Molecularly Imprinted Nanofiber Film for Sensitive Sensing 2,4,6-Tribromophenol
Abstract
:1. Introduction
2. Experimental
2.1. Instruments and Reagents
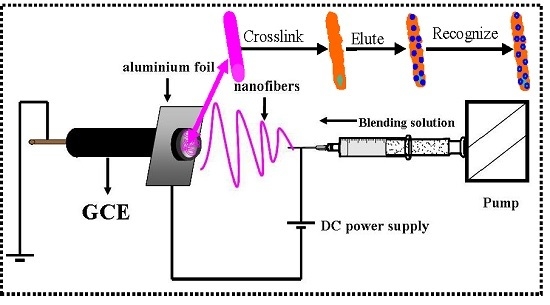
2.2. Fabrication of the Imprinted Nanofiber Sensor (INS)
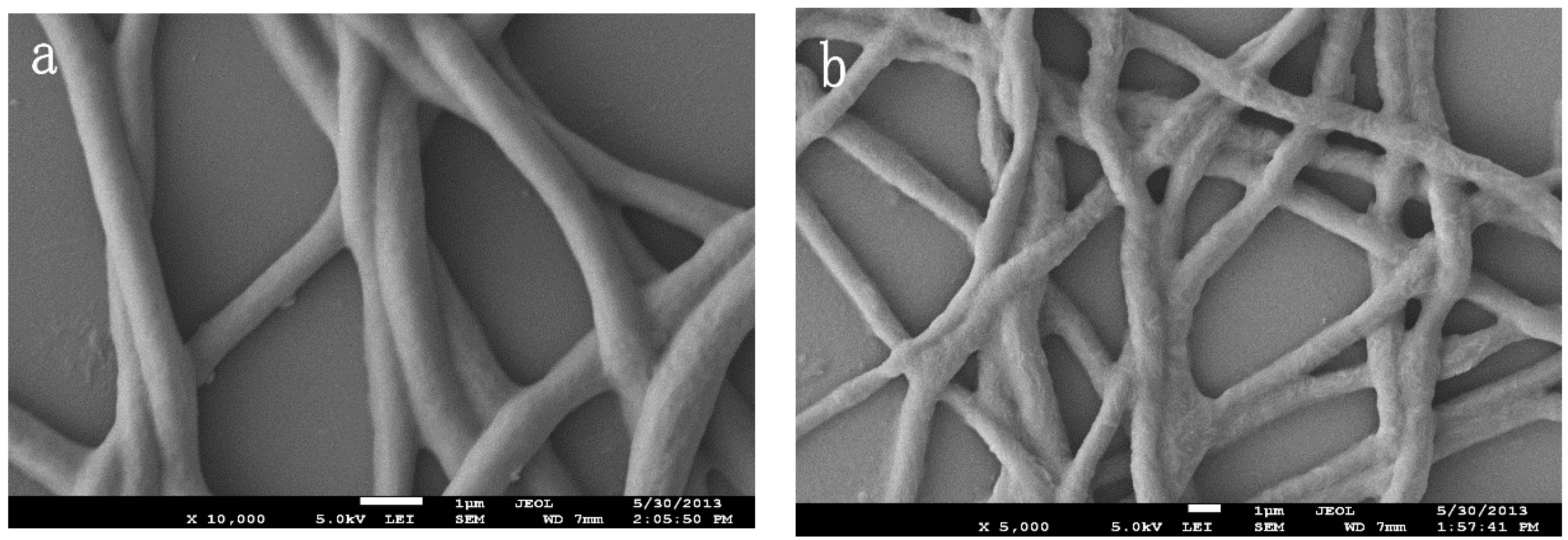
2.2.1. Preparation of the Composite Nanofibers
2.2.2. Cross-linking and Elution of Nanofibers
2.3. Electrochemical Measurements
3. Results and Discussion
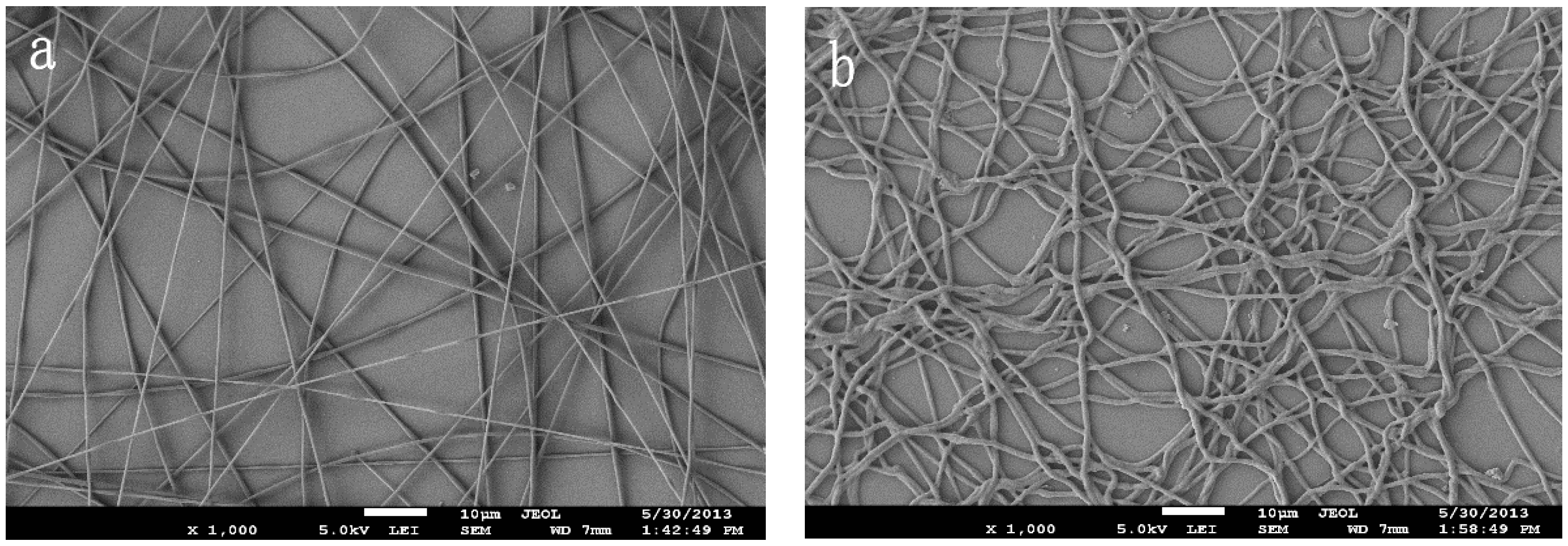
3.1. Preparation of the INS
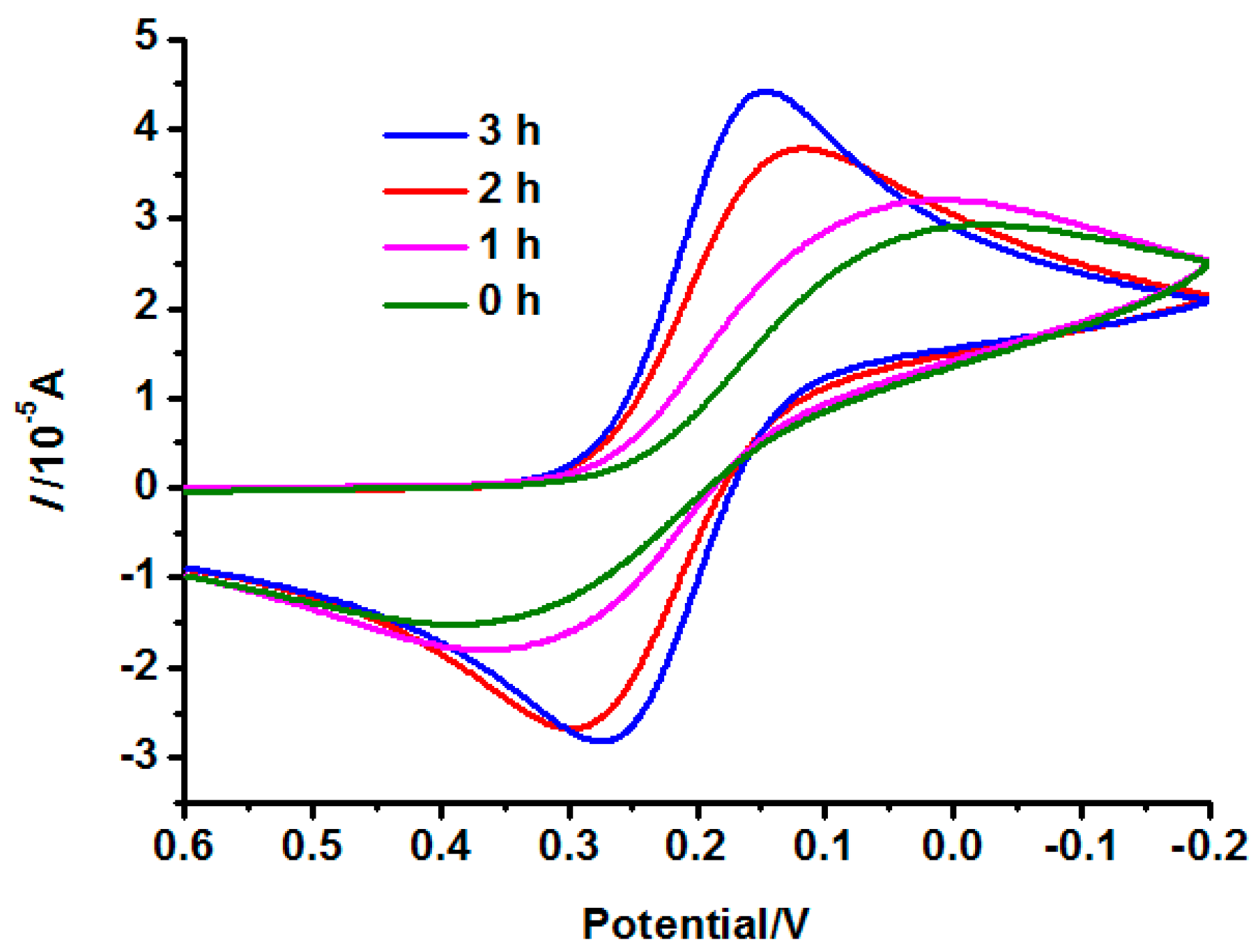
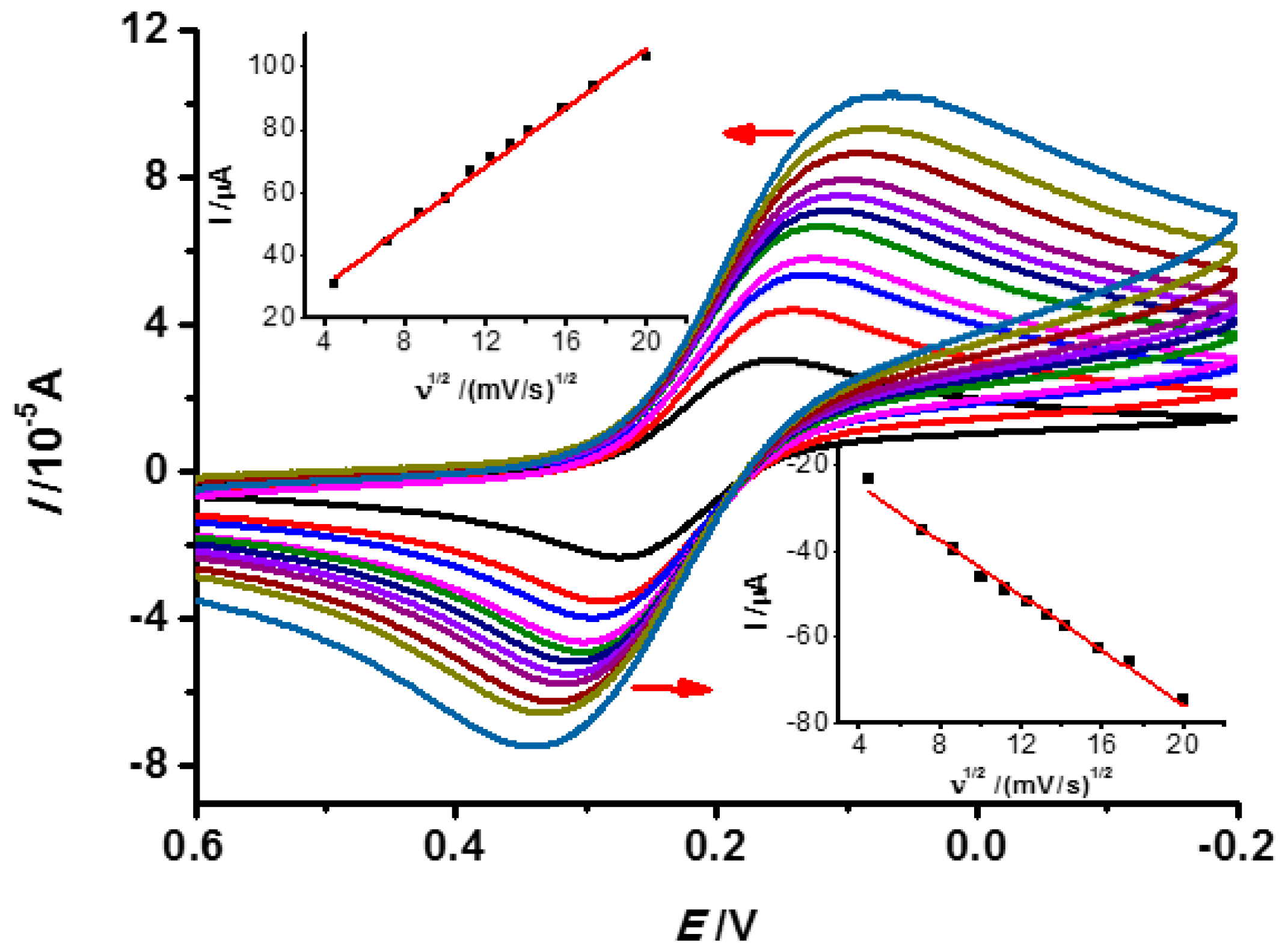
3.2. Electrochemical Properties of the INS
3.3. Effect of Imprinting of INS
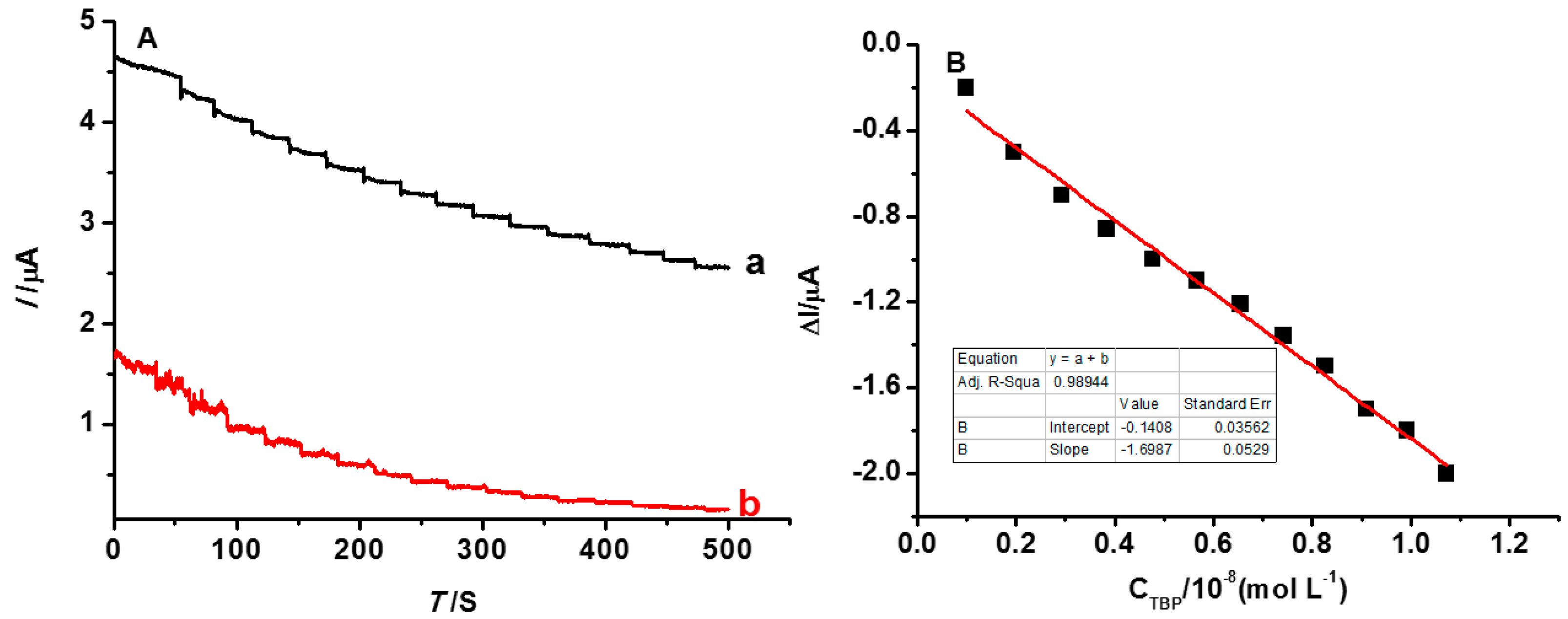
3.4. The Detection Limit of the INS
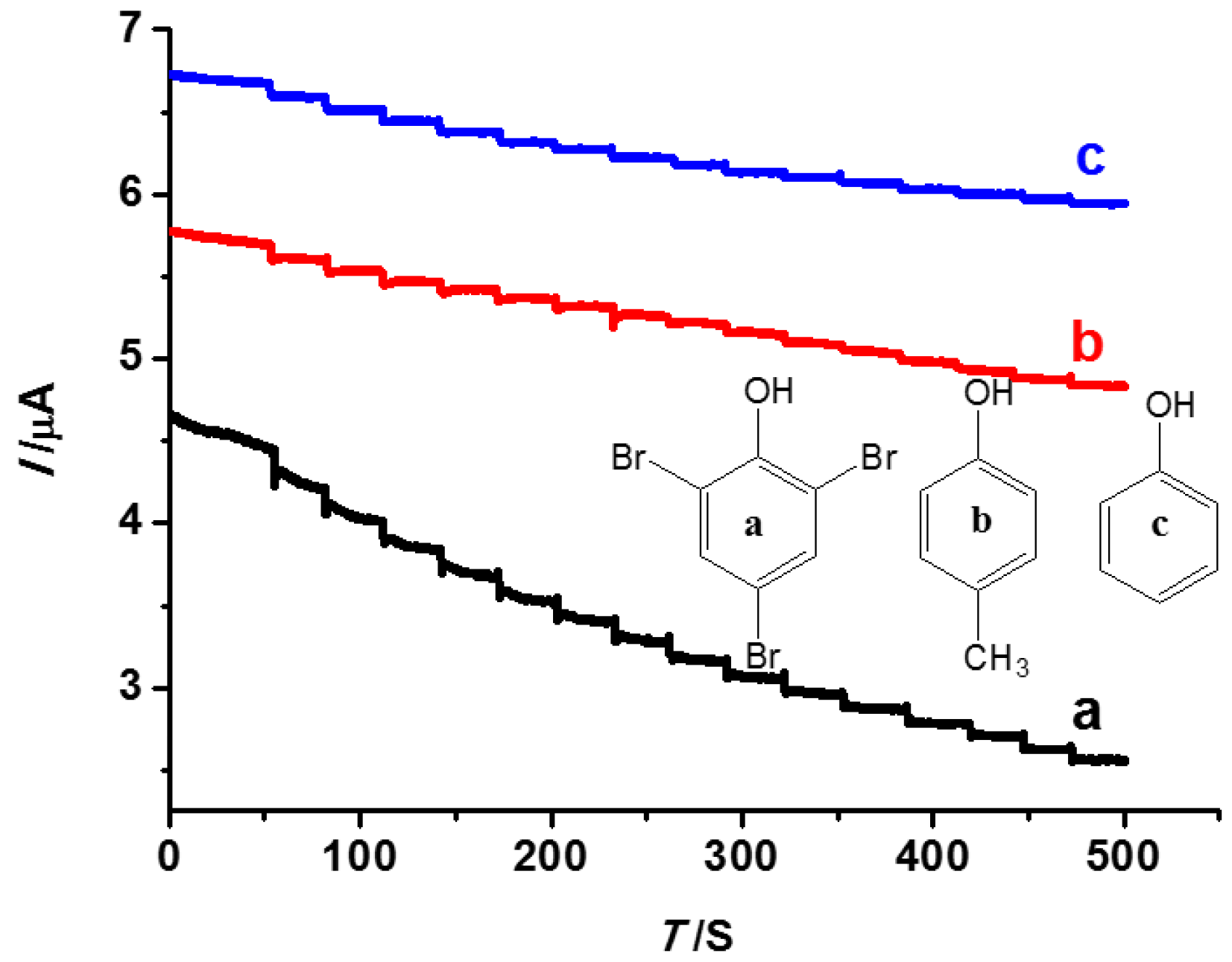
3.5. The Selectivity Performance of the INS
3.6. Regeneration and Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- De Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Thomsen, C.; Lundanes, E.; Becher, G. Brominated flame retardants in archived serum samples from Norway: A study on temporal trends and the role of age. Environ. Sci. Technol. 2002, 36, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Covaci, A.; Harrad, S.; Abdallah, M.A.E.; Ali, N.; Law, J.; Herzke, D.; de Wit, C.A. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ. Int. 2002, 7, 532–556. [Google Scholar] [CrossRef] [PubMed]
