Modulation of the Catalytic Properties of Lipase B from Candida antarctica by Immobilization on Tailor-Made Magnetic Iron Oxide Nanoparticles: The Key Role of Nanocarrier Surface Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Magnetic Nanoparticles Synthesis
2.3. Magnetic Nanoparticles Surface Modification
2.4. Enzyme Chemical Amination
2.5. Enzyme Immobilization on Magnetic Nanoparticles
2.6. Lipase Activity Assay
2.7. Structural, Colloidal and Magnetic Properties Characterization
2.8. Enzymatic Kinetic Resolution of Racemic Methyl Mandelate (1)
2.9. Thermal Stability Assay
2.10. Enzyme Nanocatalyst Storage Evaluation
3. Results and Discussion
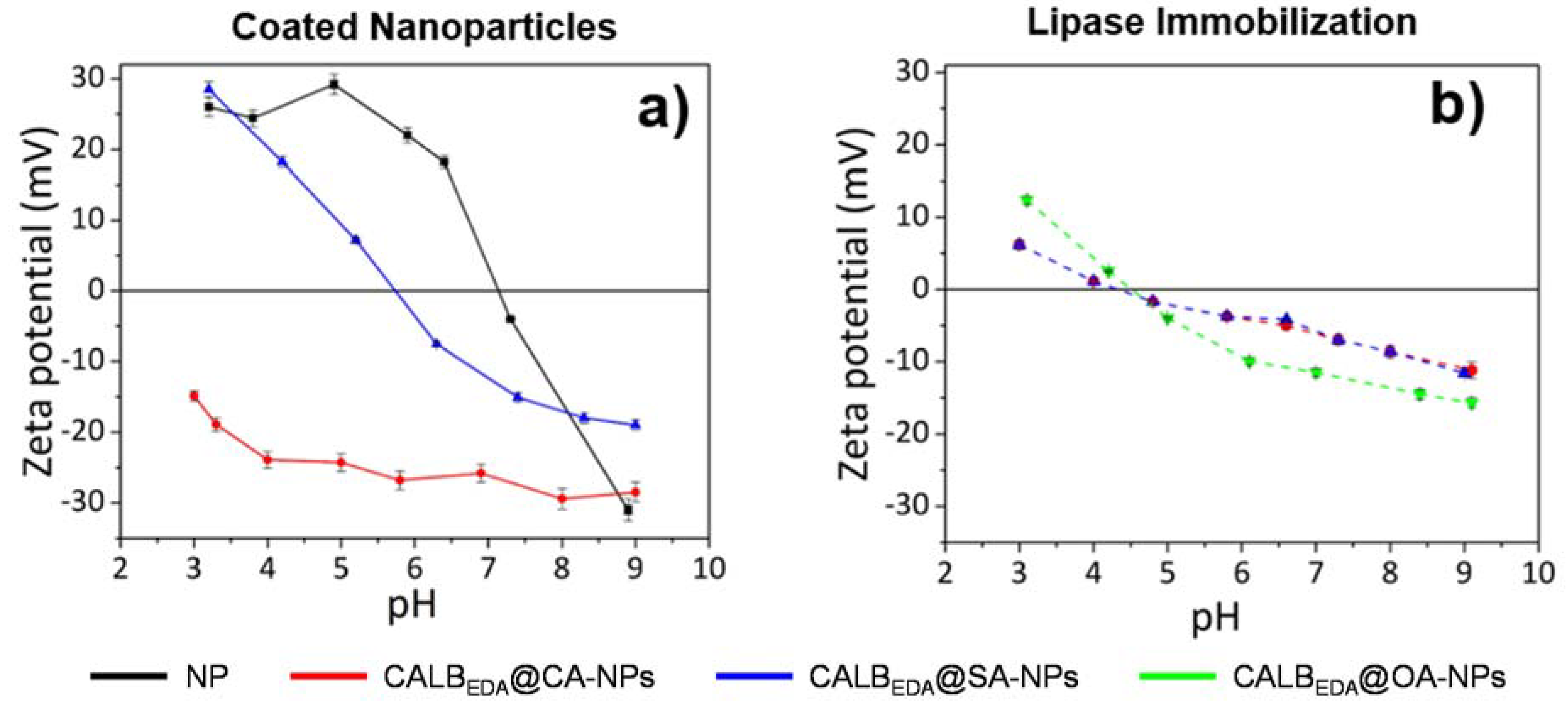
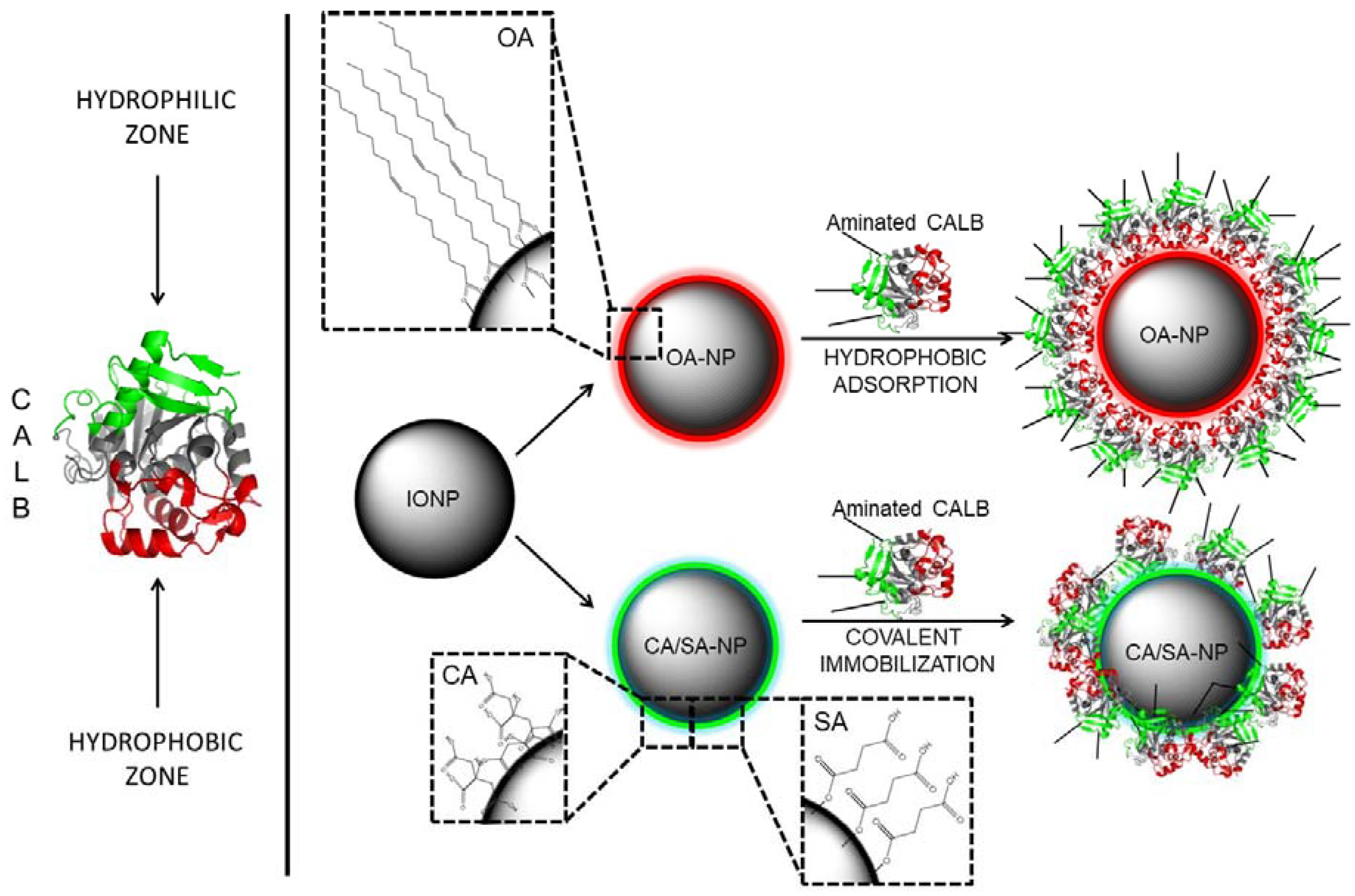
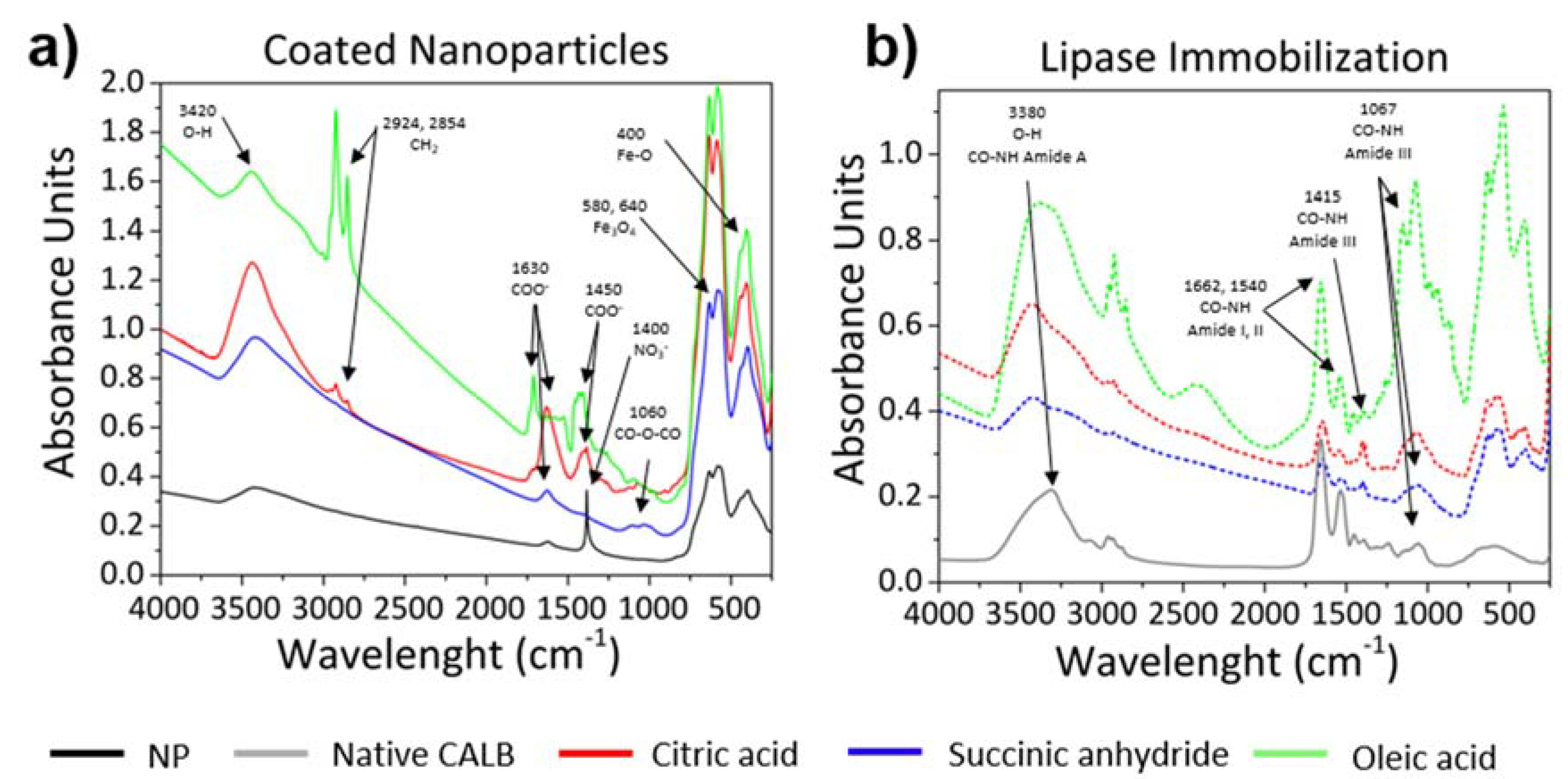
3.1. Magnetic Nanoparticles with Tailor-Made Surface Coating as Support for Enzyme Immobilization: Preparation and Characterization
3.2. Controlled Enzyme Immobilization on Tailor-Made Magnetic Nanocarriers
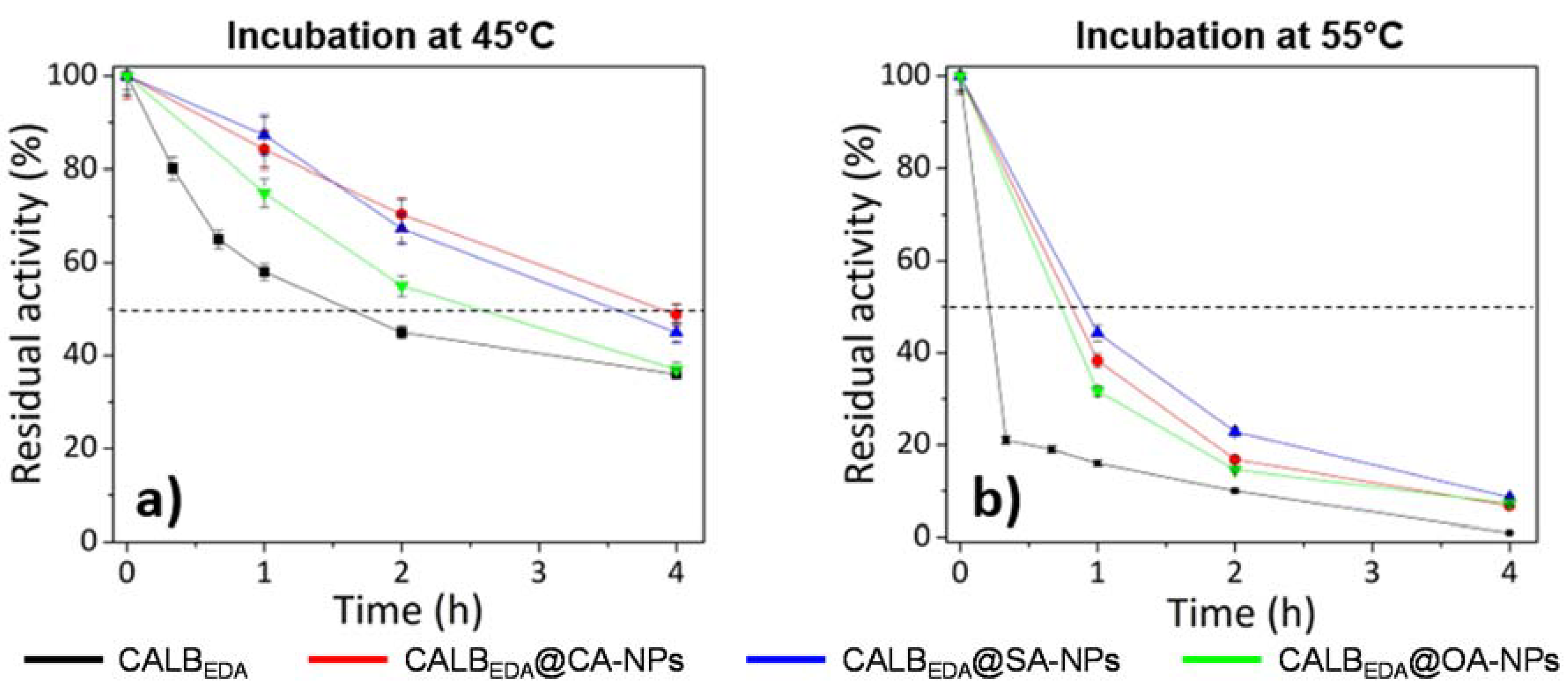
3.3. Thermal Stability Studies
3.4. Kinetic Resolution of Racemic Methyl Mandelate (1) Using Enzymatic Nanoderivatives
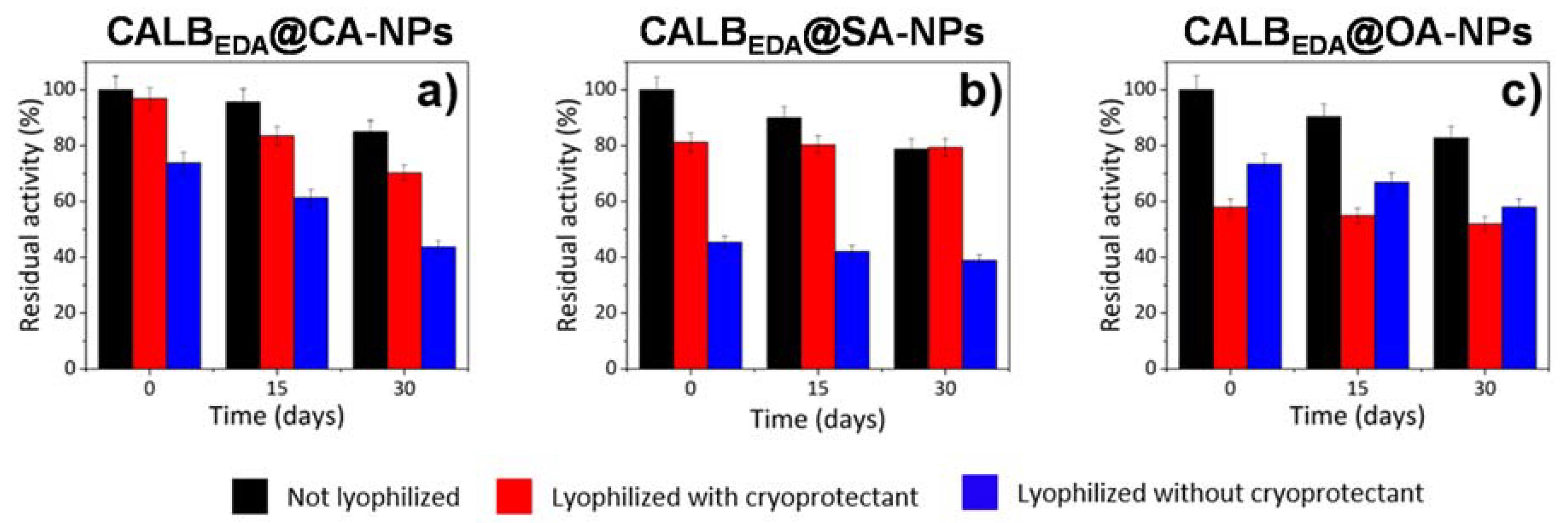
3.5. Nanocatalyst Storage Studies
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jegannathan, K.R.; Nielsen, P.H. Environmental assessment of enzyme use in industrial production—A literature review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef]
- Soetaert, W.; Vandamme, E.J. The scope and impact of industrial biotechnology. Ind. Biotechnol. 2010. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Chisti, Y. Biotechnology—A sustainable alternative for chemical industry. Biotechnol. Adv. 2005, 23, 471–499. [Google Scholar] [CrossRef] [PubMed]
