Self-Organization and Swelling of Ruthenium-Metal Coordination Polymers with PTA (Metal = Ag, Au, Co)
Abstract
:1. Introduction
2. Materials and Methods
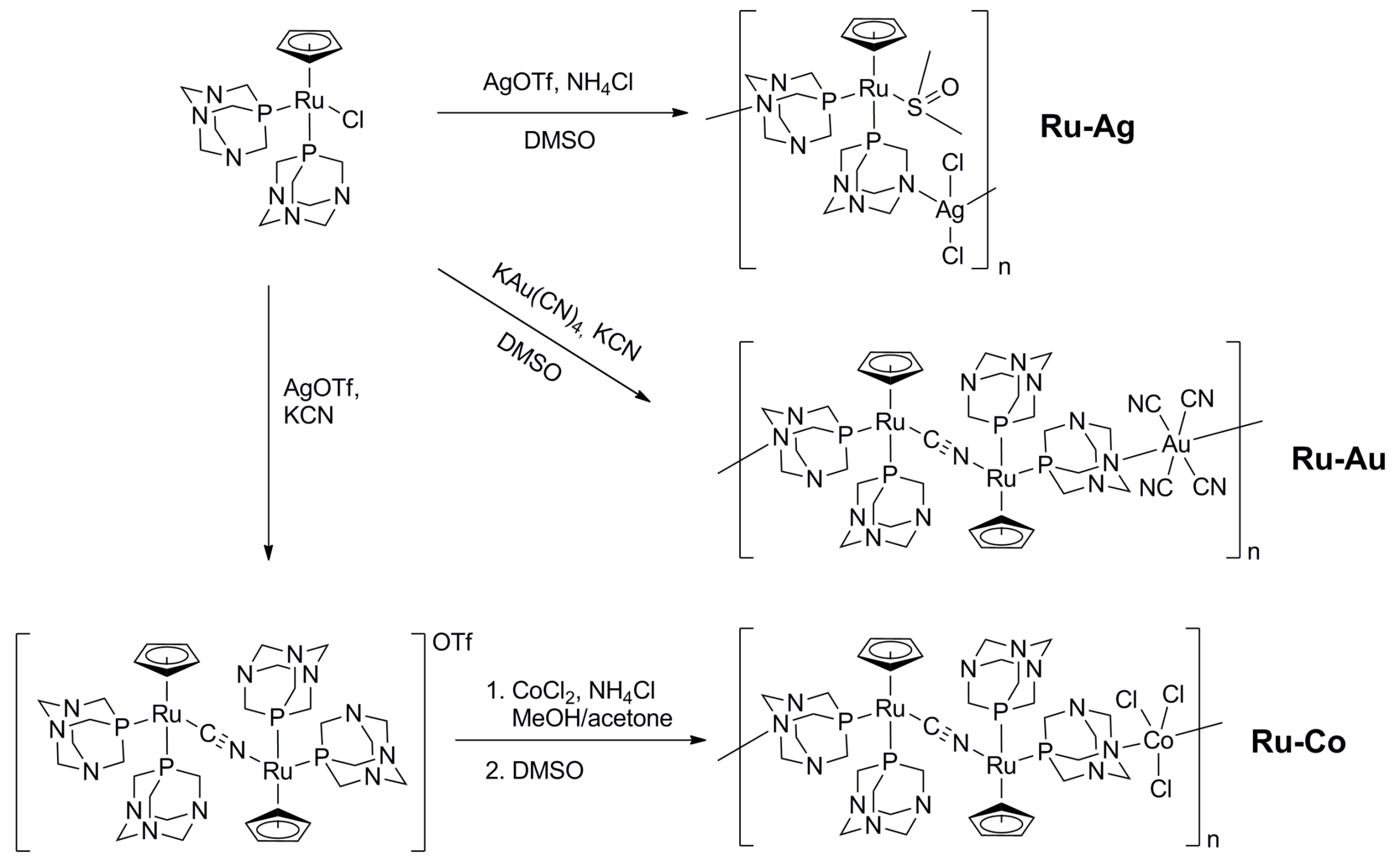
2.1. Synthesis of the Coordination Polymers
2.2. X-ray Structure Determination
2.3. Photon Correlation Spectroscopy (PCS)
2.4. Neutron Scattering
2.4.1. Small-Angle Neutron Scattering
2.4.2. Quasi-Elastic Neutron Spectroscopy
3. Results and Discussion
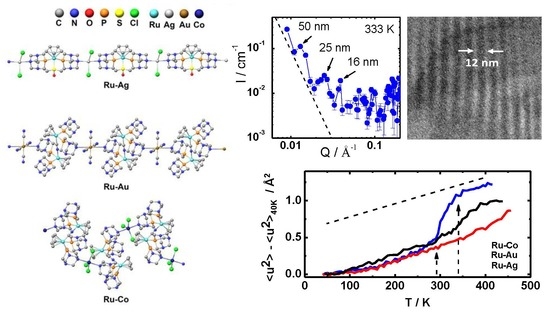
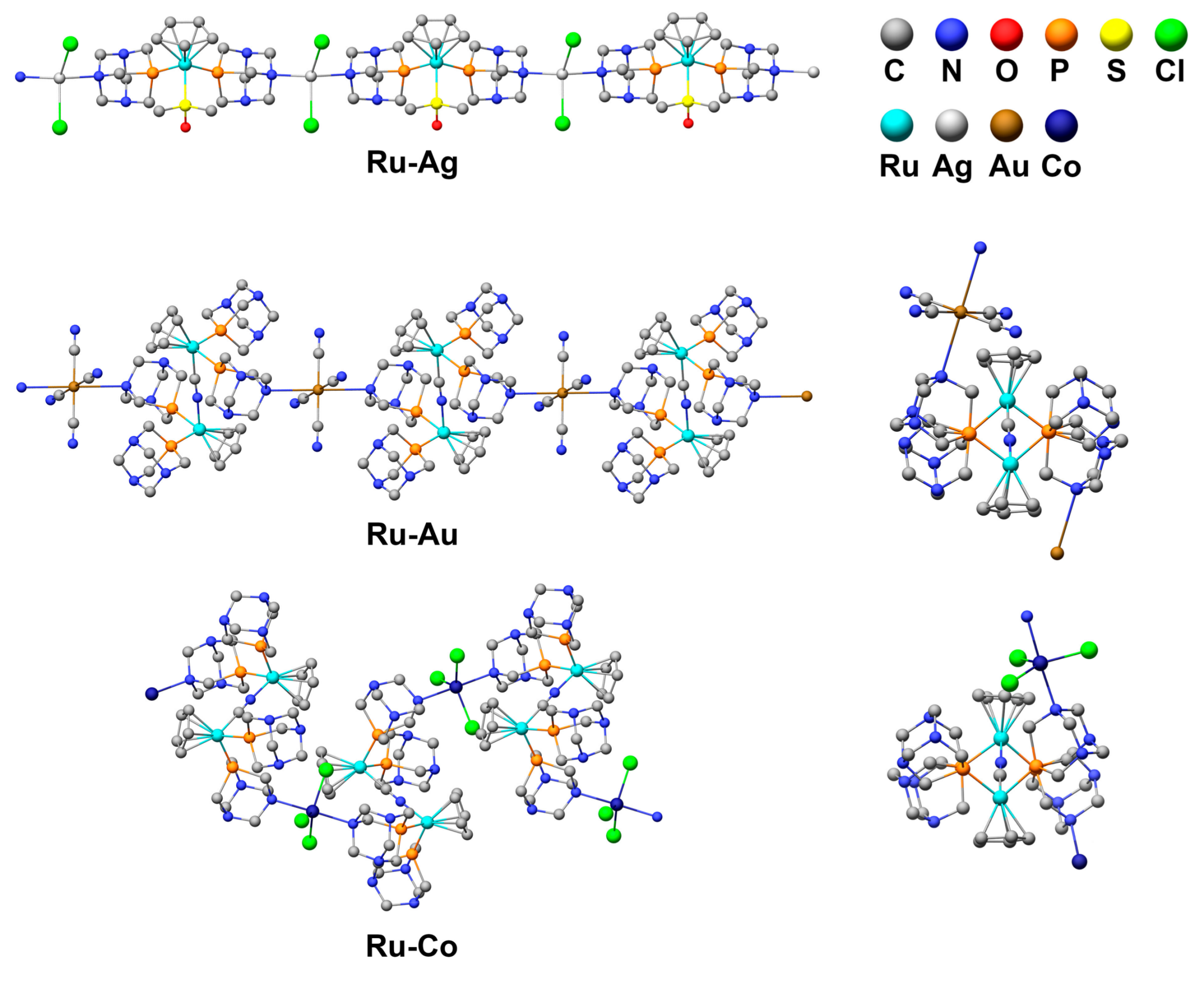
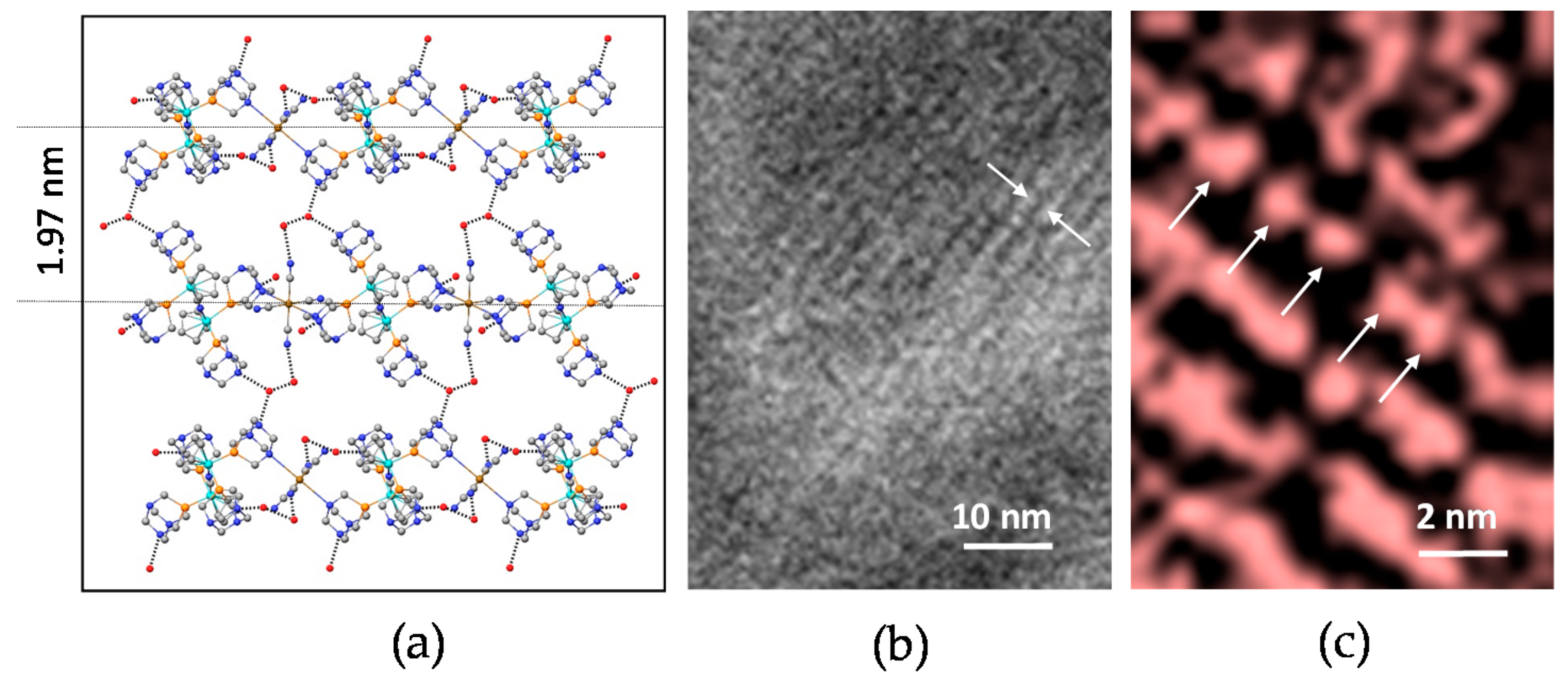
3.1. Structure of the Coordination Polymers
