Estimation of Nitrogen Concentration in Walnut Canopies in Southern Xinjiang Based on UAV Multispectral Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Selection of the Test Site
2.3. Data Acquisition
2.3.1. Collection of Walnut Canopy Leaf Samples and Determination of the Nitrogen Concentration
2.3.2. UAV Multispectral Data Acquisition
2.4. Processing of UAV Remote Sensing Images
2.4.1. Manual Confirmation of the Canopy Boundaries of the Walnut Trees
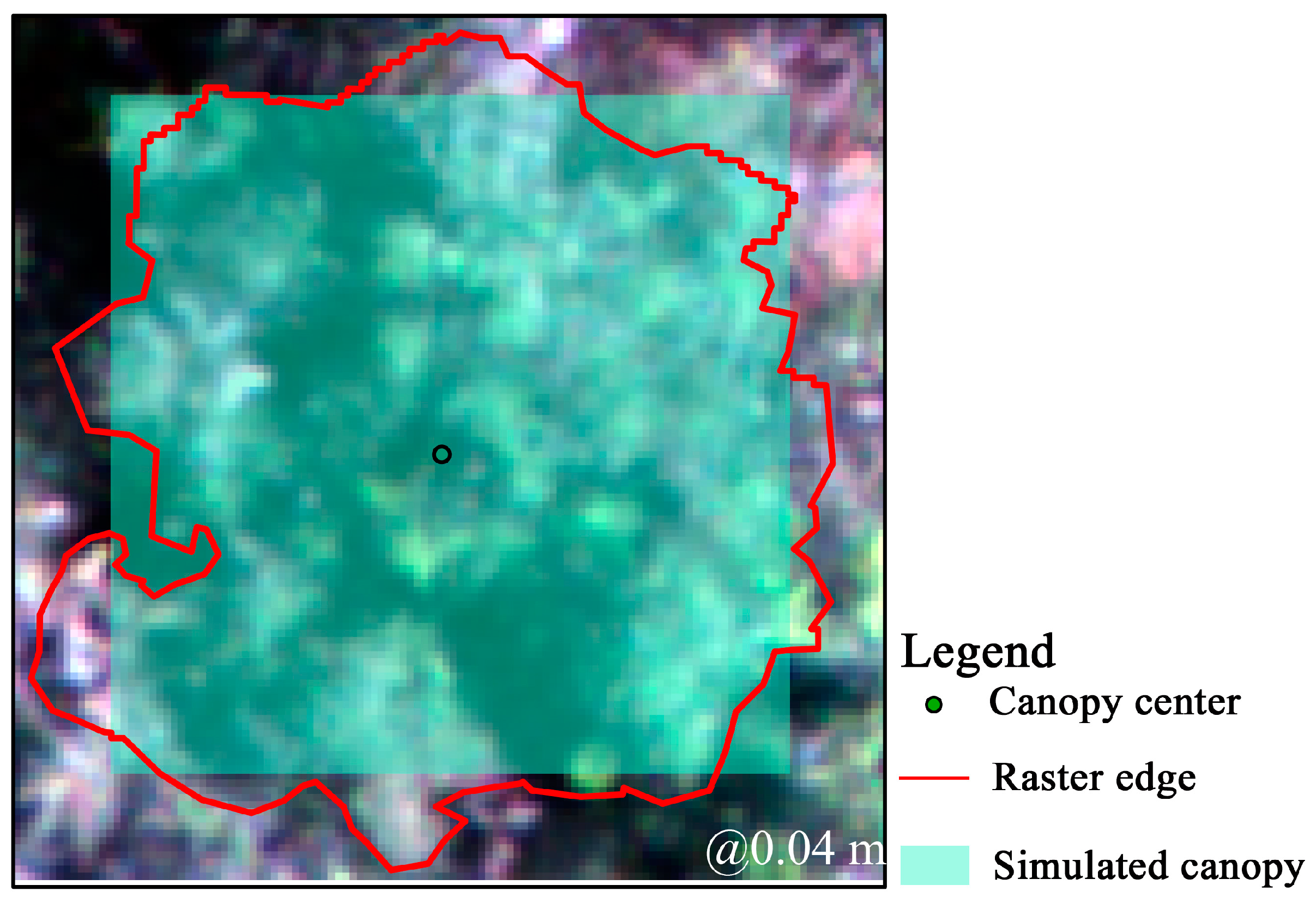
2.4.2. Simulated Canopy Method
2.5. Sensitive Factor Screening
2.6. Modeling Method and Model Evaluation Index
3. Results
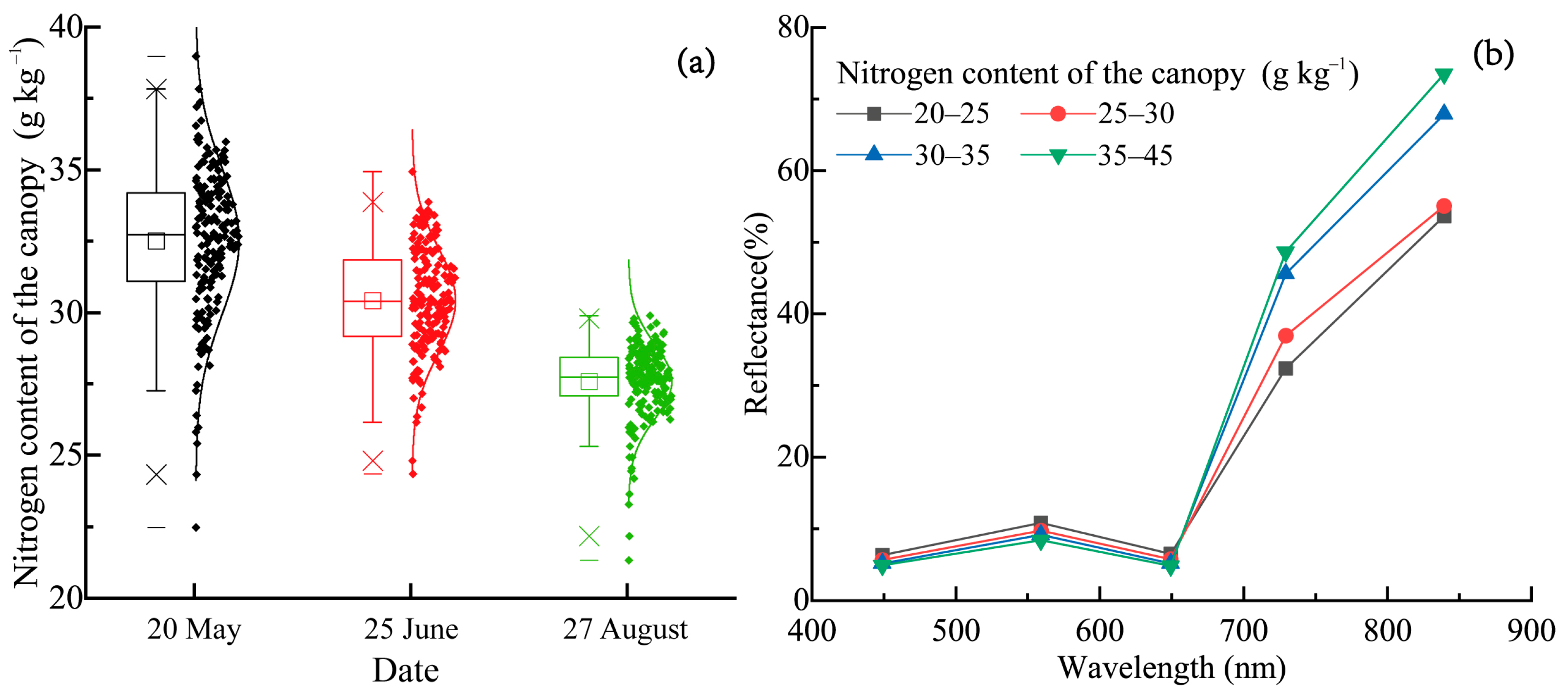
3.1. Variation in the Nitrogen Concentration of the Walnut Canopy and the Analysis of Spectral Characteristics
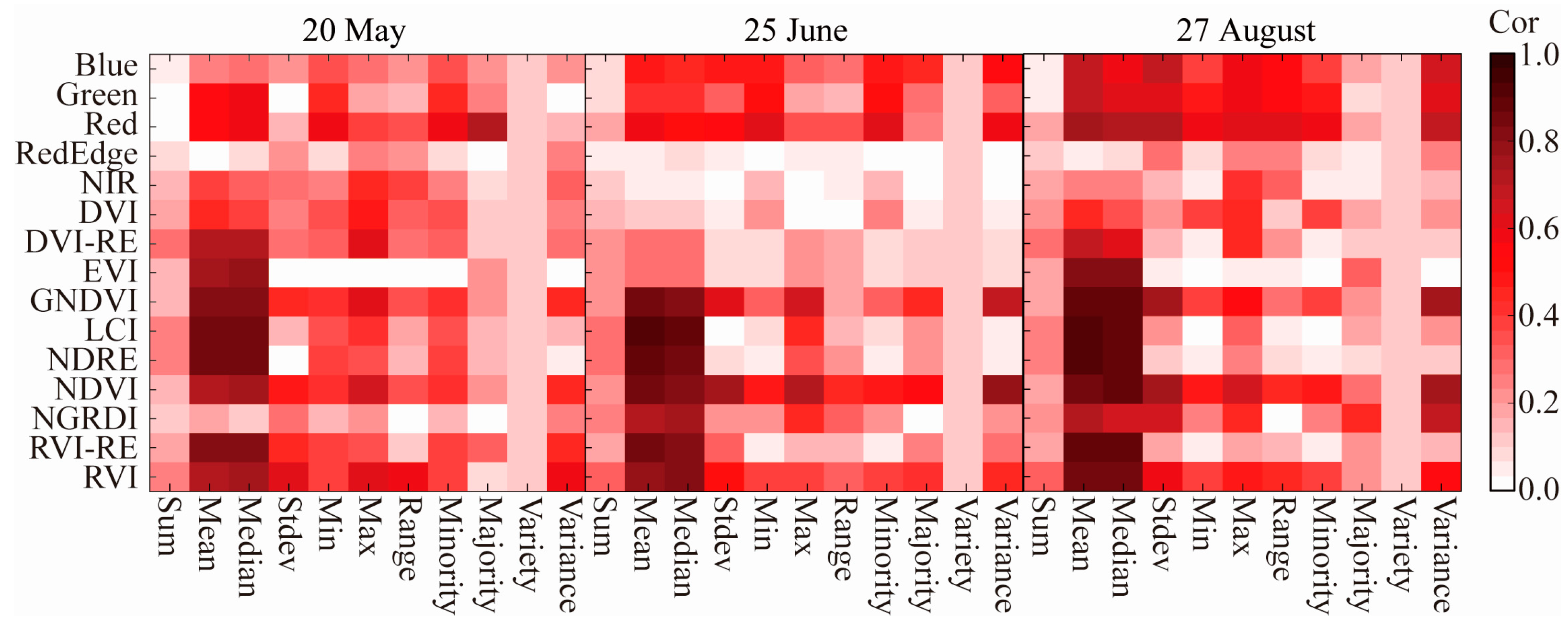
3.2. Screening of Nitrogen Sensitive Factors in Walnut Tree Canopies
3.2.1. Correlation Analysis of Manually Confirmed ROI-Extracted Spectral Information with the Nitrogen Concentration of the Walnut Canopy
3.2.2. Correlation Analysis between Simulated Canopy ROI Extraction Information and the Nitrogen Concentration of the Walnut Canopy
3.3. Model Construction and Verification
3.3.1. Manual Confirmation of the Inverse Modeling of the Sensitive Factors of Spectral Information Extracted within the ROI and the Nitrogen Concentration of the Walnut Canopy
3.3.2. Establishment of an Inversion Model between the Sensitive Factors of Extracted Spectral Information in the Simulated Canopy ROI and the Nitrogen Concentration of the Walnut Canopy
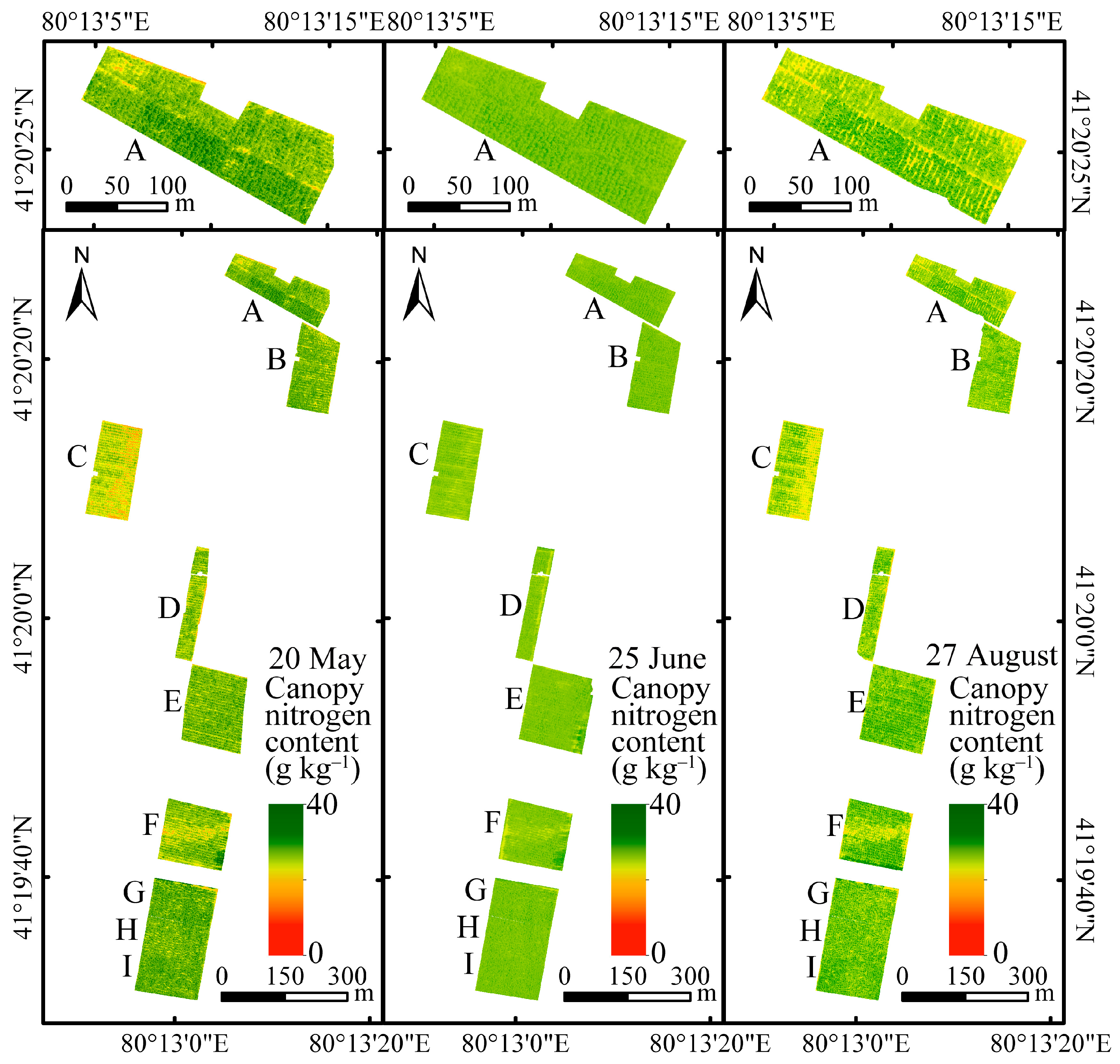
3.4. Characterization of Nitrogen Distribution in Walnut Tree Canopies Based on the Simulated Canopy Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Zhu, A.; Wang, X.; Yu, J.; Zhang, H.; Gao, J.; Cheng, Y.; Deng, X. Development of Juglans regia SSR markers by data mining of the EST database. Plant Mol. Biol. Rep. 2010, 28, 646–653. [Google Scholar] [CrossRef]
- Shah, U.N.; Mir, J.I.; Ahmed, N.; Zaid, A.; Jan, S.; Fazili, K.M.; Wani, S.H. Bio-techniques for improvement of qualitative and quantitative traits in walnut (Juglans regia). Adv. Hortic. Sci. 2018, 32, 113–136. [Google Scholar]