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial enzyme applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W. Enantiopreference of candida antarctica lipase b toward carboxylic acids: Substrate models and enantioselectivity thereof. J. Mol. Catal. B Enzym. 2016, 127, 98–116. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Busto, E.; Gotor, V. Candida antarctica lipase b: An ideal biocatalyst for the preparation of nitrogenated organic compounds. Adv. Syn. Catal. 2006, 348, 797–812. [Google Scholar] [CrossRef]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst–many applications: The use of candida antarctica b-lipase in organic synthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Fukuda, H.; Hama, S.; Tamalampudi, S.; Noda, H. Whole-cell biocatalysts for biodiesel fuel production. Trends Biotechnol. 2008, 26, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Filice, M. New emerging bio-catalysts design in biotransformations. Biotechnol. Adv. 2015, 33, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Filice, M.; Romero, O.; Guisan, J.M. Improving lipase activity by immobilization and post-immobilization strategies. In Immobilization of Enzymes and Cells, 3rd ed.; Guisan, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 255–273. [Google Scholar]
- Marciello, M.; Filice, M.; Palomo, J.M. Different strategies to enhance the activity of lipase catalysts. Catal. Sci. Technol. 2012, 2, 1531–1543. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Grate, J.W.; Wang, P. Nanostructures for enzyme stabilization. Chem. Eng. Sci. 2006, 61, 1017–1026. [Google Scholar] [CrossRef]
- Filice, M.; Marciello, M.; del Puerto Morales, M.; Palomo, J.M. Synthesis of heterogeneous enzyme-metal nanoparticle biohybrids in aqueous media and their applications in C–C bond formation and tandem catalysis. Chem. Commun. 2013, 49, 6876–6878. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Romero, O.; Gutierrez-Fernandez, J.; de las Rivas, B.; Hermoso, J.A.; Palomo, J.M. Synthesis of a heterogeneous artificial metallolipase with chimeric catalytic activity. Chem. Commun. 2015, 51, 9324–9327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, L.S.; Khan, F.; Micklefield, J. Selective covalent protein immobilization: Strategies and applications. Chem. Rev. 2009, 109, 4025–4053. