Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
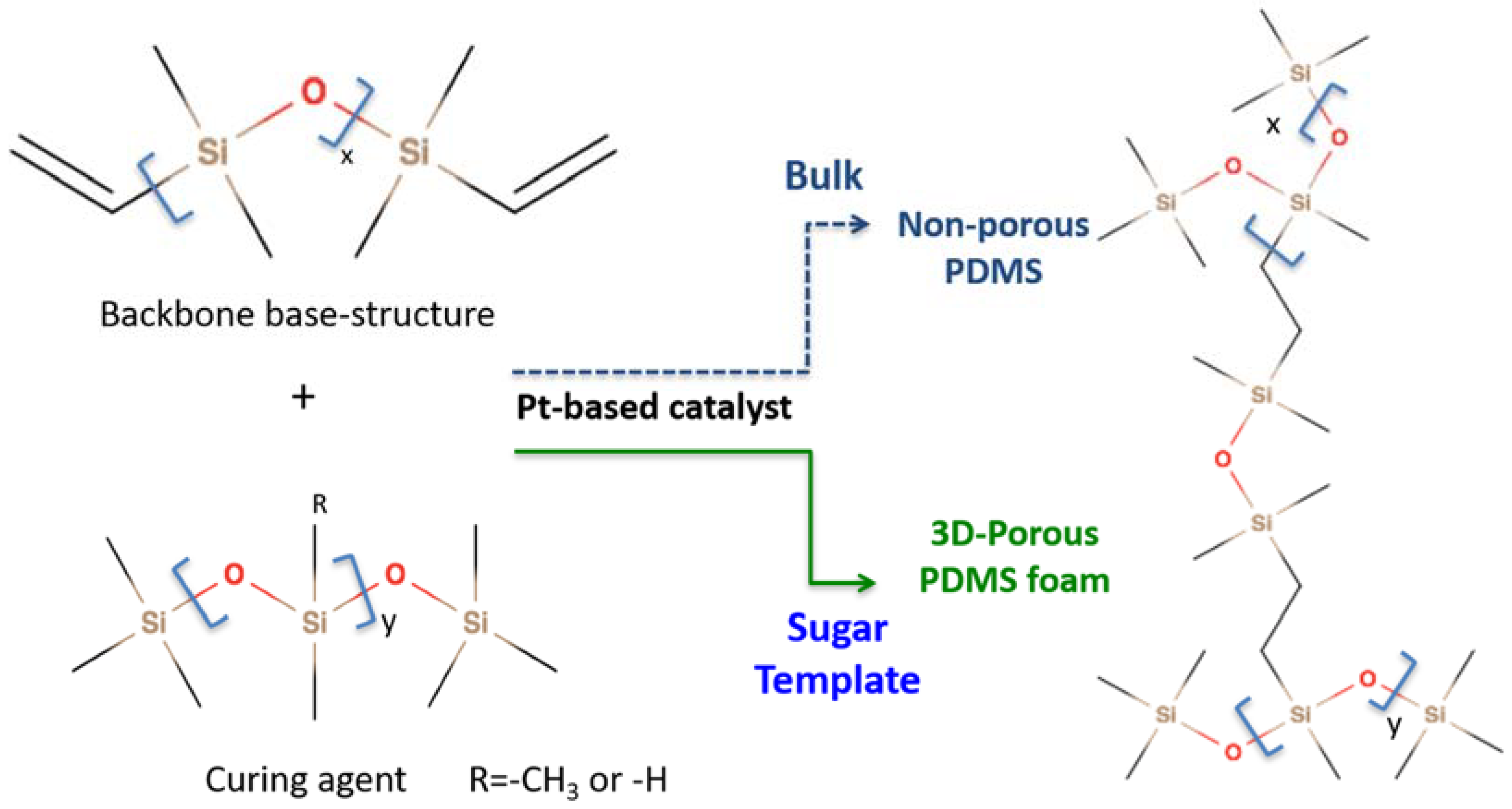
2.2. Preparation of Porous PDMS Foam
2.3. Morphological Characterization
2.4. Thermogravimetry
2.5. ATR-FTIR Analysis
3. Results and Discussion
3.1. PDMS Crosslinking Polycondensation Reaction
3.2. Surface and Morphological Characterization of 3D Porous PDMS Foam
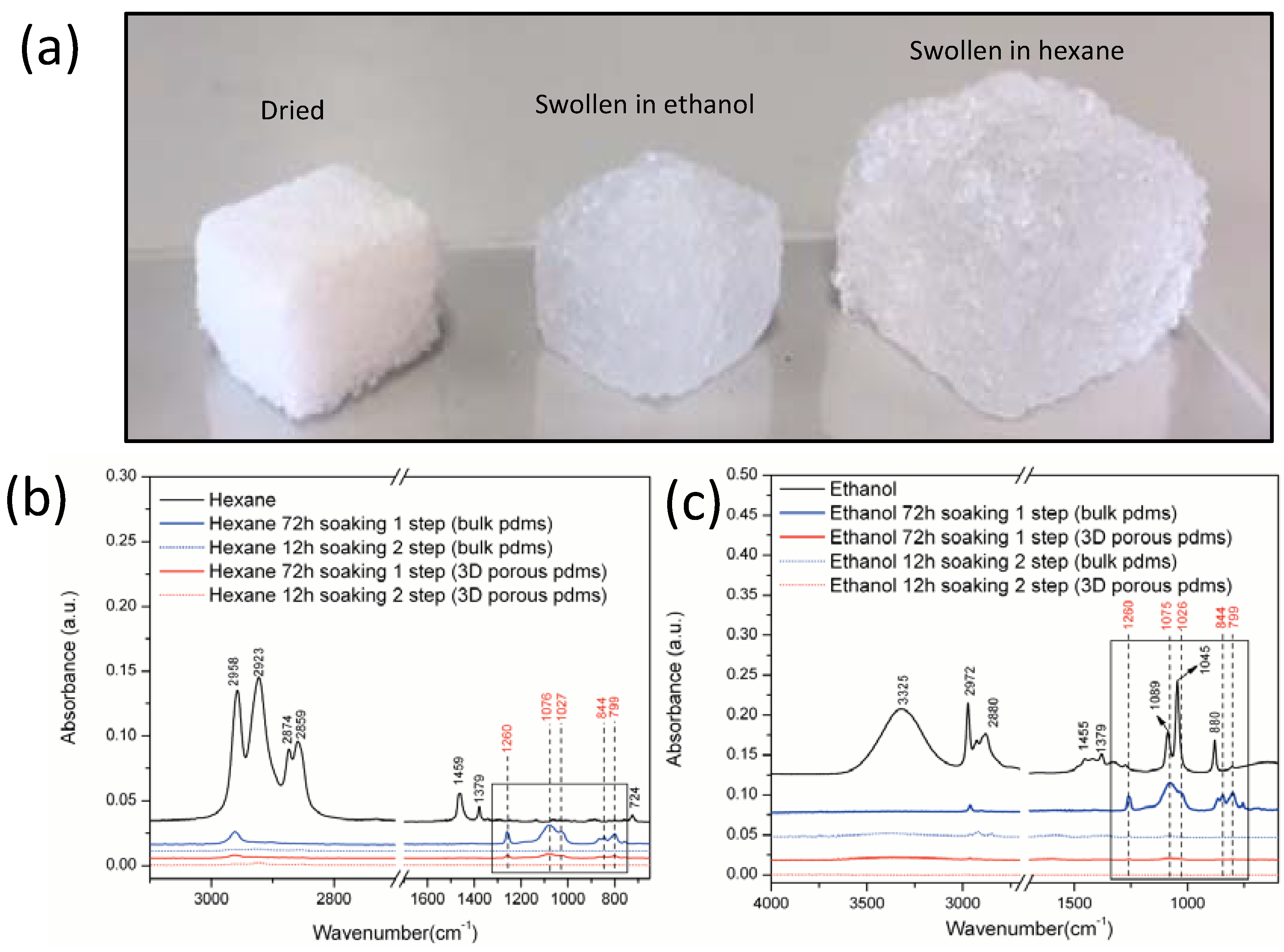
3.3. The ATR-FTIR Spectra of the Waste Washing Solvents
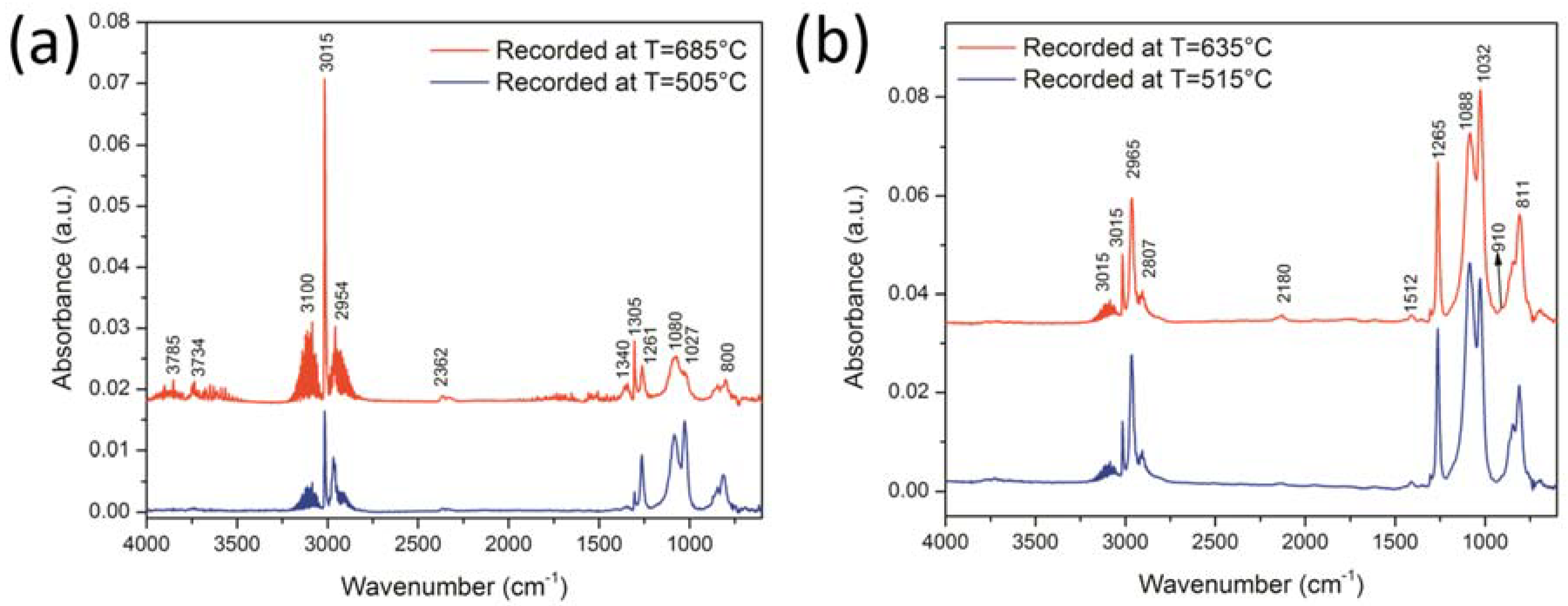
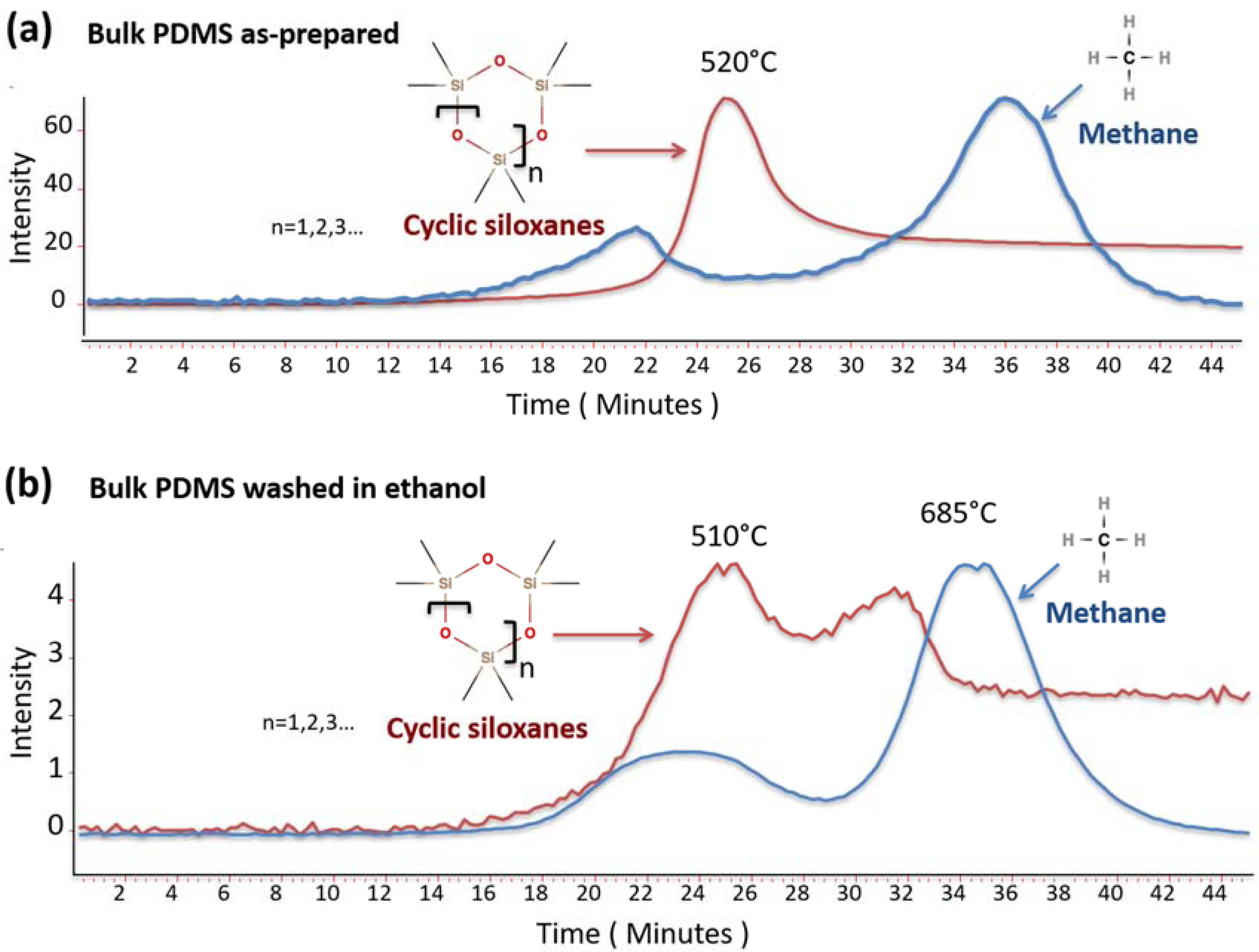
3.4. Thermal Behaviour by TG-FTIR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Somasundaran, P.; Mehta, S.C.; Purohit, P. Silicone emulsions. Adv. Colloid Interface Sci. 2006, 128–130, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Amelioration of anticorrosion and hydrophobic properties of epoxy/PDMS composite coatings containing nano ZnO particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Hu, J.; Shu, L.; Shi, X. Anti-icing performance of a superhydrophobic PDMS/modified nano-silica hybrid coating for insulators. J. Adhes. Sci. Technol. 2012, 26, 665–679. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2-SiO2-PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Jagdale, P.; Salimpour, S.; Islam, M.H.; Cuttica, F.; Hernandez, F.C.R.; Tagliaferro, A.; Frache, A. Flame Retardant Effect of Nano Fillers on Polydimethylsiloxane Composites. J. Nanosci. Nanotechnol. 2018, 18, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Dordick, J.S.; Clark, D.S. Siloxane-based biocatalytic films and paints for use as reactive coatings. Biotechnol. Bioeng. 2001, 72, 475–482. [Google Scholar] [CrossRef]