- Wambulwa, M.C.; Fan, P.Z.; Milne, R.; Wu, Z.Y.; Luo, Y.H.; Wang, Y.H.; Liu, J. Genetic analysis of walnut cultivars from southwest China: Implications for germplasm improvement. Plant Divers 2022, 44, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Kan, H.; Chen, S.X.; Thakur, K.; Wang, S.; Zhang, J.G.; Shang, Y.F.; Wei, Z.J. Comparison of phenolic compounds extracted from Diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020, 321, 126672. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. Key canopy traits drive forest productivity. Proc. Royal Soc. B 2012, 279, 2128–2134. [Google Scholar] [CrossRef] [Green Version]
- Kergoat, L.; Lafont, S.; Arneth, A.; Le Dantec, V.; Saugier, B. Nitrogen controls plant canopy light-use efficiency in temperate and boreal ecosystems. J. Geophys. Res. Biogeosci. 2008, 113, 04017. [Google Scholar] [CrossRef] [Green Version]
- Novoa, R.; Loomis, R.S. Nitrogen and plant production. Plant Soil. 1981, 58, 177–204. [Google Scholar] [CrossRef]
- Li, W.; Zhu, X.; Yu, X.; Li, M.; Tang, X.; Zhang, J.; Xue, Y.; Zhang, C.; Jiang, Y. Inversion of Nitrogen Concentration in Apple Canopy Based on UAV Hyperspectral Images. Sensors 2022, 22, 3503. [Google Scholar] [CrossRef]
- Simorte, V.; Bertoni, G.; Dupraz, C.; Masson, P. Assessment of nitrogen nutrition of walnut trees using foliar analysis and chlorophyll measurements. J. Plant Nutr. 2001, 24, 1645–1660. [Google Scholar] [CrossRef]
- Yan, S.; Rui, Z.; Shan, G.; Pan, Z.; Lei, Z. Transcriptome Analysis of Walnut Seedling Roots Under Nitrogen Starvation and Excess Nitrogen Stress. Sci. Rep. 2022, 12, 12066. [Google Scholar]
- Verardo, V.; Riciputi, Y.; Sorrenti, G.; Ornaghi, P.; Marangoni, B.; Caboni, M.F. Effect of nitrogen fertilisation rates on the content of fatty acids, sterols, tocopherols and phenolic compounds, and on the oxidative stability of walnuts. LWT 2013, 50, 732–738. [Google Scholar] [CrossRef]