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Romero, O.; Aires, A.; Guisan, J.M.; Rumbero, A.; Palomo, J.M. Preparation of an immobilized lipase-palladium artificial metalloenzyme as catalyst in the heck reaction: Role of the solid phase. Adv. Syn. Catal. 2015, 357, 2687–2696. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Guisan, J.M.; Terreni, M.; Palomo, J.M. Regioselective monodeprotection of peracetylated carbohydrates. Nat. Protoc. 2012, 7, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Uygun, D.A.; Öztürk, N.; Akgöl, S.; Denizli, A. Novel magnetic nanoparticles for the hydrolysis of starch with bacillus licheniformis α-amylase. J. Appl. Polym. Sci. 2012, 123, 2574–2581. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, A.; Su, M. Nanomaterials for biosensing applications. Nanomaterials 2016, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, C.; Yu, C.; Zhang, S.; Liu, B.; Kong, J. Protein chips and nanomaterials for application in tumor marker immunoassays. Biosens. Bioelectron. 2009, 24, 3399–3411. [Google Scholar] [CrossRef] [PubMed]
- Baniukevic, J.; Hakki Boyaci, I.; Goktug Bozkurt, A.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic gold nanoparticles in sers-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosens. Bioelectron. 2013, 43, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.-P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.-C.; Gazeau, F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Reena Mary, A.P.; Sunny, V.; Sakthikumar, D.; Yoshida, Y.; Joy, P.A.; Anantharaman, M.R. Synthesis of bio-compatible spion–based aqueous ferrofluids and evaluation of radiofrequency power loss for magnetic hyperthermia. Nanoscale Res. Lett. 2010, 5, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Aki, A.; Mizuki, T.; Maekawa, T.; Usami, R.; Morimoto, H. Encouragement of enzyme reaction utilizing heat generation from ferromagnetic particles subjected to an ac magnetic field. PLoS ONE 2015, 10, e0127673. [Google Scholar] [CrossRef] [PubMed]
