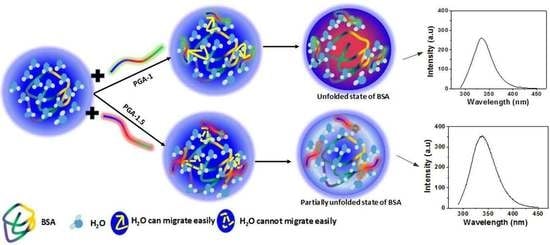
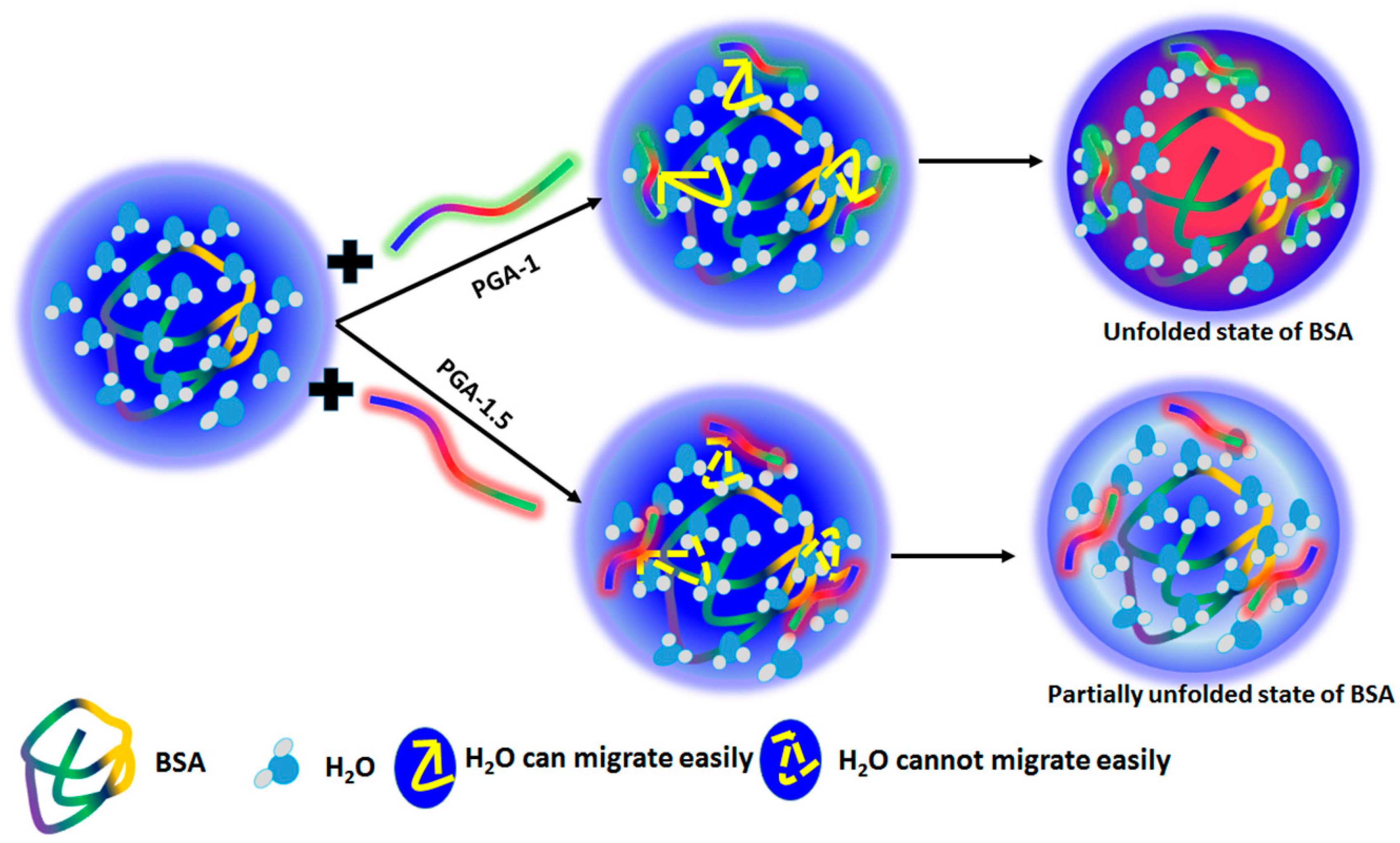
Exploring the Behavior of Bovine Serum Albumin in Response to Changes in the Chemical Composition of Responsive Polymers: Experimental and Simulation Studies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of P(NIPAM-co-PEGMA-co-AA) Copolymers
2.3. Sample Preparation
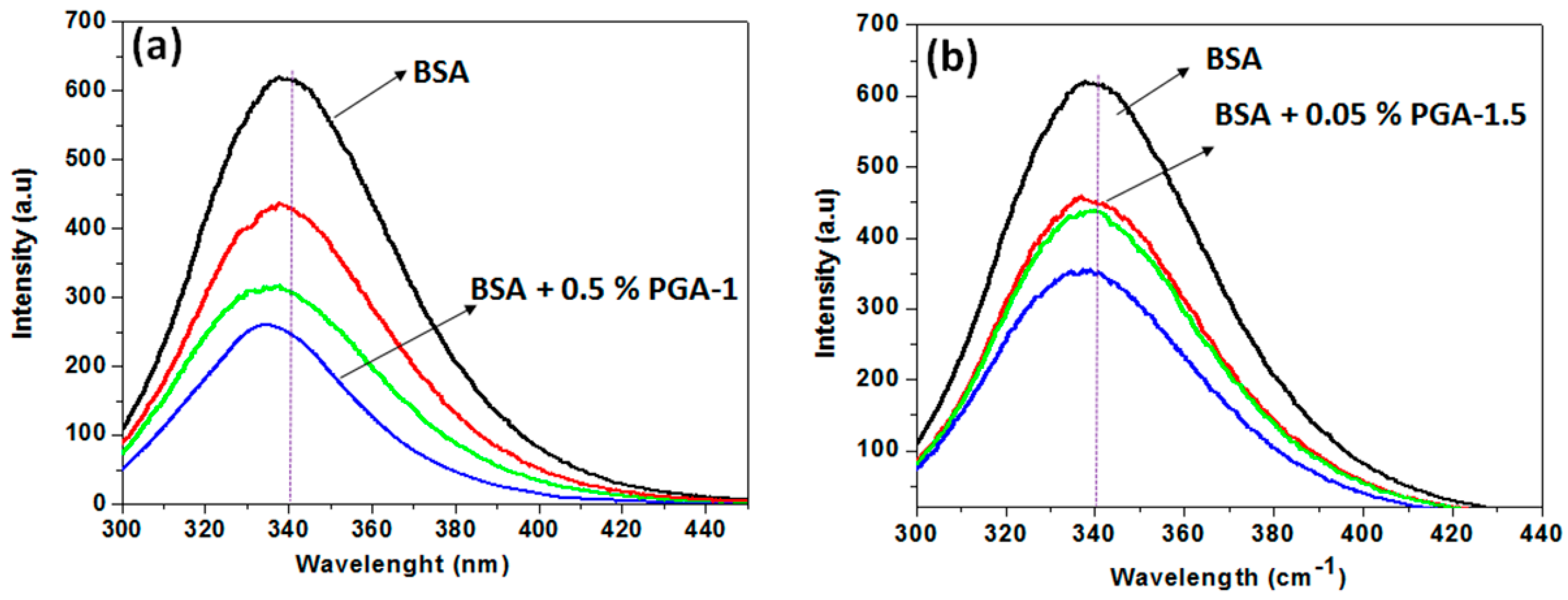
2.4. Fluorescence Intensity Measurements
2.5. Circular Dichroism (CD) Spectroscopy
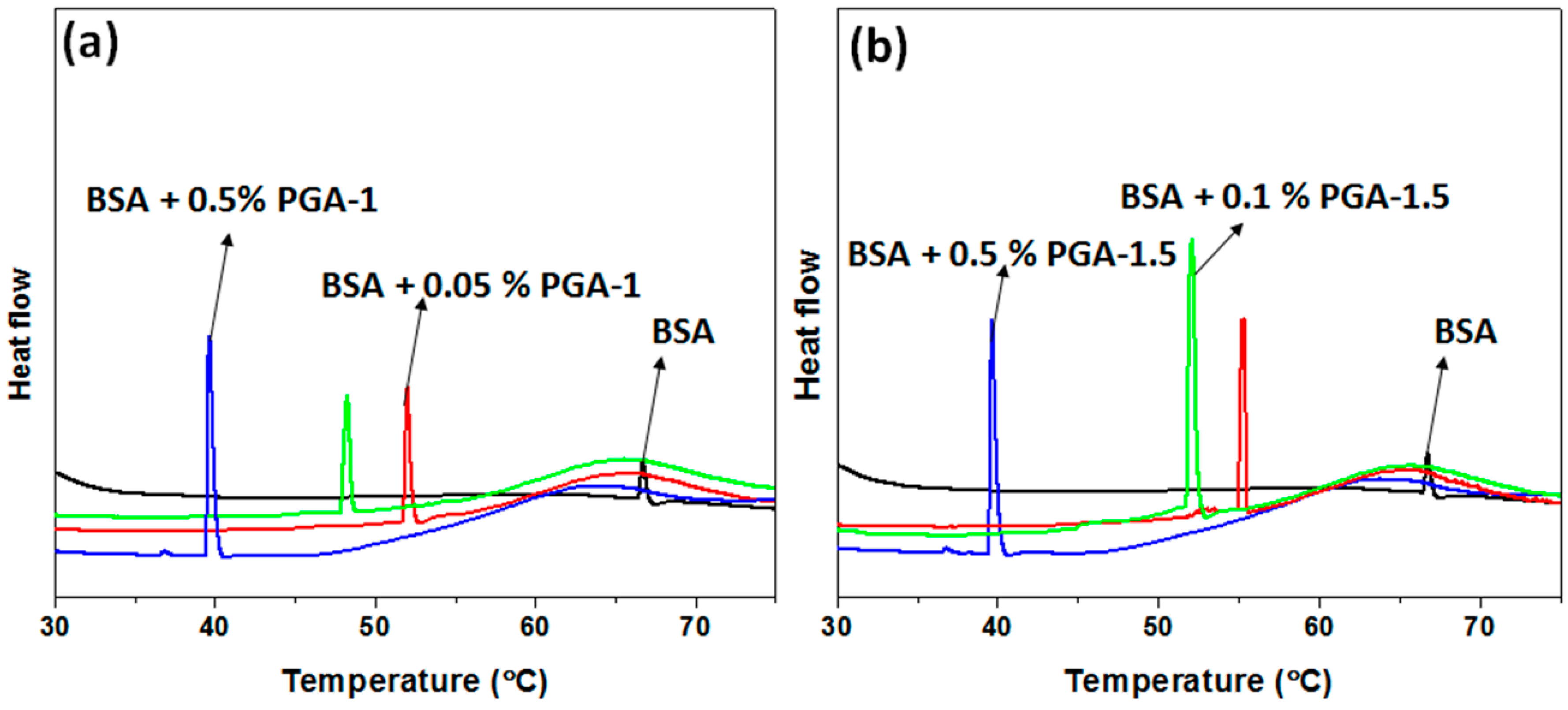
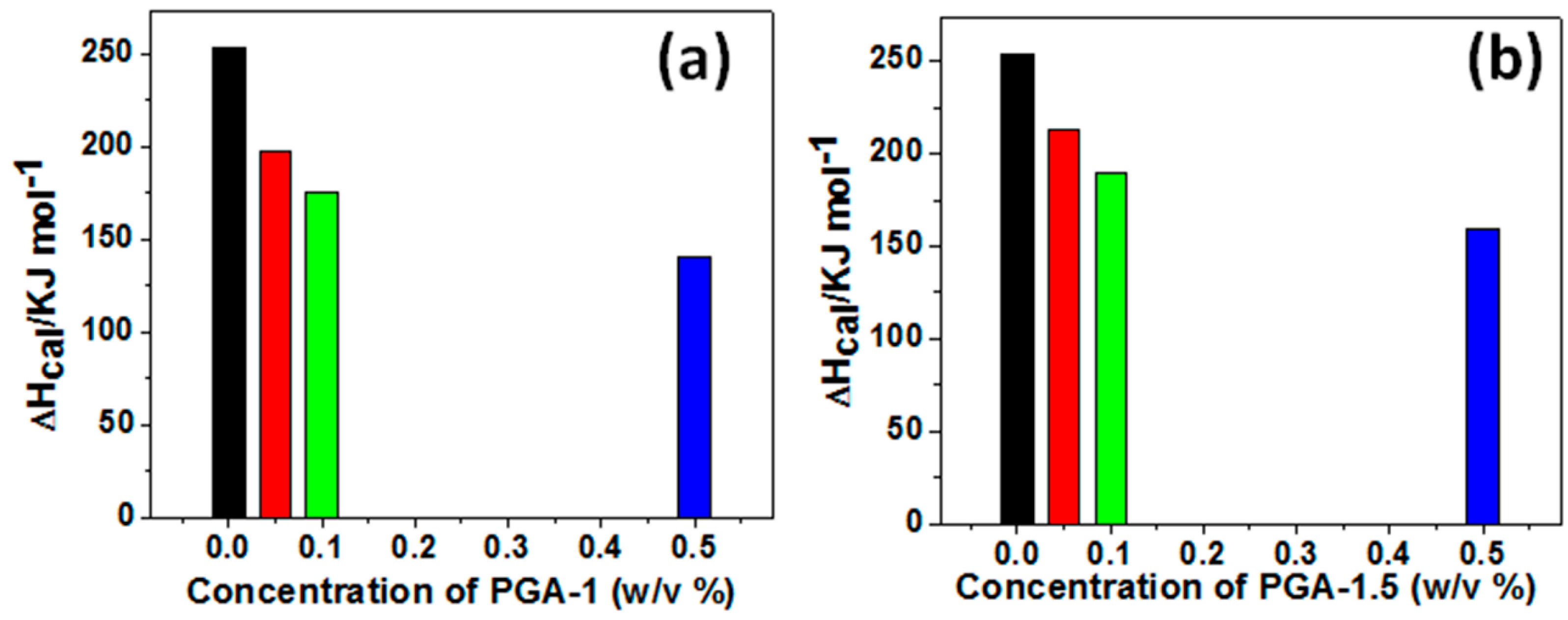
2.6. Differential Scanning Calorimetry (DSC)
2.7. Molecular Docking (MD) Simulations
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Rijn, P. Polymer directed protein assemblies. Polymers 2013, 5, 576–599. [Google Scholar] [CrossRef]
- Seelbach, R.J.; Fransen, P.; Pulido, D.; D’Este, M.; Duttenhoefer, F.; Sauerbier, S.; Freiman, T.; Niemeyer, P.; Albericio, F.; Alini, M.; et al. Injectable hyaluronan hydrogels with peptide-binding dendrimers modulate the controlled release of BMP-2 and TGF-β1. Macromol. Biosci. 2015, 15, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, T.; Kim, J.O.; Jang, S.; Kim, D.P.; Kang, I.K.; Park, S.Y. Multifaceted thermoresponsive poly(N-vinylcaprolactam) coupled with carbon dots for biomedical applications. Mater. Sci. Eng. C 2016, 61, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Lakhiari, H.; Okano, T.; Nurdin, N.; Luthi, C.; Descouts, P.; Muller, D.; Jozefonvicz, J. Temperature-responsive size-exclusion chromatography using poly(N-isopropylacrylamide) grafted silica. Biochim. Biophys. Acta 1998, 1379, 303–313. [Google Scholar] [CrossRef]
- Tan, S.W.; Han, C.; Wang, H.J.; Liu, D.R.; Tu, K.H.; Jiang, H.L.; Zhang, M.H.; Wang, L.Q. Preparation and characterization of thermo-sensitive mixed micelles and in vitro drug release. Acta Polym. Sin. 2011, 11, 1237–1243. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Chen, J.K.; Chang, C.J. Fabrications and applications of stimulus-responsive polymer films and patterns on surfaces: A review. Materials 2014, 7, 805–875. [Google Scholar] [CrossRef]