- Gunes, A.; Alpaslan, M.; Inal, A. Critical nutrient concentrations and antagonistic and synergistic relationships among the nutrients of NFT-grown young tomato plants. J. Plant Nutr. 1998, 21, 2035–2047. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crops Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Khanal, S.; Kc, K.; Fulton, J.P.; Shearer, S.; Ozkan, E. Remote sensing in agriculture—Accomplishments, limitations, and opportunities. Remote Sens. 2020, 12, 3783. [Google Scholar] [CrossRef]
- van Maarschalkerweerd, M.; Husted, S. Recent developments in fast spectroscopy for plant mineral analysis. Front. Plant Sci. 2015, 6, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhou, Z.; Zhang, G.; Meng, Y.; Chen, B.; Wang, Y. Monitoring the leaf water content and specific leaf weight of cotton (Gossypium hirsutum L.) in saline soil using leaf spectral reflectance. Eur. J. Agron. 2012, 41, 103–117. [Google Scholar] [CrossRef]
- Guo, P.T.; Li, M.F.; Luo, W.; Cha, Z.Z. Estimation of foliar nitrogen of rubber trees using hyperspectral reflectance with feature bands. Infrared Phys Technol. 2019, 102, 103021. [Google Scholar] [CrossRef]
- Perry, E.M.; Goodwin, I.; Cornwall, D. Remote sensing using canopy and leaf reflectance for estimating nitrogen status in red-blush pears. HortScience 2018, 53, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Eitel, J.U.H.; Long, D.S.; Gessler, P.E.; Smith, A.M.S. Using in-situ measurements to evaluate the new RapidEye™ satellite series for prediction of wheat nitrogen status. Int. J. Remote Sens. 2007, 28, 4183–4190. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Yuan, F.; Gnyp, M.L.; Yao, Y.; Cao, Q.; Bareth, G. Potential of RapidEye and WorldView-2 satellite data for improving rice nitrogen status monitoring at different growth stages. Remote Sens. 2017, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, C.; Mancini, M.; dalla Marta, A.; Orlandini, S.; Napoli, M. Integrating satellite data with a Nitrogen Nutrition Curve for precision top-dress fertilization of durum wheat. Eur. J. Agron. 2020, 120, 126148. [Google Scholar] [CrossRef]
- Yu, R.; Zhu, X.; Bai, X.; Tian, Z.; Jiang, Y.; Yang, G. Inversion reflectance by apple tree canopy ground and unmanned aerial vehicle integrated remote sensing data. J. Plant Res. 2021, 134, 729–736. [Google Scholar] [CrossRef]