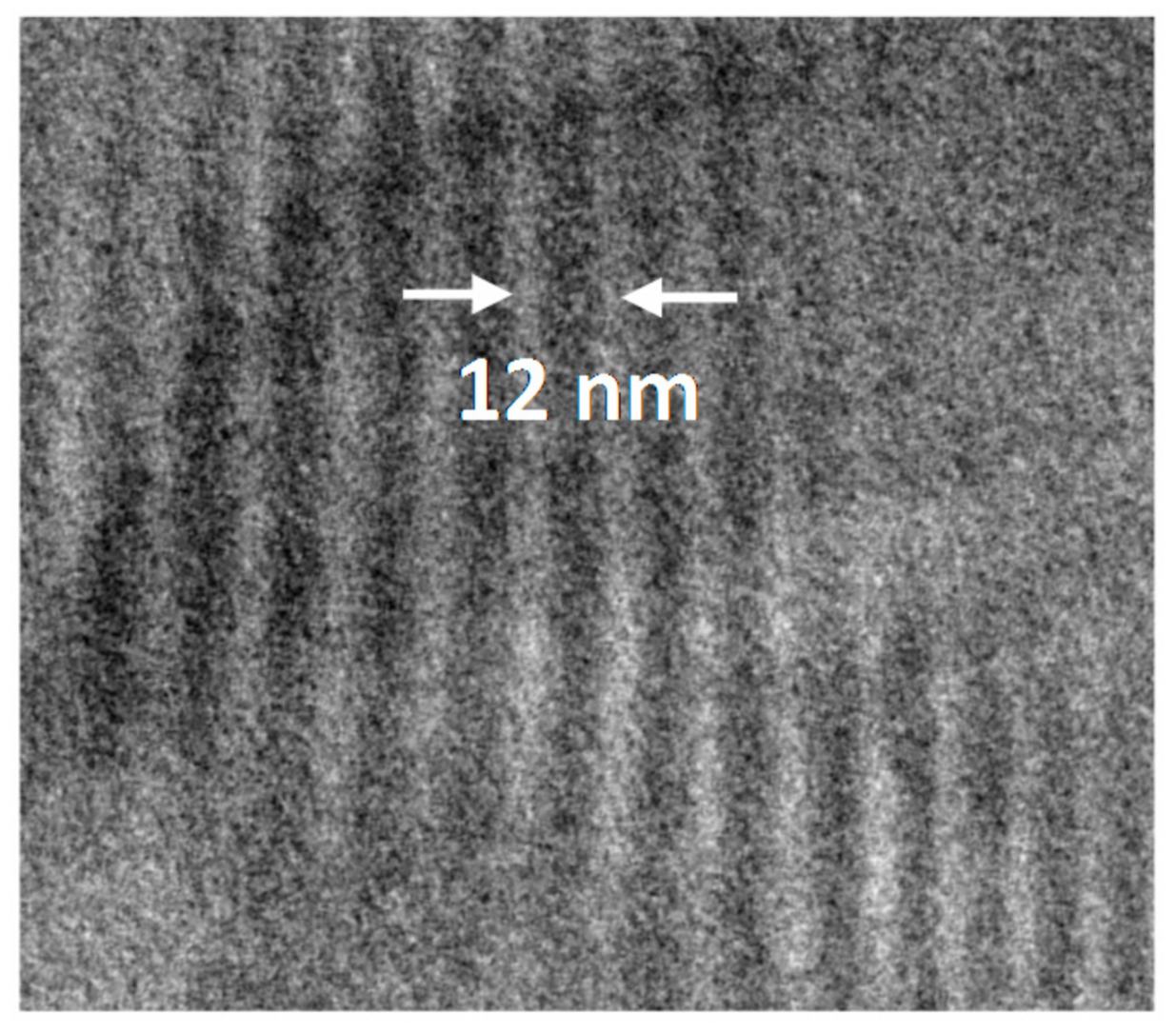
3.2. Polymer Visualization and Self-Assembly
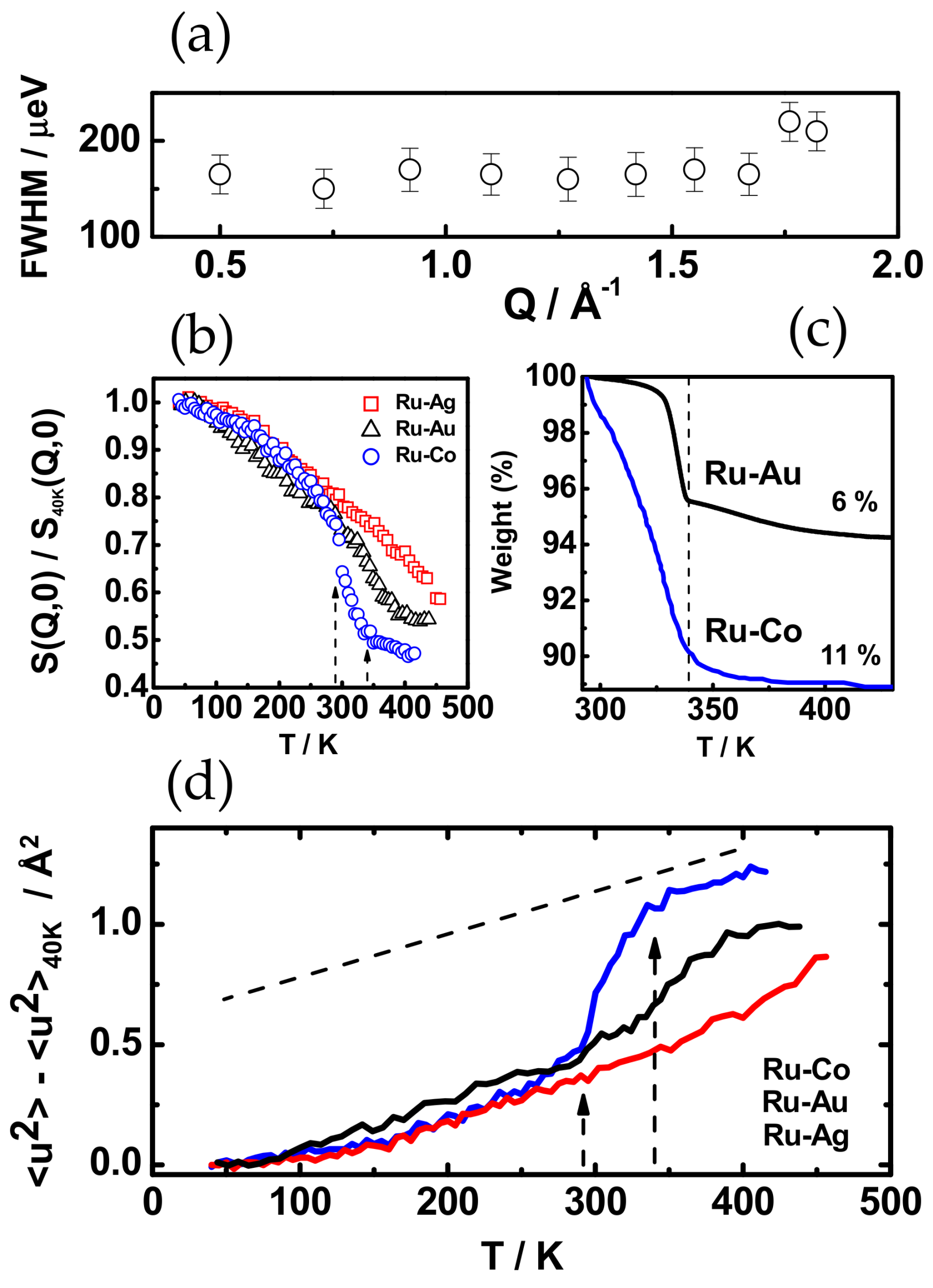
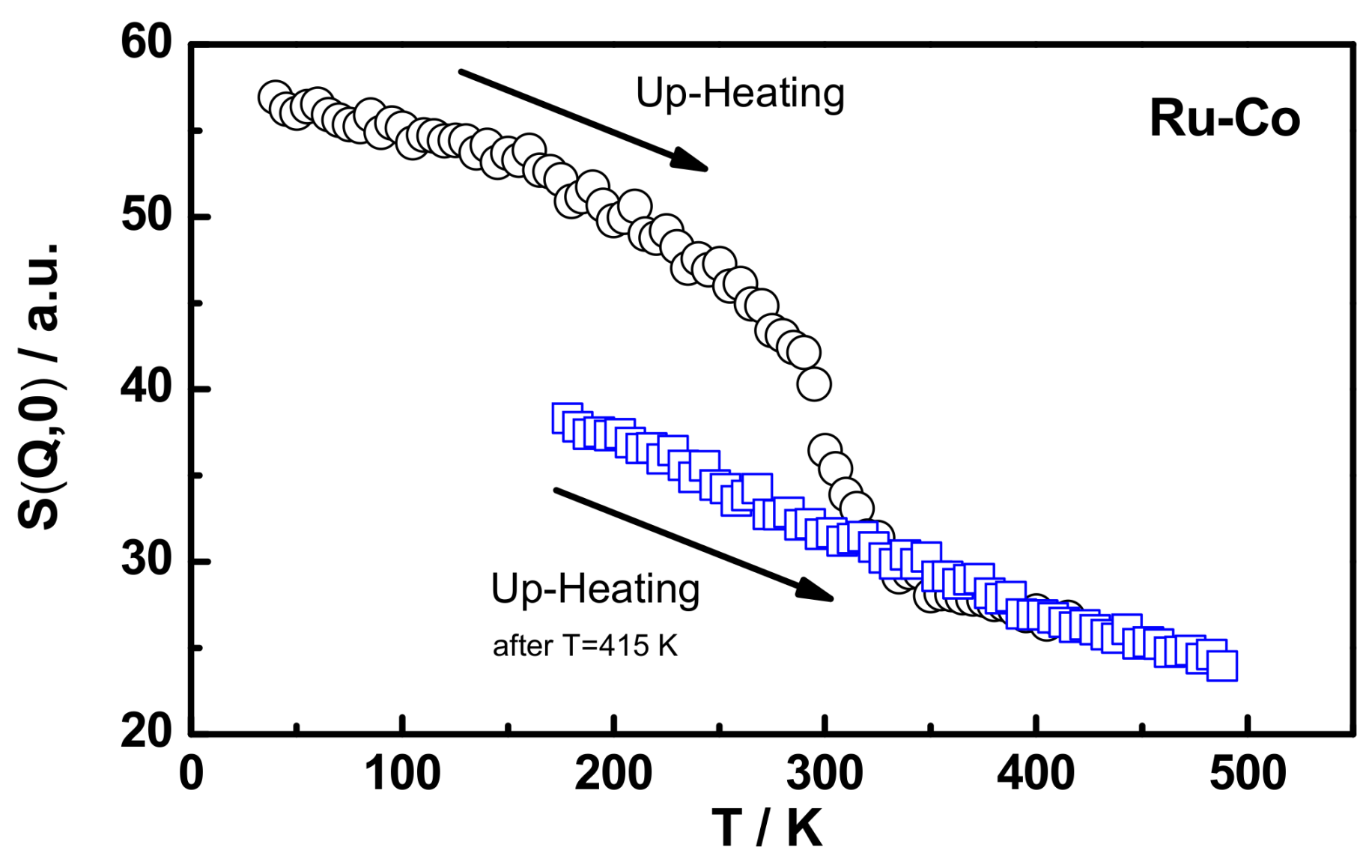
3.3. Polymer Dynamics
3.4. Assembly of Polymers in Water: Colloidal Scale
3.5. Assembly of Polymers in Water: Nano Scale
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fraser, C.L. Synthetic Metal-Containing Polymers; Manners, I., Ed.; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Nguyen, P.; Gómez-Elipe, P.; Manners, L. Organometallic polymers with transition metals in the main chain. Chem. Rev. 1999, 99, 1515–1548. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, F.S.; Haven, A.C. Derivatives of Dicyclopentadienyliron. J. Am. Chem. Soc. 1955, 77, 6295–6297. [Google Scholar] [CrossRef]
- Janiak, C. Engineering coordination polymers towards applications. Dalt. Trans. 2003, 2781. [Google Scholar] [CrossRef]
- Givaja, G.; Amo-Ochoa, P.; Gómez-García, C.J.; Zamora, F. Electrical conductive coordination polymers. Chem. Soc. Rev. 2012, 41, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Herrero, J.; Zamora, F. Coordination polymers for nanoelectronics. Adv. Mater. 2011, 23, 5311–5317. [Google Scholar] [CrossRef] [PubMed]
- Lidrissi, C.; Romerosa, A.; Saoud, M.; Serrano-Ruiz, M.; Gonsalvi, L.; Peruzzini, M. Stable, water-soluble Pta-based Ru-Ag organometallic polymers. Angew. Chem. Int. Ed. 2005, 44, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- Serrano Ruiz, M.; Romerosa, A.; Sierra-Martin, B.; Fernandez-Barbero, A. A water soluble diruthenium-gold organometallic microgel. Angew. Chem. Int. Ed. 2008, 47, 8665–8669. [Google Scholar] [CrossRef] [PubMed]
- Scalambra, F.; Serrano-Ruiz, M.; Romerosa, A. First water-soluble backbone Ru-Ru-Ni heterometallic organometallic polymer. Macromol. Rapid Commun. 