- Mizuki, T.; Sawai, M.; Nagaoka, Y.; Morimoto, H.; Maekawa, T. Activity of lipase and chitinase immobilized on superparamagnetic particles in a rotational magnetic field. PLoS ONE 2013, 8, e66528. [Google Scholar] [CrossRef] [PubMed]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; Yousefi, P.; Mohammadi, J.; Brask, J. Enzymatic production of biodiesel using lipases immobilized on silica nanoparticles as highly reusable biocatalysts: Effect of water, t-butanol and blue silica gel contents. Renew. Energy 2016, 91, 196–206. [Google Scholar] [CrossRef]
- Gao, J.; Kong, W.; Zhou, L.; He, Y.; Ma, L.; Wang, Y.; Yin, L.; Jiang, Y. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chem. Eng. J. 2017, 309, 70–79. [Google Scholar] [CrossRef]
- Ren, Y.; Rivera, J.G.; He, L.; Kulkarni, H.; Lee, D.-K.; Messersmith, P.B. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol. 2011, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, T.; Basak, A.; Manocha, L.M.; Shah, A.R.; Madamwar, D. Robust nanobioconjugates of candida antarctica lipase B—Multiwalled carbon nanotubes: Characterization and application for multiple usages in non-aqueous biocatalysis. Bioresour. Technol. 2013, 140, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, H.; Sun, H.; Zhao, L.; Wei, D. Process development for the production of (R)-(−)-mandelic acid by recombinant escherichia coli cells harboring nitrilase from burkholderia cenocepacia j2315. Org. Process Res. Dev. 2015, 19, 2012–2016. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.; Martínez, B.; Sandiumenge, F. Surface and internal spin canting in γ-Fe2O3 nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Costo, R.; Bello, V.; Robic, C.; Port, M.; Marco, J.F.; Puerto Morales, M.; Veintemillas-Verdaguer, S. Ultrasmall iron oxide nanoparticles for biomedical applications: Improving the