- Schattling, P.; Jochum, F.D.; Theato, P. Multi-stimuli responsive polymers—The all-in-one talents. Polym. Chem. 2014, 5, 25–36. [Google Scholar] [CrossRef]
- Zhang, Q.; Vanparijs, N.; Louage, B.; De Geest, B.G.; Hoogenboom, R. Dual pH- and temperature-responsive RAFT-based block co-polymer micelles and polymer-protein conjugates with transient solubility. Polym. Chem. 2014, 5, 1140–1144. [Google Scholar] [CrossRef]
- You, S.; Cai, Q.; Müllen, K.; Yang, W.; Yin, M. pH-sensitive unimolecular fluorescent polymeric micelles: From volume phase transition to optical response. Chem. Commun. 2014, 50, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Reddy, P.M.; Hsieh, S.R.; Huang, H.C. Influence of imidazolium based green solvents on volume phase transition temperature of crosslinked poly(N-isopropylacrylamide-co-acrylic acid) hydrogel. Soft Matter 2015, 11, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Reddy, P.M.; Kumar, A.; Venkatesu, P.; Chang, C.J. The biological stimuli for governing the phase transition temperature of the “smart” polymer PNIPAM in water. Colloids Surf. B 2015, 135, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, Y.; Nagumo, Y.; Suzuki, Y.; Funatsu, T.; Ishikawa, Y.; Kanazawa, H. The effects of anionic electrolytes and human serum albumin on the LCST of poly(N-isopropylacrylamide)-based temperature-responsive copolymers. Colloids Surf. B 2015, 132, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hamerska-Dudra, A.; Bryjak, J.; Trochimczuk, A.W. Immobilization of glucoamylase and trypsin on crosslinked thermosensitive carriers. Enzym. Microb. Technol. 2007, 41, 197–204. [Google Scholar] [CrossRef]