- Cao, C.; Wang, T.; Gao, M.; Li, Y.; Li, D.; Zhang, H. Hyperspectral inversion of nitrogen content in maize leaves based on different dimensionality reduction algorithms. Comput. Electron. Agric. 2021, 190, 106461. [Google Scholar] [CrossRef]
- Yao, X.; Ren, H.; Cao, Z.; Tian, Y.; Cao, W.; Zhu, Y.; Cheng, T. Detecting leaf nitrogen content in wheat with canopy hyperspectrum under different soil backgrounds. Int. J. Appl. Earth Obs. Geoinf. 2014, 32, 114–124. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Tian, T.; Yin, J. A review of unmanned aerial vehicle low-altitude remote sensing (UAV-LARS) use in agricultural monitoring in China. Remote Sens. 2021, 13, 1221. [Google Scholar] [CrossRef]
- Fu, Z.; Yu, S.; Zhang, J.; Xi, H.; Gao, Y.; Lu, R.; Zheng, H.; Zhu, Y.; Cao, W.; Liu, X. Combining UAV multispectral imagery and ecological factors to estimate leaf nitrogen and grain protein content of wheat. Eur. J. Agron. 2022, 132, 126405. [Google Scholar] [CrossRef]
- Jiang, J.; Atkinson, P.M.; Zhang, J.; Lu, R.; Zhou, Y.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Combining fixed-wing UAV multispectral imagery and machine learning to diagnose winter wheat nitrogen status at the farm scale. Eur. J. Agron. 2022, 138, 126537. [Google Scholar] [CrossRef]
- Xu, W.; Chen, P.; Zhan, Y.; Chen, S.; Zhang, L.; Lan, Y. Cotton yield estimation model based on machine learning using time series UAV remote sensing data. Int. J. Appl. Earth Obs Geoinf. 2021, 104, 102511. [Google Scholar] [CrossRef]
- Feng, A.; Zhou, J.; Vories, E.D.; Sudduth, K.A.; Zhang, M. Yield estimation in cotton using UAV-based multi-sensor imagery. Biosyst. Eng. 2020, 193, 101–114. [Google Scholar] [CrossRef]
- da Silva, E.E.; Baio FH, R.; Teodoro LP, R.; da Silva Junior, C.A.; Borges, R.S.; Teodoro, P.E. UAV-multispectral and vegetation indices in soybean grain yield prediction based on in situ observation. Remote Sens. Appl. Soc. Environ. 2020, 18, 100318. [Google Scholar] [CrossRef]
- Shendryk, Y.; Sofonia, J.; Garrard, R.; Rist, Y.; Skocaj, D.; Thorburn, P. Fine-scale prediction of biomass and leaf nitrogen content in sugarcane using UAV LiDAR and multispectral imaging. Int. J. Appl. Earth Obs. Geoinf. 2020, 92, 102177. [Google Scholar] [CrossRef]