- De Wit, C.A.; Herzke, D.; Vorkam, K. Brominated flame retardants in the arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408, 2885–2918. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Jakobsson, E. Decomposition of tetrabromobisphenol A in the presence of UV-light and hydroxyl radicals. Organohalogen Compd. 1998, 35, 419–422. [Google Scholar]
- Vetter, W.; Janussen, D. Halogenated natural products in five species of Antarctic sponges: Compounds with POP-like properties? Environ. Sci. Technol. 2005, 39, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Nichkova, M.; Marco, M.P. Biomonitoring human exposure to organohalogenated substances by measuring urinary chlorophenols using a high-throughput screening (HTS) immunochemical method. Environ. Sci. Technol. 2006, 40, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Nichkova, M.; Germani, M.; Marco, M.P. Immunochemical analysis of 2,4,6-tribromophenol for assessment of wood contamination. J. Agric. Food Chem. 2008, 56, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.F.; Sanderson, J.T.; Letcher, R.J.; Bergman, A.; van den Berg, M. Inhibition and induction of aromatase (CYP19) activity by brominated flame. Toxicol. Sci. 2005, 88, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, C.S.; Yu, L.Q.; Zhou, B.S. Chronic exposure to environmental levels of tribromophenol impairs zebrafish reproduction. Toxicol. Appl. Pharm. 