- Hamerska-Dudra, A.; Bryjak, J.; Trochimczuk, A.W. Novel method of enzymes stabilization on crosslinked thermosensitive carriers. Enzym. Microb. Technol. 2006, 38, 921–925. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, J.; Ren, H.; Zhou, Q.; Lin, Q. Horseradish peroxidase immobilized in macroporous hydrogel for acrylamide polymerization. J. Polym. Sci. A Polym. Chem. 2008, 46, 2222–2232. [Google Scholar] [CrossRef]
- Miletić, N.; Vuković, Z.; Nastasović, A.; Loos, K. Effect of Candida antarctica Lipase B immobilization on the porous structure of the carrier. Macromol. Biosci. 2011, 11, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Schachschal, S.; Adler, H.J.; Pich, A.; Wetzel, S.; Matura, A.; van Pee, K.H. Encapsulation of enzymes in microgels by polymerization/cross-linking in aqueous droplets. Colloid Polym. Sci. 2011, 289, 693–698. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, C.; Hu, R.; Lin, W.; Wang, Q.; Zhao, J.; Bilinovich, S.M.; Leeper, T.C.; Li, L.; Cheung, H.M.; et al. Probing the weak interaction of proteins with neutral and zwitterionic antifouling polymers. Acta Biomater. 2014, 10, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Q.; Chen, J.; Luo, R.; Maitz, M.F.; Huang, N. Immobilization of serum albumin and peptide aptamer for EPC on polydopamine coated titanium surface for enhanced in-situ self-endothelialization. Mater. Sci. Eng. C 2016, 60, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Derkus, B.; Emregul, K.C.; Emregul, E. Evaluation of protein immobilization capacity on various carbon nanotube embedded hydrogel biomaterials. Mater. Sci. Eng. C 2015, 56, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ogorzalek, T.L.; Wei, S.; Liu, Y.; Wang, Q.; Brooks, C.L.; Chen, Z.; Marsh, E.N.G. Molecular-level insights into orientation-dependent changes in the thermal stability of enzymes covalently immobilized on surfaces. Langmuir 2015, 31, 6145–6153. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, C.; Lin, W.; Hu, R.; Wang, Q.; Chen, H.; Li, L.; Chen, S.; Zheng, J. Binding characteristics between polyethylene glycol (PEG) and proteins in aqueous solution. J. Mater. Chem. B 2014, 2, 2983–2992. [Google Scholar] [CrossRef]