colloidal and magnetic properties. Langmuir 2012, 28, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, F.; Lévy, M.; Wilhelm, C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine 2008, 3, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Van Ewijk, G.A.; Vroege, G.J.; Philipse, A.P. Convenient preparation methods for magnetic colloids. J. Magn. Magn. Mater. 1999, 201, 31–33. [Google Scholar] [CrossRef]
- Martina, M.-S.; Fortin, J.-P.; Ménager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of superparamagnetic liposomes revealed as highly efficient mri contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, H.; Sánchez, E.H.; Brollo, M.E.F.; Asín, L.; Moerner, K.K.; Frandsen, C.; Lázaro, F.J.; Serna, C.J.; Veintemillas-Verdaguer, S.; Morales, M.P.; et al. Formation mechanism of maghemite nanoflowers synthesized by a polyol-mediated process. ACS Omega 2017, 2, 7172–7184. [Google Scholar] [CrossRef]
- Montes, T.; Grazu, V.; López-Gallego, F.; Hermoso, J.A.; Guisán, J.M.; Fernández-Lafuente, R. Chemical modification of protein surfaces to improve their reversible enzyme immobilization on ionic exchangers. Biomacromolecules 2006, 7, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lorente, G.; Godoy, C.A.; Mendes, A.A.; Lopez-Gallego, F.; Grazu, V.; de las Rivas, B.; Palomo, J.M.; Hermoso, J.; Fernandez-Lafuente, R.; Guisan, J.M. Solid-phase chemical amination of a lipase from bacillus thermocatenulatus to improve its stabilization via covalent immobilization on highly activated glyoxyl-agarose. Biomacromolecules 2008, 9, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Marciello, M.; Bolivar, J.M.; Filice, M.; Mateo, C.; Guisan, J.M. Preparation of lipase-coated, stabilized, hydrophobic magnetic particles for reversible conjugation of biomacromolecules. Biomacromolecules 2013, 14, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Dyal, A.; Loos, K.; Noto, M.; Chang, S.W.; Spagnoli, C.; Shafi, K.V.P.M.; Ulman, A.; Cowman, M.; Gross, R.A. Activity of candida rugosa lipase immobilized on γ-Fe2O3 magnetic nanoparticles. J. Am. Chem. Soc. 2003, 125, 1684–1685. [Google Scholar] [CrossRef] [PubMed]