- Alckmin, G.T.; Lucieer, A.; Rawnsley, R.; Kooistra, L. Perennial ryegrass biomass retrieval through multispectral UAV data. Comput. Electron. Agric. 2022, 193, 106574. [Google Scholar] [CrossRef]
- Shao, G.; Han, W.; Zhang, H.; Wang, Y.; Zhang, L.; Niu, Y.; Zhang, Y.; Cao, P. Estimation of transpiration coefficient and aboveground biomass in maize using time-series UAV multispectral imagery. Crop Sci. 2022, 10, 1376–1385. [Google Scholar] [CrossRef]
- Qiao, L.; Zhao, R.; Tang, W.; An, L.; Sun, H.; Li, M.; Wang, N.; Liu, Y.; Liu, G. Estimating maize LAI by exploring deep features of vegetation index map from UAV multispectral images. Field Crops Res. 2022, 289, 108739. [Google Scholar] [CrossRef]
- Lang, Q.; Zhiyong, Z.; Longsheng, C.; Hong, S.; Minzan, L.; Li, L.; Junyong, M. Detection of chlorophyll content in Maize Canopy from UAV Imagery. IFAC-PapersOnLine 2019, 52, 330–335. [Google Scholar] [CrossRef]
- Prado Osco, L.; Marques Ramos, A.P.; Roberto Pereira, D.; Akemi Saito Moriya, É.; Nobuhiro Imai, N.; Takashi Matsubara, E.; Eduardo Creste, J. Predicting canopy nitrogen content in citrus-trees using random forest algorithm associated to spectral vegetation indices from UAV-imagery. Remote Sens. 2019, 11, 2925. [Google Scholar] [CrossRef] [Green Version]
- Noguera, M.; Aquino, A.; Ponce, J.M.; Cordeiro, A.; Silvestre, J.; Arias-Calderón, R.; Andújar, J.M. Nutritional status assessment of olive crops by means of the analysis and modelling of multispectral images taken with UAVs. Biosyst. Eng. 2021, 211, 1–18. [Google Scholar] [CrossRef]
- Chen, S.; Hu, T.; Luo, L.; He, Q.; Li, H. Rapid estimation of leaf nitrogen content in apple-trees based on canopy hyperspectral reflectance using multivariate methods. Infrared Phys. Technol. 2020, 111, 103542. [Google Scholar] [CrossRef]
- Rui, Z.; Qi, Z.; Chen, J.; Hong, Z.; Shan, G.; Xu, C. Effect of water and fertilizer coupling on photosynthetic characteristics and quality in walnut. J. Fruit Sci. 2015, 32, 1170–1178. [Google Scholar]