- Bharmoria, P.; Kumar, A. Thermodynamic investigations of protein’s behaviour with ionic liquids in aqueous medium studied by isothermal titration calorimetry. Biochim. Biophys. Acta 2016, 1860, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Lomova, M.V.; Brichkina, A.I.; Kiryukhin, M.V.; Vasina, E.N.; Pavlov, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Antipina, M.N. Multilayer capsules of bovine serum albumin and tannic acid for controlled release by enzymatic degradation. ACS Appl. Mater. Interfaces 2015, 7, 11732–11740. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Mukherjee, S. Binding, unfolding and refolding dynamics of serum albumins. Biochim. Biophys. Acta 2013, 1830, 5394–5404. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Peters, T. Serum albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [PubMed]
- Kragh-Hansen, U. Molecular aspects of ligand binding to serum albumin. Pharmacol. Rev. 1981, 33, 17–53. [Google Scholar] [PubMed]
- Peters, T. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Thermo-sensitive polymeric micelles based on poly(N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T.; Miyazaki, T.; Kohori, F.; Sakai, K.; Yokoyama, M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J. Control. Release 2006, 115, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Liu, S.-Q.; Heng, P.W.-S.; Yang, Y.-Y. Evaluating proteins release from, and their interactions with, thermosensitive poly (N-isopropylacrylamide) hydrogels. J. Control. Release 2005, 102, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, S.-H.; Park, K.D.; Jung, M.C.; Yang, W.I.; Han, S.W.; Noh, J.Y.; Lee, J.W. Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(N-isopropylacrylamide) and water-soluble chitosan copolymer. Biomaterials 2004, 25, 5743–5751. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Gupta, S.; Dey, D.; Maiti, S.; Dhara, D. Synthesis of PEG containing cationic block copolymers and their interaction with human serum albumin. React. Funct. Polym. 2014, 74, 81–89. [Google Scholar] [CrossRef]