2010, 243, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Blythe, J.W.; Heitz, A.; Joll, C.A.; Kagi, R.I. Determination of trace concentrations of bromophenols in water using purge-and-trap after in situ acetylation. J. Chromatogr. A 2006, 1102, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Polo, M.; Llompart, M.; Garcia-Jares, C.; Gomez-Noya, G.; Bollain, M.H.; Cela, R. Development of a solid-phase microextraction method for the analysis of phenolic flame retardants in water samples. J. Chromatogr. A 2006, 1124, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.; Alquie, L.; Kaisa, J.P.; Reed, G.; Gilmor, T.; Vas, G. Method development and validation for the determination of 2,4,6-tribromoanisole, 2,4,6-tribromophenol, 2,4,6-trichloroanisole, and 2,4,6-trichlorophenol in various drug products using stir bar sorptive extraction and gas chromatography–tandem mass spectrometry detection. J. Chromatogr. A 2012, 1262, 196–204. [Google Scholar] [PubMed]
- Gong, K.P.; Zhu, X.Z.; Zhao, R.; Xiong, S.X.; Mao, L.Q.; Chen, C.F. Rational attachment of synthetic triptycene orthoquinone onto carbon nanotubes for electrocatalysis and sensitive detection of thiols. Anal. Chem. 2005, 77, 8158–8165. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Moayeri, A.; Ajji, A. Fabrication of polyaniline/poly(ethylene oxide)/non- covalently functionalized graphene nanofibers via electrospinning. Synth. Met. 2015, 200, 7–15. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Zheng, B.Z.; Liu, G.Y.; Yao, A.W.; Xiao, Y.L.; Du, J.; Guo, Y.; Xiao, D.; Hu, Q.M.; Choi, M.F. A sensitive AgNPs/CuO nanofibers non-enzymatic glucose sensor based on electrospinning technology. Sens. Actuators B Chem. 2014, 195, 431–438. [Google Scholar] [CrossRef]
- Ji, Y.T.; Yan, C.C.; Yu, H.; Chen, L.Z.; Dong, F.C. One-step fabrication of ammonia sensor by electrospinning PS-b-PMA nanofibers on quartz crystal microbalance. Sens. Actuators B Chem. 2014, 203, 459–464. [Google Scholar] [CrossRef]
- Osmani, Q.; Hughes, H.; McLoughlin, P. Probing the recognition of molecularly imprinted polymer beads. J. Mater. Sci. 2012, 47, 2218–2227. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.P.; Prasad, A. Molecularly imprinted polymer based enantioselective sensing devices: A review. Anal. Chim. Acta 2015, 853, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Augusto, F.; Hantao, L.W.; Mogollon, N.; Braga, S. New materials and trends in sorbents for solid-phase extraction. TrAC Trends Anal. Chem. 2013, 43, 14–23. [Google Scholar] [CrossRef]