- Dunn, K.W.; Hall, P.N.; Khoo, C.T.K. Breast implant materials: Sense and safety. Br. J. Plast. Surg. 1992, 45, 315–321. [Google Scholar] [CrossRef]
- Mattei, G.; Magliaro, C.; Giusti, S.; Ramachandran, S.D.; Heinz, S.; Braspenning, J.; Ahluwalia, A. On the adhesion-cohesion balance and oxygen consumption characteristics of liver organoids. PLoS ONE 2017, 12, e0173206. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, T.; Tsou, H.; Ting, Y.; Tseng, Y. Biopolymer brushes grown on PDMS contact lenses by in situ atmospheric plasma-induced polymerization. J. Polym. Res. 2017, 24, 69. [Google Scholar] [CrossRef]
- Srisa-Art, M.; Noblitt, S.D.; Krummel, A.T.; Henry, C.S. IR-Compatible PDMS microfluidic devices for monitoring of enzyme kinetics. Anal. Chim. Acta 2018, 1021, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; McClelland, J.J.; Gupta, R.; Qin, D.; Zhao, X.M.; Sohn, L.L.; Celotta, R.J.; Whitesides, G.M. Replica molding using polymeric materials: A practical step toward nanomanufacturing. Adv. Mater. 1997, 9, 147–149. [Google Scholar] [CrossRef]
- Surdo, S.; Diaspro, A.; Duocastella, M. Microlens fabrication by replica molding of frozen laser-printed droplets. Appl. Surf. Sci. 2017, 418, 554–558. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable superhydrophobic/superoleophilic PDMS sponges and their applications in selective oil absorption and in plugging oil leakages. J. Mater. Chem. A 