- Nho, Y.-C.; Park, J.-S.; Lim, Y.-M. Preparation of poly(acrylic acid) hydrogel by radiation crosslinking and its application for mucoadhesives. Polymers 2014, 6, 890–898. [Google Scholar] [CrossRef]
- Reddy, P.M.; Chang, C.J.; Hsieh, S.; Huang, H.; Lee, M. Overview of the effect of monomers and green solvents on thermoresponsive copolymers: Phase transition temperature and surface properties. RSC Adv. 2015, 5, 86901–86909. [Google Scholar] [CrossRef]
- Mikheeva, L.M.; Grinberg, N.V.; Mashkevich, A.Y.; Grinberg, V.Y.; Thanh, L.T.M.; Makhaeva, E.E.; Khokhlov, A.R. Microcalorimetric study of thermal cooperative transitions in poly(N-vinylcaprolactam) hydrogels. Macromolecules 1997, 30, 2693–2699. [Google Scholar] [CrossRef]
- CLC Drug Discovery Workbench. Available online: http://www.clcbio.com/products/clc-drug-discovery-workbench/ (accessed on 21 September 2015).
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77, 114–122. [Google Scholar] [CrossRef] [PubMed]
- YASARA SERVER. Available online: http://www.yasara.org/minimizationserver.htm (accessed on 21 September 2015).
- Korb, O.; Stützle, T.; Exner, T.E. Empirical scoring functions for advanced protein-ligand docking with plants. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- CLC Drug Discovery Workbench—CLC Manuals. Available online: http://www.clcsupport.com/clcdrugdiscoveryworkbench/current/User_Manual.pdf (accessed on 21 September 2015).
- Abou-Zied, O.K.; Al-Shihi, O.I.K. Characterization of subdomain IIA binding site of human serum albumin in its native, unfolded, and refolded states using small molecular probes. J. Am. Chem. Soc. 2008, 130, 10793–10801. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: New York, NY, USA, 2006; pp. 529–575. [Google Scholar]