- Alnoch, C.R.; Rodrigues de Melo, R.; Palomo, M.J.; Maltempi de Souza, E.; Krieger, N.; Mateo, C. New tailor-made alkyl-aldehyde bifunctional supports for lipase immobilization. Catalysts 2016, 6, 191. [Google Scholar] [CrossRef]
- Ju, T.; Brewer, K.; D’Souza, A.; Cummings, R.D.; Canfield, W.M. Cloning and expression of human core 1 β1, 3-galactosyltransferase. J. Biol. Chem. 2002, 277, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Natividad, E.; Castro, M.; Mediano, A. Adiabatic vs. Non-adiabatic determination of specific absorption rate of ferrofluids. J. Magn. Magn. Mater. 2009, 321, 1497–1500. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, M.A.; Torres, T.E.; Andrés-Vergés, M.; Costo, R.; de la Presa, P.; Serna, C.J.; Morales, M.P.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Magnetic nanoparticles for power absorption: Optimizing size, shape and magnetic properties. J. Solid State Chem. 2009, 182, 2779–2784. [Google Scholar] [CrossRef] [Green Version]
- Marciello, M.; Rossi, S.; Caramella, C.; Remuñán-López, C. Freeze-dried cylinders carrying chitosan nanoparticles for vaginal peptide delivery. Carbohydr. Polym. 2017, 170, 43–51. [Google Scholar] [CrossRef] [PubMed]
- De la Presa, P.; Luengo, Y.; Multigner, M.; Costo, R.; Morales, M.P.; Rivero, G.; Hernando, A. Study of heating efficiency as a function of concentration, size, and applied field in γ-Fe2O3 nanoparticles. J. Phys. Chem. C 2012, 116, 25602–25610. [Google Scholar] [CrossRef]
- De la Presa, P.; Luengo, Y.; Velasco, V.; Morales, M.P.; Iglesias, M.; Veintemillas-Verdaguer, S.; Crespo, P.; Hernando, A. Particle interactions in liquid magnetic colloids by zero field cooled measurements: Effects on heating efficiency. J. Phys. Chem. C 2015, 119, 11022–11030. [Google Scholar] [CrossRef] [Green Version]