- Carlson, T.N.; Ripley, D.A. On the relation between NDVI, fractional vegetation cover, and leaf area index. Remote Sens. Environ. 1997, 62, 241–252. [Google Scholar] [CrossRef]
- Hunt, E.R.; Cavigelli, M.; Daughtry, C.S.; Mcmurtrey, J.E.; Walthall, C.L. Evaluation of digital photography from model aircraft for remote sensing of crop biomass and nitrogen status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Xue, L.; Cao, W.; Luo, W.; Dai, T.; Zhu, Y. Monitoring leaf nitrogen status in rice with canopy spectral reflectance. Agron. J. 2004, 96, 135–142. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, B.; Yang, W.; Chen, J.; Onda, Y.; Qiu, G. Sensitivity of the enhanced vegetation index (EVI) and normalized difference vegetation index (NDVI) to topographic effects: A case study in high-density cypress forest. Sensors 2007, 7, 2636–2651. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Younie, M.; Paul, J.W.; Bittman, S. Evaluation of leaf chlorophyll index for making fertilizer nitrogen recommendations for silage corn in a high fertility environment. Commun. Soil Sci. Plant Anal. 2002, 33, 665–684. [Google Scholar] [CrossRef]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn Iii, W.J. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J. Sci. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Morlini, I. On multicollinearity and concurvity in some nonlinear multivariate models. Stat. Method Appl.-Ger. 2006, 15, 3–26. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Xie, M.; Hu, B.; Luo, D.; Wan, C.; Peng, J.; Shi, Z. Estimation of Soil Organic Carbon Using Vis-NIR Spectral Data and Spectral Feature Bands Selection in Southern Xinjiang, China. Sensors 2022, 22, 6124. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Gong, C.; Chen, Y.; Yu, H. Applications of deep learning for dense scenes analysis in agriculture: A review. Sensors 2020, 20, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhu, X.; Li, W.; Tang, X.; Yu, X.; Jiang, Y. Retrieval of nitrogen content in apple canopy based on unmanned aerial vehicle hyperspectral images using a modified correlation coefficient method. Sustainability 2022, 14, 1992. [Google Scholar] [CrossRef]