2015, 36, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Filipowicz, M.; Guedes Da Silva, M.F.C.; Kłak, J.; Smoleński, P.; Pombeiro, A.J.L. Unprecedented mixed-valence Cu(I)/Cu(II) complex derived from N-methyl-1,3,5-triaza-7-phosphaadamantane: Synthesis, structural features, and magnetic properties. Organometallics 2012, 31, 7921–7925. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals: Fifth Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Akbayeva, D.N.; Gonsalvi, L.; Oberhauser, W.; Peruzzini, M.; Vizza, F.; Brüggeller, P.; Romerosa, A.; Sava, G.; Bergamo, A. Synthesis, catalytic properties and biological activity of new water soluble ruthenium cyclopentadienyl PTA complexes [(C5R5)RuCl(PTA)2] (R = H, Me; PTA = 1,3,5-triaza-7-phosphaadamantane). Chem. Commun. 2003, 264–265. [Google Scholar] [CrossRef]
- Serrano-Ruiz, M.; Imberti, S.; Bernasconi, L.; Jadagayeva, N.; Scalambra, F.; Romerosa, A. Study of the interaction of water with the aqua-soluble dimeric complex [RuCp(PTA)2-μ-CN-1κC:2κ(2)N-RuCp(PTA)2](CF3SO3) (PTA = 1,3,5-triaza-7-phosphaadamantane) by neutron and X-ray diffraction in solution. Chem. Commun. 2014, 50, 11587–11590. [Google Scholar] [CrossRef] [PubMed]
- Romerosa, A.; Campos-Malpartida, T.; Lidrissi, C.; Saoud, M.; Serrano-Ruiz, M.; Peruzzini, M.; Garrido-Cárdenas, J.A.; García-Maroto, F. Synthesis, characterization, and DNA binding of new water-soluble cyclopentadienyl ruthenium(II) complexes incorporating phosphines. Inorg. Chem. 2006, 45, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Dynamic Light Scattering; Pecora, R. (Ed.) Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Burchard, W. Static and dynamic light scattering from branched polymers and biopolymers. In Light Scattering from Polymers; Springer: Berlin, Germany, 1983; pp. 1–124. [Google Scholar]
- ISIS Muon and Neutron Source: LOQ. Available online: https://www.isis.stfc.ac.uk/Pages/Loq.aspx (accessed on 15 May 2018).