- Schiestel, T.; Brunner, H.; Tovar, G.E.M. Controlled surface functionalization of silica nanospheres by covalent conjugation reactions and preparation of high density streptavidin nanoparticles. J. Nanosci. Nanotechnol. 2004, 4, 504–511. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, A.G.; Atta, M.A.; Al-Lohedan, A.H. Synthesis and evaluation of poly(sodium 2-acrylamido-2-methylpropane sulfonate-co-styrene)/magnetite nanoparticle composites as corrosion inhibitors for steel. Molecules 2014, 19, 1713–1731. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Valle, G.; Bonetto, R.; Ferreira, M.L.; Briand, L.E. Ftir, sem and fractal dimension characterization of lipase b from candida antarctica immobilized onto titania at selected conditions. Appl. Surf. Sci. 2010, 256, 1624–1635. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Cheraghipour, E.; Tamaddon, A.M.; Javadpour, S.; Bruce, I.J. Peg conjugated citrate-capped magnetite nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2013, 328, 91–95. [Google Scholar] [CrossRef]
- Barbosa, O.; Ruiz, M.; Ortiz, C.; Fernández, M.; Torres, R.; Fernandez-Lafuente, R. Modulation of the properties of immobilized calb by chemical modification with 2,3,4-trinitrobenzenesulfonate or ethylendiamine. Advantages of using adsorbed lipases on hydrophobic supports. Process Biochem. 2012, 47, 867–876. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Fessi, H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur. J. Pharm. Biopharm. 2006, 63, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Gupta, M.N. Freeze-drying of proteins: Some emerging concerns. Biotechnol. Appl. Biochem. 2004, 39, 165–177. [Google Scholar] [CrossRef] [PubMed]
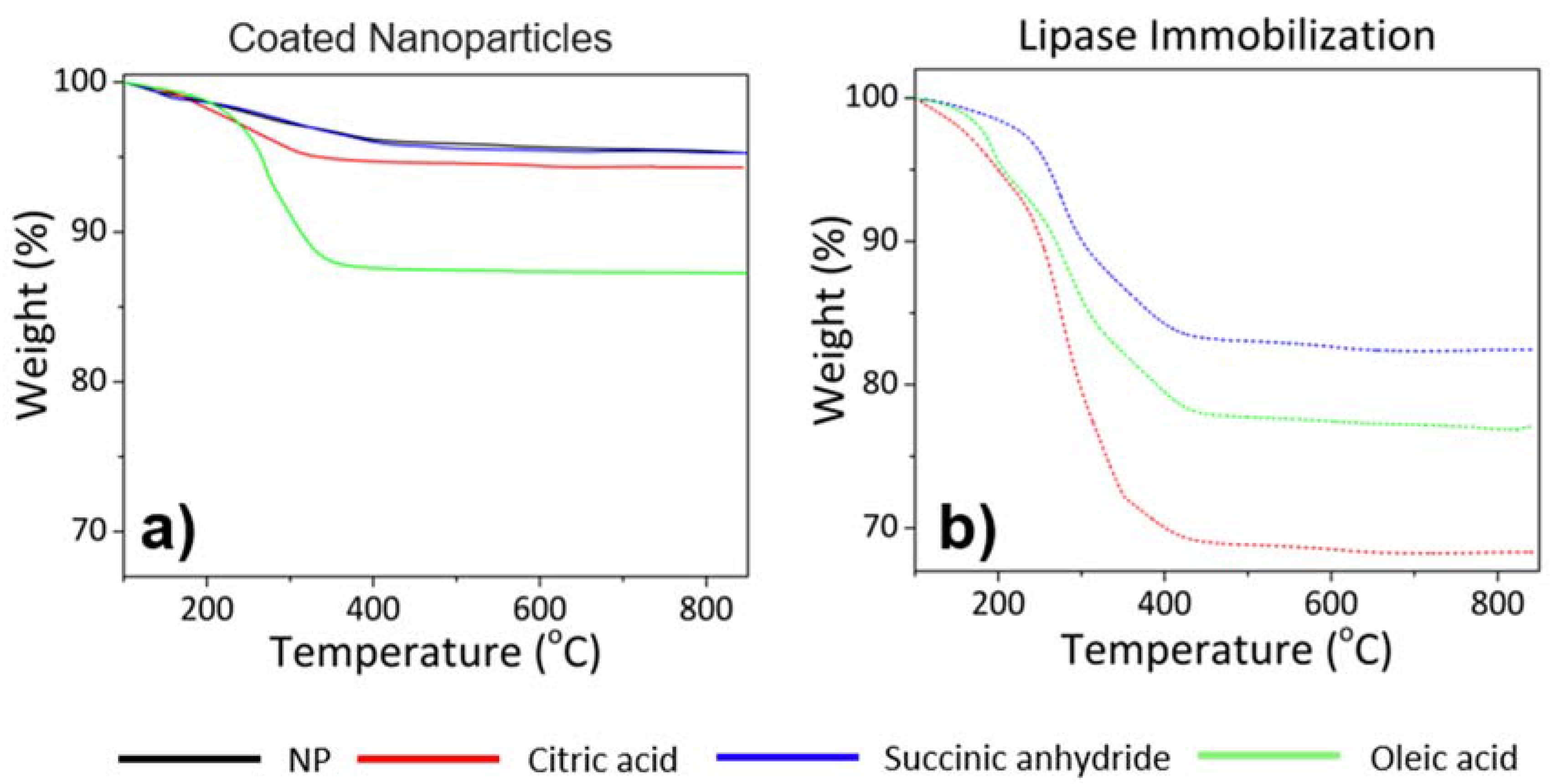


| Sample | Immobilization | TEM | DLS | ζ-potential | SAR a | Ms | ||
|---|---|---|---|---|---|---|---|---|
| - | µg Enzyme/mg Fe | Enzyme activity (IU b/mglip) | Core size (nm) | ØHydr.Z-average (nm) | PDI c | pH 7 (mV) | W/gFe | emu/g γ-Fe2O3 |