- Johansen, K.; Duan, Q.; Tu, Y.H.; Searle, C.; Wu, D.; Phinn, S.; McCabe, M.F. Mapping the condition of macadamia tree crops using multi-spectral UAV and WorldView-3 imagery. ISPRS J. Photogramm. 2020, 165, 28–40. [Google Scholar] [CrossRef]
- Vong, C.N.; Conway, L.S.; Feng, A.; Zhou, J.; Kitchen, N.R.; Sudduth, K.A. Corn emergence uniformity estimation and mapping using UAV imagery and deep learning. Comput. Electron. Agric. 2022, 198, 107008. [Google Scholar] [CrossRef]


| Date | Flight Time (UTC +8:00) | Solar Altitude Angle | Fertility Period |
|---|---|---|---|
| 20 May | 13:30–14:30 | 61.40°–60.98° | Fruit fast-growth period |
| 25 June | 13:30–14:30 | 64.01°–62.97° | Fruit sclerotization period |
| 27 August | 13:45–14:45 | 51.64°–42.73° | Fruit near-maturity period |
| Spectral Index | Calculation Formula | Source |
|---|---|---|
| NDVI | (NIR − Red)/(NIR + Red) | [40] |
| NDRE | (NIR − RedEdge)/(NIR + RedEdge) | [41] |
| NGRDI | (Green − Red)/(Green + Red) | [42] |
| GNDVI | (NIR − Green)/(NIR + Green) | [43] |
| RVI | NIR/Red | [44] |
| DVI | NIR − Red | [45] |
| EVI | 2.5(NIR − Red)/(NIR + 6Red − 7.5Blue + 1) | [46] |
| LCI | (NIR − RedEdge)/(NIR + Red) | [47] |
| DVI − RedEdge | NIR − RedEdge | - |
| RVI − RedEdge | NIR/RedEdge | - |
| Growth Period of Walnut Fruit | Number of Samples | Max (g kg−1) | Min (g kg−1) | Mean (g kg−1) | SE | Variance | CV (%) |
|---|---|---|---|---|---|---|---|
| Fruit fast-growth period | 180 | 38.98 | 22.47 | 32.48 | 2.58 | 6.59 | 7.90 |
| Fruit sclerotization period | 180 | 34.94 | 24.34 | 30.41 | 1.85 | 3.37 | 6.04 |
| Fruit near-maturity period | 180 | 29.89 | 21.33 | 27.56 | 1.30 | 1.70 | 4.72 |
| Date | Types of Variables | PLSR | SVMR | RF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Val | Val | Val | ||||||||
| R2 | RMSE (g kg−1) | RPD | R2 | RMSE (g kg−1) | RPD | R2 | RMSE (g kg−1) | RPD | ||
| 20 May | Band | 0.53 | 0.53 | 1.22 | 0.65 | 0.46 | 1.35 | 0.71 | 0.43 | 1.76 |
| SI | 0.59 | 0.50 | 1.15 | 0.72 | 0.42 | 1.84 | 0.82 | 0.34 | 1.89 | |
| Band + SI | 0.60 | 0.50 | 1.25 | 0.84 | 0.32 | 2.00 | 0.85 | 0.30 | 2.55 | |
| 25 June | Band | 0.74 | 0.31 | 1.79 | 0.77 | 0.25 | 1.85 | 0.77 | 0.27 | 1.39 |