- ISIS Muon and Neutron Source: IRIS. Available online: https://www.isis.stfc.ac.uk/Pages/iris.aspx (accessed on 15 May 2018).
- Frick, B.; Fetters, L.J. Methyl Group Dynamics in Glassy Polyisoprene: A Neutron Backscattering Investigation. Macromolecules 1994, 27, 974–980. [Google Scholar] [CrossRef]
- Lyulin, A.V.; Al-Barwani, M.S.; Allen, M.P.; Wilson, M.R.; Neelov, I.; Allsopp, N.K. Molecular dynamics simulation of main chain liquid crystalline polymers. Macromolecules 1998, 31, 4626–4634. [Google Scholar] [CrossRef]
- Miyatsu, S.; Kofu, M.; Nagoe, A.; Yamada, T.; Sadakiyo, M.; Yamada, T.; Kitagawa, H.; Tyagi, M.; García Sakai, V.; Yamamuro, O. Proton dynamics of two-dimensional oxalate-bridged coordination polymers. Phys. Chem. Chem. Phys. 2014, 16, 17295–17304. [Google Scholar] [CrossRef] [PubMed]
- Romerosa, A.; Scalambra, F. Non-Covalent Interactions of Water with Metal Complexes in Solution; John Wiley & Sons: New York, NY, USA, 2016; ISBN 9781119113874. [Google Scholar]
- Suarez, I.J.; Sierra-Martin, B.; Fernandez-Barbero, A. Swelling of ionic and non-ionic minigels. Colloids Surf. A Physicochem. Eng. Asp. 2009, 343, 30–33. [Google Scholar] [CrossRef]
- Capriles-Gonzalez, D.; Sierra-Martin, B.; Fernandez-Nieves, A.; Fernandez-Barbero, A. Coupled deswelling of multiresponse microgels. J. Phys. Chem. B 2008, 112, 12195–12200. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Martin, B.; Retama, J.R.; Laurenti, M.; Fernandez Barbero, A.; Lopez Cabarcos, E. Structure and polymer dynamics within PNIPAM-based microgel particles. Adv. Colloid Interface Sci. 2014, 205, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Barbero, A.; Fernandez-Nieves, A.; Grillo, I.; Lopez-Cabarcos, E. Structural modifications in the swelling of inhomogeneous microgels by light and neutron scattering. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2002, 66. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Martin, B.; Serrano-Ruiz, M.; García-Sakai, V.; Scalambra, F.; Romerosa, A.; Fernandez-Barbero, A. Self-Organization and Swelling of Ruthenium-Metal Coordination Polymers with PTA (Metal = Ag, Au, Co). Polymers 2018, 10, 528. https://doi.org/10.3390/polym10050528
Sierra-Martin B, Serrano-Ruiz M, García-Sakai V, Scalambra F, Romerosa A, Fernandez-Barbero A. Self-Organization and Swelling of Ruthenium-Metal Coordination Polymers with PTA (Metal = Ag, Au, Co). Polymers. 2018; 10(5):528. https://doi.org/10.3390/polym10050528
Chicago/Turabian StyleSierra-Martin, Benjamin, Manuel Serrano-Ruiz, Victoria García-Sakai, Franco Scalambra, Antonio Romerosa, and Antonio Fernandez-Barbero. 2018. "Self-Organization and Swelling of Ruthenium-Metal Coordination Polymers with PTA (Metal = Ag, Au, Co)" Polymers 10, no. 5: 528. https://doi.org/10.3390/polym10050528