- Deep, S.; Ahluwalia, J.C. Interaction of bovine serum albumin with anionic surfactants. Phys. Chem. Chem. Phys. 2001, 3, 4583–4591. [Google Scholar] [CrossRef]
- Gupta, B.S.; Taha, M.; Lee, M.J. Buffers more than buffering agent: Introducing a new class of stabilizers for the protein BSA. Phys. Chem. Chem. Phys. 2015, 17, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.K.; Ahmad, E.; Khan, J.M.; Alam, P.; Ishtikhar, M.; Khan, R.H. Elucidating the interaction of limonene with bovine serum albumin: a multi-technique approach. Mol. BioSyst. 2015, 11, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Keer, M.; Smirnovas, V.; Winter, R.; Richtering, W. Copolymer microgels from mono- and disubstituted acrylamides: Phase behavior and hydrogen bonds. Macromolecules 2008, 41, 6830–6836. [Google Scholar] [CrossRef]
- Mittal, S.; Chowhan, R.K.; Singh, L.R. Macromolecular crowding: Macromolecules friend or foe. Biochim. Biophys. Acta 2015, 1850, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, S.; Pan, L.; Li, J.; Liu, F.; Liu, H. Spectroscopic studies on the interactions between imidazolium chloride ionic liquids and bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kanga, J.; Liua, Y.; Xiea, M.X.; Lia, S.; Jianga, M.; Wang, Y.D. Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Biophys. Acta 2004, 1674, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Azegami, S.; Tsuboi, A.; Izumi, T.; Hirata, M.; Dubin, P.L.; Wang, B.; Kokufuta, E. Formation of an intrapolymer complex from human serum albumin and poly(ethylene glycol). Langmuir 1999, 15, 940–947. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Liu, R.T.; Zhao, X.C.; Yang, B.J.; Gao, C.Z.; Hao, X.P.; Wu, Y.Z. New strategy for the evaluation of CdTe quantum dot toxicity targeted to bovine serum albumin. Sci. Total Environ. 2009, 407, 5019–5023. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, G.; Sun, Y.; Zhang, H.; Mao, H.; Feng, Y. Interaction between proteins and cationic gemini surfactant. Biomacromolecules 2007, 8, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Woody, R.W.; Tinoco, I. Optical rotation of oriented helices. III. Calculation of the rotatory dispersion and circular dichroism of the Alpha- and 310-Helix. J. Chem. Phys. 1967, 46, 4927–4945. [Google Scholar] [CrossRef]
- Taha, M.; Quental, M.V.; Correia, I.; Freirea, M.G.; Coutinho, J.A.P. Extraction and stability of bovine serum albumin (BSA) using cholinium-based Good’s buffers ionic liquids. Process Biochem. 2015, 50, 1158–1166. [Google Scholar] [CrossRef]
- Zhang, H.M.; Lou, K.; Cao, J.; Wang, Y.Q. Interaction of a hydrophobic-functionalized PAMAM dendrimer with bovine serum albumin: Thermodynamic and structural changes. Langmuir 2014, 30, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Flora, K.; Brennan, J.D.; Baker, G.A.; Doody, M.A.; Bright, F.V. Unfolding of acrylodan-labeled human serum albumin probed by steady-state and time-resolved fluorescence methods. Biophys. J. 1998, 75, 1084–1096. [Google Scholar] [CrossRef]
- Pfeil, W.; Privalov, P.L. Thermodynamic investigations of proteins: II. Calorimetric study of lysozyme denaturation by guanidine hydrochloride. Biophys. Chem. 1976, 4, 33–40. [Google Scholar] [CrossRef]
- Cai, W.S.; Gan, L.H.; Tam, K.C. Phase transition of aqueous solutions of poly(N,N-diethylacrylamide-co-acrylic acid) by differential scanning calorimetric and spectrophotometric methods. Colloid Polym. Sci. 2001, 279, 793–799. [Google Scholar] [CrossRef]
- Keerl, M.; Richtering, W. Synergistic depression of volume phase transition temperature in copolymer microgels. Colloid Polym. Sci. 2007, 285, 471–474. [Google Scholar] [CrossRef]