| NP | - | - | 12.9 ± 2.6 | 95.5 | 0.2 | +3.45 | 131 | 67.9 ± 0.03 |
| CA-NP | - | - | 11.7 3.3 | 80.72 | 0.19 | −25.7 | 161.2 | 53.9 ± 0.01 |
| CALBEDA@ CA-NP | 234 | 5.73 | 10.3 ± 4.3 | 120.8 | 0.13 | −6.2 | 75.36 | 31.13 ± 0.02 |
| SA-NP | - | - | 13 ± 2.7 | 121.5 | 0.15 | −13.9 | 125.6 | 57.67 ± 0.02 |
| CALBEDA@ SA-NP | 149 | 8.28 | 12.4 ± 2.2 | 202.2 | 0.17 | −6.2 | 60.7 | 36.35 ± 0.01 |
| OA-NP d | - | - | 12 ± 2.8 | 109.2 | 0.15 | - | 106.45 | 64.78 ± 0.04 |
| CALBEDA@ OA-NP | 162.7 | 13.5 | 11.8 ± 2.2 | 248.7 | 0.31 | −5.7 | 58.61 | 49.6 ± 0.03 |
| Entry | T (°C) | Catalyst a | Specific activity (IU/mglip) b | c (%) | ee (%) c | Ed |
|---|---|---|---|---|---|---|
| 1 | 25 | CALBEDA@CA-NPs | 32 | 48 e | 77.6 (R) | 27 |
| 2 | 25 | CALBEDA@SA-NPs | 34.6 | 51.5 e | 87 (R) | 28 |
| 3 | 25 | CALBEDA@OA-NPs | 46.6 | 55 e | 74 (R) | 9 |
| 4 | 45 | CALBEDA@CA-NPs | 83.2 | 49.5 f | 84 (R) | 34 |
| 5 | 45 | CALBEDA@SA-NPs | 88 | 51 f | 89 (R) | 38 |
| 6 | 45 | CALBEDA@OA-NPs | 120 | 53 f | 80 (R) | 14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viñambres, M.; Filice, M.; Marciello, M. Modulation of the Catalytic Properties of Lipase B from Candida antarctica by Immobilization on Tailor-Made Magnetic Iron Oxide Nanoparticles: The Key Role of Nanocarrier Surface Engineering. Polymers 2018, 10, 615. https://doi.org/10.3390/polym10060615
Viñambres M, Filice M, Marciello M. Modulation of the Catalytic Properties of Lipase B from Candida antarctica by Immobilization on Tailor-Made Magnetic Iron Oxide Nanoparticles: The Key Role of Nanocarrier Surface Engineering. Polymers. 2018; 10(6):615. https://doi.org/10.3390/polym10060615
Chicago/Turabian StyleViñambres, Mario, Marco Filice, and Marzia Marciello. 2018. "Modulation of the Catalytic Properties of Lipase B from Candida antarctica by Immobilization on Tailor-Made Magnetic Iron Oxide Nanoparticles: The Key Role of Nanocarrier Surface Engineering" Polymers 10, no. 6: 615. https://doi.org/10.3390/polym10060615