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Choi, S.J.; Kwon, T.H.; Im, H.; Moon, D.I.; Baek, D.J.; Seol, M.L.; Duarte, J.P.; Choi, Y.K. A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Chen, M.; Du, C.; Guo, H.; Bai, H.; Li, L. Poly(dimethylsiloxane) oil absorbent with a three-dimensionally interconnected porous structure and swellable skeleton. ACS Appl. Mater. Interfaces 2013, 5, 10201–10206. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, C.; Cui, L.; Song, Z.; Zhao, X.; Ma, Y.; Jiang, L. Facile Preparation of the Porous PDMS Oil-Absorbent for Oil/Water Separation. Adv. Mater. Interfaces 2017, 4, 1600862. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. Development of a porous 3D graphene-PDMS scaffold for improved osseointegration. Colloids Surf. B Biointerfaces 2017, 159, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, J.; Zhu, D.; Zheng, J.; Handschuh-Wang, S.; Zhou, X.; Zhang, J.; Liu, Y.; Liu, Z.; He, C.; et al. Hydrophilic Sponges for Leaf-Inspired Continuous Pumping of Liquids. Adv. Sci. 2017, 4, 1700028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Nam, Y.G.; Lee, B.K.; Han, K.; Kwon, T.H.; Kim, D.S. Fabrication of a hydrophilic poly(dimethylsiloxane) microporous structure and its application to portable microfluidic pump. Jpn. J. Appl. Phys. 2010, 49, 06GM01. [Google Scholar] [CrossRef]
- Cha, K.J.; Kim, D.S. A portable pressure pump for microfluidic lab-on-a-chip systems using a porous polydimethylsiloxane (PDMS) sponge. Biomed. Microdevices 2011, 13, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Iglio, R.; Mariani, S.; Robbiano, V.; Strambini, L.; Barillaro, G. Flexible Polydimethylsiloxane Foams Decorated with Multiwalled Carbon Nanotubes Enable Unprecedented Detection of Ultralow Strain and Pressure Coupled with a Large Working Range. ACS Appl. Mater. Interfaces 2018, 10, 13877–13885. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Yang, K.; Wang, Z.; Chen, M.; Zhang, L.; Zhang, H.; Li, C. Fabrication of Highly Stretchable Conductors Based on 3D Printed Porous Poly(dimethylsiloxane) and Conductive Carbon Nanotubes/Graphene Network. ACS Appl. Mater. Interfaces 2016, 8, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, L.; Duan, S.; Jing, S.; Jiang, H.; Li, C. Highly stretchable conductors integrated with a conductive carbon nanotube/graphene network and 3D porous poly (dimethylsiloxane). Adv. Funct. Mater. 2014, 24, 7548–7556. [Google Scholar] [CrossRef]
- Silva, F.A.; Chagas-Silva, F.A.; Florenzano, F.H.; Pissetti, F.L. Poly(dimethylsiloxane) and Poly[vinyltrimethoxysilane-co-2-(dimethylamino) ethyl methacrylate] Based Cross-Linked Organic-Inorganic Hybrid Adsorbent for Copper(II) Removal from Aqueous Solutions. J. Braz. Chem. Soc. 2016, 27, 2181–2191. [Google Scholar] [CrossRef]
- Krajnc, M.; Šebenik, U. Kinetic Study of Thermal Degradation of Polydimethylsiloxane: The Effect of Molecular Weight on Thermal Stability in Inert Atmosphere. Polym. Sci. 2017, 3, 6–11. [Google Scholar] [CrossRef]
- Thomas, T.H.; Kendrick, T.C. Thermal analysis of polydimethylsiloxanes. I. Thermal degradation in controlled atmospheres. J. Polym. Sci. Part B Polym. Phys. 1969, 7, 537–549. [Google Scholar] [CrossRef]
