer, R.; Petit, A.; Williams, P.; Dessaux, Y. N-hexanoyl-l-homoserine lactone, a mediator of bacterial quorum-sensing regulation, exhibits plant-dependent stability and may be inactivated by germinating Lotus corniculatus seedlings. FEMS Microbiol. Ecol. 2005, 52, 13–20. [Google Scholar] [CrossRef]
- Keshavan, N.D.; Chowdhary, P.K.; Haines, D.C.; Gonzalez, J.E.L. Canavanine made by Medicago sativa interferes with quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 2005, 187, 8427–8436. [Google Scholar] [CrossRef]
- Teplitski, M.; Robinson, J.B.; Bauer, W.D. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant-Microbe Interact. 2000, 13, 637–648. [Google Scholar] [CrossRef]
- Götz, C.; Fekete, A.; Gebefuegi, I.; Forczek, S.T.; Fuksová, K.; Li, X.; Englmann, M.; Gryndler, M.; Hartmann, A.; Matucha, M.; Schmitt-Kopplin, P.; Schröder, P. Uptake, degradation and chiral discrimination of N-acyl-d/l-homoserine lactones by barley (Hordeum vulgare) and yam bean (Pachyrhizus erosus) plants. Anal. Bioanal. Chem. 2007, 389, 1447–1457. [Google Scholar] [CrossRef]
- Schikora, A.; Schenk, S.T.; Stein, E.; Molitor, A.; Zuccaro, A.; Kogel, K.-H. N-acyl-homoserine lactone confers resistance toward biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol. 2011, 157, 1407–1418. [Google Scholar] [CrossRef]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; Hartmann, A.; Langebartels, C. Induction of systemic resistance in tomato by N-acyl-l-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Latour, X.; Barbey, C.; Chane, A.; Groboillot, A.; Burini, J.-F. Rhodococcus erythropolis and Its γ-Lactone Catabolic Pathway: An Unusual Biocontrol System That Disrupts Pathogen Quorum Sensing Communication. Agronomy 2013, 3, 816-838. https://doi.org/10.3390/agronomy3040816
Latour X, Barbey C, Chane A, Groboillot A, Burini J-F. Rhodococcus erythropolis and Its γ-Lactone Catabolic Pathway: An Unusual Biocontrol System That Disrupts Pathogen Quorum Sensing Communication. Agronomy. 2013; 3(4):816-838. https://doi.org/10.3390/agronomy3040816
Chicago/Turabian StyleLatour, Xavier, Corinne Barbey, Andrea Chane, Anne Groboillot, and Jean-François Burini. 2013. "Rhodococcus erythropolis and Its γ-Lactone Catabolic Pathway: An Unusual Biocontrol System That Disrupts Pathogen Quorum Sensing Communication" Agronomy 3, no. 4: 816-838. https://doi.org/10.3390/agronomy3040816