| SI | 0.78 | 0.25 | 1.68 | 0.79 | 0.26 | 2.15 | 0.83 | 0.21 | 2.07 | |
| Band + SI | 0.79 | 0.25 | 1.70 | 0.80 | 0.26 | 2.15 | 0.84 | 0.21 | 2.07 | |
| 27 August | Band | 0.67 | 0.97 | 1.63 | 0.69 | 0.55 | 1.74 | 0.76 | 0.51 | 1.19 |
| SI | 0.72 | 0.71 | 1.84 | 0.88 | 0.34 | 2.30 | 0.90 | 0.31 | 3.08 | |
| Band + SI | 0.77 | 0.52 | 2.07 | 0.90 | 0.32 | 2.46 | 0.92 | 0.30 | 3.22 | |
| Date | Types of Variable | PLSR | SVMR | RF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Val | Val | Val | ||||||||
| R2 | RMSE (g kg−1) | RPD | R2 | RMSE (g kg−1) | RPD | R2 | RMSE (g kg−1) | RPD | ||
| 20 May | Band | 0.50 | 0.54 | 1.41 | 0.55 | 0.53 | 1.32 | 0.62 | 0.50 | 1.52 |
| SI | 0.59 | 0.50 | 1.54 | 0.59 | 0.50 | 1.38 | 0.62 | 0.50 | 1.53 | |
| Band + SI | 0.61 | 0.48 | 1.58 | 0.62 | 0.48 | 1.43 | 0.64 | 0.48 | 1.60 | |
| 25 June | Band | 0.46 | 0.38 | 1.36 | 0.56 | 0.35 | 1.05 | 0.71 | 0.28 | 1.44 |
| SI | 0.70 | 0.29 | 1.28 | 0.72 | 0.28 | 1.46 | 0.73 | 0.28 | 1.86 | |
| Band + SI | 0.73 | 0.29 | 1.26 | 0.73 | 0.27 | 1.47 | 0.74 | 0.27 | 1.93 | |
| 27 August | Band | 0.48 | 1.22 | 1.23 | 0.61 | 0.70 | 0.61 | 0.62 | 0.63 | 1.52 |
| SI | 0.64 | 0.65 | 1.14 | 0.65 | 0.62 | 1.59 | 0.83 | 0.44 | 2.16 | |
| Band + SI | 0.80 | 0.43 | 1.94 | 0.83 | 0.51 | 1.10 | 0.84 | 0.43 | 2.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Feng, C.; Ma, Y.; Chen, X.; Lu, B.; Song, Y.; Zhang, Z.; Zhang, R. Estimation of Nitrogen Concentration in Walnut Canopies in Southern Xinjiang Based on UAV Multispectral Images. Agronomy 2023, 13, 1604. https://doi.org/10.3390/agronomy13061604
Wang Y, Feng C, Ma Y, Chen X, Lu B, Song Y, Zhang Z, Zhang R. Estimation of Nitrogen Concentration in Walnut Canopies in Southern Xinjiang Based on UAV Multispectral Images. Agronomy. 2023; 13(6):1604. https://doi.org/10.3390/agronomy13061604
Chicago/Turabian StyleWang, Yu, Chunhui Feng, Yiru Ma, Xiangyu Chen, Bin Lu, Yan Song, Ze Zhang, and Rui Zhang. 2023. "Estimation of Nitrogen Concentration in Walnut Canopies in Southern Xinjiang Based on UAV Multispectral Images" Agronomy 13, no. 6: 1604. https://doi.org/10.3390/agronomy13061604