- Camino, C.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects, Polymer (Guildf.) 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lageard, M. Thermal polydimethylsiloxane degradation. Part 2. The degradation mechanisms. Polymer (Guildf.) 2002, 43, 2011–2015. [Google Scholar] [CrossRef]
- Iglio, R.; Mariani, S.; Strambini, L.M.; Baribaro, G. Macroporous PDMS foam decorated with carbon nanotubes for conductometric pressure and strain sensors. In Proceedings of the IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Herz, J.; Munch, J.P.; Candau, S. Experimental investigation of the role of trapped entanglements in swollen polydimethylsiloxane networks. J. Macromol. Sci. Part B 1980, 18, 267–279. [Google Scholar] [CrossRef]
- Rana, D.; Kim, H.L.; Kwag, H.; Choe, S. Hybrid blends of similar ethylene 1-octene copolymers. Polymer (Guildf.) 2000, 41, 7067–7082. [Google Scholar] [CrossRef]
- Rana, D.; Kim, H.L.; Kwag, H.; Rhee, J.; Cho, K.; Woo, T.; Lee, B.H.; Choe, S. Blends of Ethylene 1-Octene Copolymer Synthesized by Ziegler–Natta and Metallocene Catalysts. I. Thermal and Mechanical Properties. J. Appl. Polym. Sci. 2000, 76, 1950–1964. [Google Scholar] [CrossRef]
- Johnson, L.M.; Gao, L.; Shields, C.W., IV; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaboury, S.R.; Urban, M.W. Quantitative analysis of the Si-H groups formed on poly(dimethylsiloxane) surfaces: An ATR FTi.r. approach. Polymer (Guildf.) 1992, 33, 5085–5089. [Google Scholar] [CrossRef]
- Sun, J.T.; Huang, Y.D.; Cao, H.L.; Gong, G.F. Effects of ambient-temperature curing agents on the thermal stability of poly (methylphenylsiloxane). Polym. Degrad. Stab. 2004, 85, 725–731. [Google Scholar] [CrossRef]