- Li, B.; Turuvekere, S.; Agrawal, M.; La, D.; Ramani, K.; Kihara, D. Characterization of local geometry of protein surfaces with the visibility criterion. Proteins 2008, 71, 670–683. [Google Scholar] [CrossRef] [PubMed]

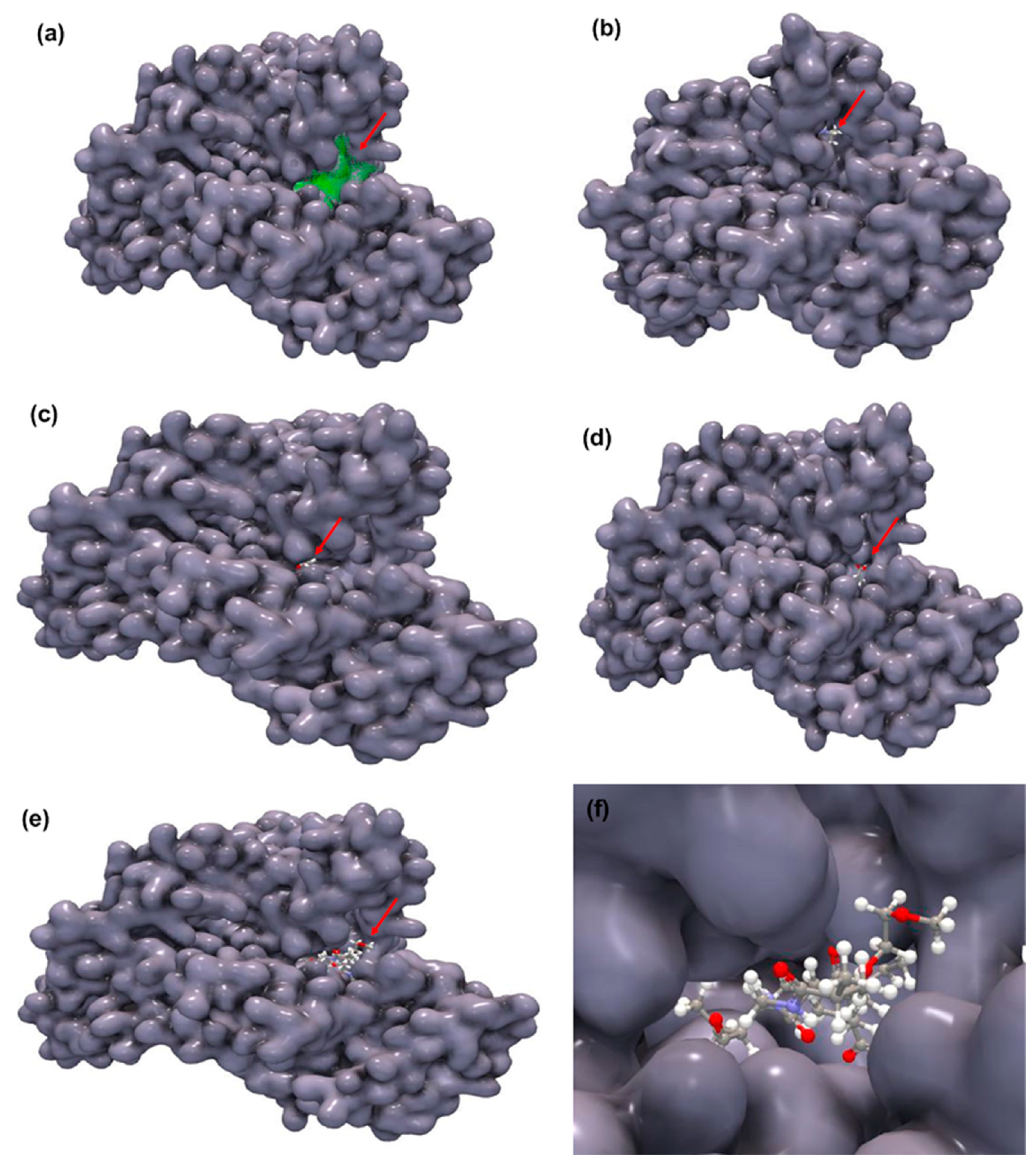
| Molecular dimensions of the binding sites | Docking score | H–bond score |
|---|---|---|
| Copolymer | ||
| 632.83 Å | −61.86 | −19.13 |
| 422.83 Å | −45.87 | −14.57 |
| 127.49 Å | −59.78 | −4.00 |
| PEGMA | ||
| 632.83 Å | −27.44 | −4.00 |
| 422.83 Å | −27.48 | −8.00 |
| 127.49 Å | −31.39 | −3.98 |
| NIPAM | ||
| 632.83 Å | −23.99 | −0.72 |
| 422.83 Å | −27.68 | −4.00 |
| 127.49 Å | −28.78 | 0.00 |
| AA | ||
| 632.83 Å | −22.91 | −9.72 |
| 422.83 Å | −21.73 | −11.62 |
| 127.49 Å | −20.50 | −4.00 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, S.-R.; Reddy, P.M.; Chang, C.-J.; Kumar, A.; Wu, W.-C.; Lin, H.-Y. Exploring the Behavior of Bovine Serum Albumin in Response to Changes in the Chemical Composition of Responsive Polymers: Experimental and Simulation Studies. Polymers 2016, 8, 238. https://doi.org/10.3390/polym8060238
Hsieh S-R, Reddy PM, Chang C-J, Kumar A, Wu W-C, Lin H-Y. Exploring the Behavior of Bovine Serum Albumin in Response to Changes in the Chemical Composition of Responsive Polymers: Experimental and Simulation Studies. Polymers. 2016; 8(6):238. https://doi.org/10.3390/polym8060238
Chicago/Turabian StyleHsieh, Shih-Rong, P. Madhusudhana Reddy, Chi-Jung Chang, Awanish Kumar, Wan-Chi Wu, and Hui-Yi Lin. 2016. "Exploring the Behavior of Bovine Serum Albumin in Response to Changes in the Chemical Composition of Responsive Polymers: Experimental and Simulation Studies" Polymers 8, no. 6: 238. https://doi.org/10.3390/polym8060238
APA StyleHsieh, S.-R., Reddy, P. M., Chang, C.-J., Kumar, A., Wu, W.-C., & Lin, H.-Y. (2016). Exploring the Behavior of Bovine Serum Albumin in Response to Changes in the Chemical Composition of Responsive Polymers: Experimental and Simulation Studies. Polymers, 8(6), 238. https://doi.org/10.3390/polym8060238