- Nicolescu, V.; Meouche, W.; Branger, C.; Margaillan, A.; Sarbu, A.; Fruth, V.; Donescu, D. A new microemulsion approach for producing molecularly imprinted polymers with selective recognition cavities for gallic acid. Polym. Int. 2013, 62, 949–956. [Google Scholar] [CrossRef]
- Ma, Y.; Pan, G.Q.; Zhang, Y.; Guo, X.Z.; Zhang, H.Q. Narrowly dispersed hydrophilic molecularly imprinted polymer nanoparticles for efficient molecular recognition in real aqueous samples including river water, milk, and bovine serum. Angew. Chem. Int. Ed. 2013, 52, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Chandra, B.K.C.; Sosnowska, M.; Sobczak, J.W.; Nesterov, V.N.; D’Souza, F.; Kutner, W. Nicotine molecularly imprinted polymer: Synergy of coordination and hydrogen bonding. Biosens. Bioelectron. 2015, 64, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Yola, M.L.; Atar, N. A novel molecular imprinted nanosensor based quartz crystal microbalance for determination of kaempferol. Sens. Actuators B Chem. 2014, 194, 79–85. [Google Scholar] [CrossRef]
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical detection of sulfanilamide using pencil graphite electrode based on molecular imprinting technology. Electroanalysis 2014, 26, 2328–2336. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Alexander, S. Design and fabrication of molecularly imprinted polymer-based potentiometric sensor from the surface modified multiwalled carbon nanotube for the determination of lindane (γ-hexachlorocyclohexane), an organochlorine pesticide. Biosens. Bioelectron. 2015, 64, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cong, J.; Liu, J.; Gao, Y.; Liu, X. A facile approach for synthesizing molecularly imprinted graphene for ultrasensitive and selective electrochemical detecting 4-nitrophenol. Anal. Chim. Acta 2015, 864, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Z.; Chen, X.; Chen, R.; Zheng, X. Preparation of imprinted PVB/β-CD nanofiber by electrospinning technique and its selective binding abilities for naringin. Chin. J. Chem. 2011, 29, 1753–1758. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhang, Z.; Wang, L.; Chen, Z.; Xiang, S. The cooperative utilization of imprinting, electro-spinning and a pore-forming agent to synthesize β-cyclodextrin polymers with enhanced recognition of naringin. RSC Adv. 2013, 3, 25396–25402. [Google Scholar] [CrossRef]