- Fang, W.; Zeng, X.; Lai, X.; Li, H.; Chen, W.; Zhang, Y. Thermal degradation mechanism of addition-cure liquid silicone rubber with urea-containing silane. Thermochim. Acta 2015, 605, 28–36. [Google Scholar] [CrossRef]



| Wavenumber (cm−1) | Assignment | Reference |
|---|---|---|
| Si–CH3, Si–CH2– | ||
| 690, 790 | Si–C stretching | [34] |
| 843 | C–H (–CH2) rocking | [25] |
| 1260 | symmetric C–H bending | [34] |
| 1414 | asymmetric C–H bending | [35] |
| 2965 | asymmetric C–H stretching | [35] |
| 2905 | symmetric C–H stretching | [35] |
| Si–H | ||
| 2158 912 | Si–H stretching Si–H bending | [35] |
| Si–O–Si | ||
| 1023 | asymmetric in–Si–O–Si stretching | [25] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rivera, J.; Iglio, R.; Barillaro, G.; Duce, C.; Tinè, M.R. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers 2018, 10, 616. https://doi.org/10.3390/polym10060616
González-Rivera J, Iglio R, Barillaro G, Duce C, Tinè MR. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers. 2018; 10(6):616. https://doi.org/10.3390/polym10060616
Chicago/Turabian StyleGonzález-Rivera, José, Rossella Iglio, Giuseppe Barillaro, Celia Duce, and Maria Rosaria Tinè. 2018. "Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process" Polymers 10, no. 6: 616. https://doi.org/10.3390/polym10060616
APA StyleGonzález-Rivera, J., Iglio, R., Barillaro, G., Duce, C., & Tinè, M. R. (2018). Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers, 10(6), 616. https://doi.org/10.3390/polym10060616