- Ma, X.; Wu, D.; Huang, L.; Wu, Z.; Xiang, S.; Chen, S. Sensing 2,4,6-tribromophenol based on molecularly imprinted technology. Monatshefte Chem. 2015, 146, 485–491. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Wu, D.; Wang, L.; Zhang, Z.; Xiang, S. Ultrasensitive sensing tris (2,3-dibromopropyl) isocyanurate based on the synergistic effect of amino and hydroxyl groups of molecularly imprinted poly (o-aminophenol) film. New J. Chem. 2016, 40, 1649–1654. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Milosevic, B.; Frenot, A.; Ye, L. Generation of molecular recognition sites in electrospun polymer nanofibers via molecular imprinting. Macromolecules 2006, 39, 357–361. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Alexandra, J.; Bengt, H.; Ye, L. Encapsulation and selective recognition of molecularly imprinted theophylline and 17 β-estradiol nanoparticles within electrospun polymer nanofibers. Langmuir 2006, 22, 8960–8965. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Q.; Zhang, Y.; Liu, Y.; Wan, Y.; Gao, K.; Huang, Y.; Xia, W.; Wang, H.; Shi, Y.; et al. Molecularly imprinted nanofiber membranes enhanced biodegradation of trace bisphenol A by pseudomonas aeruginosa. Chem. Eng. J. 2015, 262, 989–998. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, D.; Liu, H.; Zeng, Y.; Yin, Z. Electrochemical molecular imprinted sensors based on electrospun nanofiber and determination of ascorbic acid. Anal. Sci. 2015, 31, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, D.; Huang, J.; Tu, H.; Yang, Y.; Zhang, A. One-step electrodeposition of a molecularly imprinting chitosan/phenyltrimethoxysilane/AuNPs hybrid film and its application in the selective determination of p-nitrophenol. Analyst 2013, 138, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Deng, J.; Xiao, X.L.; Ding, L.; Yuan, Y.L.; Li, H.; Li, X.T.; Yan, X.N.; Wang, L.L. Electrochemical sensor based on a poly(para-aminobenzoic acid) film modified glassy carbon electrode for the determination of melamine in milk. Electrochim. Acta 2011, 56, 4595–4602. [Google Scholar] [CrossRef]




© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Li, M.; Wu, D.; Ma, X.; Wu, Z.; Xiang, S.; Chen, S. Molecularly Imprinted Nanofiber Film for Sensitive Sensing 2,4,6-Tribromophenol. Polymers 2016, 8, 222. https://doi.org/10.3390/polym8060222
Huang L, Li M, Wu D, Ma X, Wu Z, Xiang S, Chen S. Molecularly Imprinted Nanofiber Film for Sensitive Sensing 2,4,6-Tribromophenol. Polymers. 2016; 8(6):222. https://doi.org/10.3390/polym8060222
Chicago/Turabian StyleHuang, Limei, Meishan Li, Dan Wu, Xiuling Ma, Zhenyue Wu, Shengchang Xiang, and Sheng Chen. 2016. "Molecularly Imprinted Nanofiber Film for Sensitive Sensing 2,4,6-Tribromophenol" Polymers 8, no. 6: 222. https://doi.org/10.3390/polym8060222