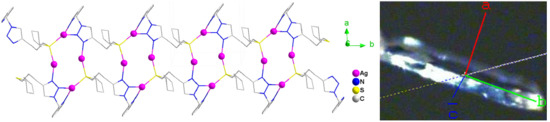
A One-Dimensional Coordination Polymer Containing Cyclic [Ag4] Clusters Supported by a Hybrid Pyridine and Thioether Functionalized 1,2,3-Triazole
Abstract
:1. Introduction
2. Materials and Methods
X-ray Crystallography
3. Results and Discussion
3.1. Synthesis
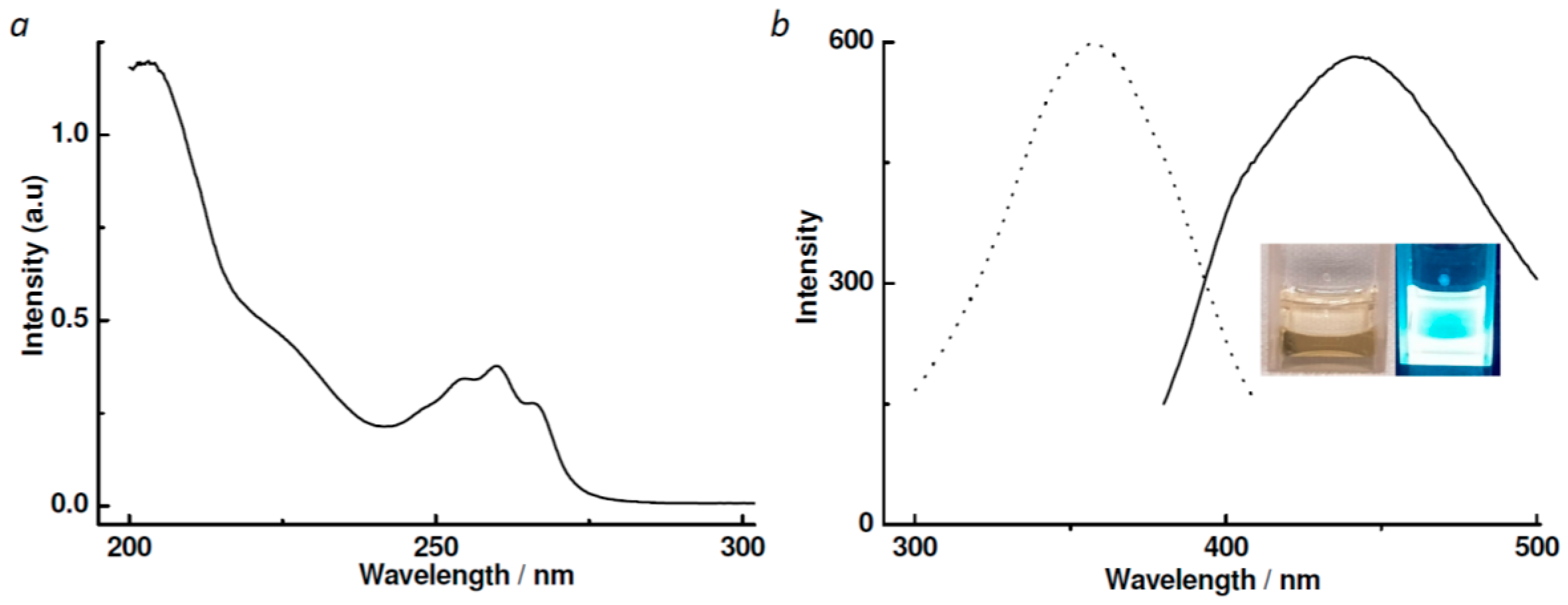
3.2. UV–Vis and Photoluminescent Spectra of Ligand L
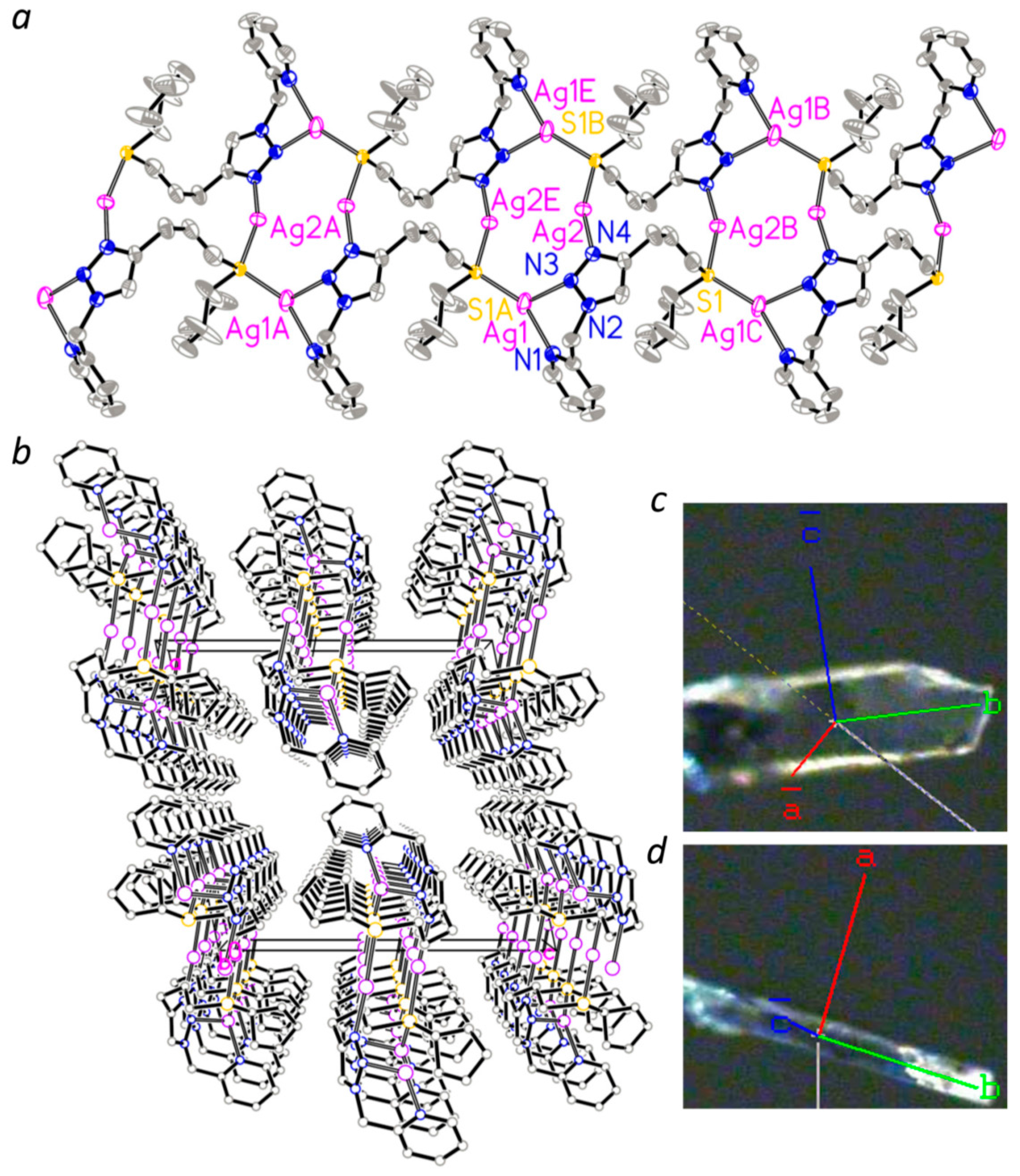
3.3. Molecular Structure of Complex 1
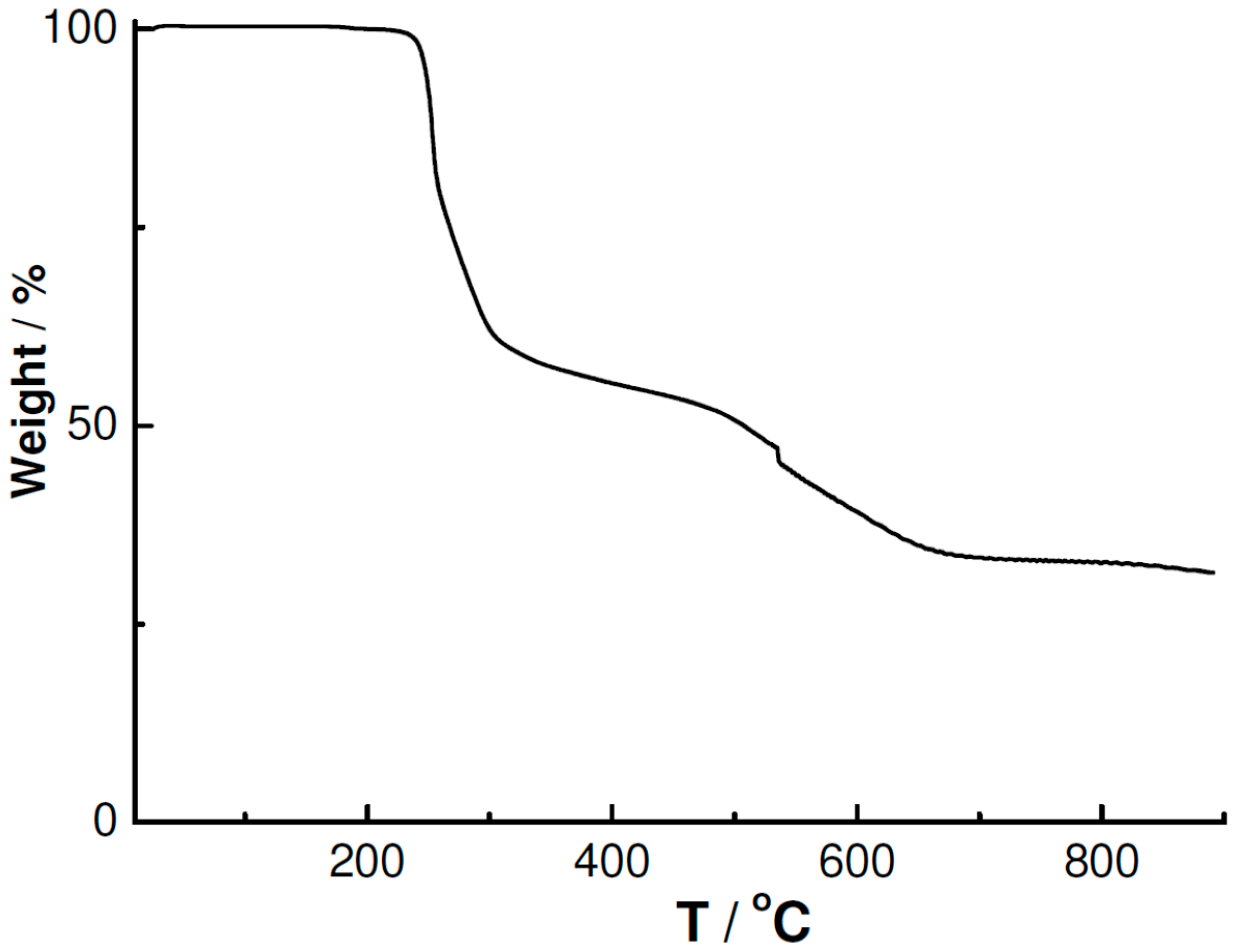
3.4. Powder XRD and TGA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, J.-W.; Hou, S.-X.; Hecke, K.V.; Cui, G.-H. Rigid versus semi-rigid bis(imidazole) ligands in the assembly of two Co.(II) coordination polymers: Structural variability, electrochemical properties and photocatalytic behavior. Dalton Trans. 2017, 46, 2892–2903. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Tăbăcaru, A.; Galli, S. Coordination polymers and metal–organic frameworks based on poly(pyrazole)-containing ligands. Coord. Chem. Rev. 2016, 307, 1–31. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nakanishi, T. Luminescent lanthanide coordination polymers for photonic applications. RSC Adv. 2015, 5, 338–353. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Liao, P.-Q.; Lin, R.-B.; Wei, Y.-S.; Zeng, M.-H.; Chen, X.-M. Metal cluster-based functional porous coordination polymers. Coord. Chem. Rev. 2015, 293, 263–278. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Henderson, W. Coordination chemistry of 3- and 4-mercaptobenzoate ligands: Versatile hydrogen-bonding isomers of the thiosalicylate (2-mercaptobenzoate) ligand. Coord. Chem. Rev. 2017, 341, 19–52. [Google Scholar] [CrossRef]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.-C. Tuning the topology and functionality of metal-organic frameworks by ligand design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-M.; Yu, B.-Y.; Van Hecke, K.; Cui, G.-H. A series of d10 metal coordination polymers based on a flexible bis(2-methylbenzimidazole) ligand and different carboxylates: Synthesis, structures, photoluminescence and catalytic properties. Cryst. Eng. Commun. 2015, 17, 2279–2293. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Blake, A.J.; Champness, N.R.; Lemenovskii, D.A.; Majouga, A.G.; Zyk, N.V.; Schröder, M. Supramolecular design of one-dimensional coordination polymers based on silver(I) complexes of aromatic nitrogen-donor ligands. Coord. Chem. Rev. 2001, 222, 155–192. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Xu, L.-Y.; Ren, Z.-G.; Wang, H.-F.; Lang, J.-P. Assembly of silver(i)/n,n-bis(diphenylphosphanylmethyl)-3-aminopyridine/halide or pseudohalide complexes for efficient photocatalytic degradation of organic dyes in water. Cryst. Growth Des. 2017, 17, 4826–4834. [Google Scholar] [CrossRef]
- Beheshti, A.; Soleymani-Babadi, S.; Mayer, P.; Abrahams, C.T.; Motamedi, H.; Trzybińki, D.; Wozniak, K. Design, synthesis, and antibacterial assessment of silver(i)-based coordination polymers with variable counterions and unprecedented structures by the tuning spacer length and binding mode of flexible bis(imidazole-2-thiones) ligands. Cryst. Growth Des. 2017, 17, 5249–5262. [Google Scholar] [CrossRef]
- Dey, A.; Biradha, K. Anion and guest directed tetracyclic macrocycles of ag5l4 and ag6l4 with an arc-shaped ligand containing pyridine and benzimidazole units: reversal of anion selectivity by guest. Cryst. Growth Des. 2017, 17, 5629–5633. [Google Scholar] [CrossRef]
- Reger, D.L.; Semeniuc, R.F.; Smith, M.D. Supramolecular architecture of a silver(i) coordination polymer supported by a new ligand containing four tris(pyrazolyl)methane units. Inorg. Chem. 2001, 40, 6545–6546. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, R.; Yuan, D.; Wu, B.; Lou, B.; Hong, M. Hierarchical assembly of a novel luminescent silver coordination framework with 4-(4-pyridylthiomethyl)benzoic acid. J. Mol. Struct. 2005, 737, 55–59. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Yu, L.-C.; Zhong, D.-C.; Liang, H.; Zhu, X.-H.; Zhou, Z.-Y. An unprecedented 1D ladder-like silver(I) coordination polymer with ciprofloxacin. Inorg. Chem. Commun. 2006, 9, 839–843. [Google Scholar] [CrossRef]
- He, W.-J.; Luo, G.-G.; Wu, D.-L.; Liu, L.; Xia, J.-X.; Li, D.-X.; Dai, J.-C.; Xiao, Z.-J. An odd-numbered heptameric water cluster containing a puckered pentamer self-assembled in a Ag(I) polymeric solid. Inorg. Chem. Commun. 2012, 18, 4–7. [Google Scholar] [CrossRef]
- Zorlu, Y.; Can, H.; Aksakal, F. A novel 2D chiral silver(I) coordination polymer assembled from 5-sulfosalicylic acid and (2S,4R)-4-hydroxyproline: Synthesis, crystal structure, HOMO–LUMO and NBO analysis. J. Mol. Struct. 2013, 1049, 368–376. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Fang, M.-X.; Xu, Z.-H.; Wang, X.-P.; Wang, S.-N.; Han, L.-L.; Li, X.-Y.; Sun, D. Construction of a crystalline Ag(I) coordination polymer based on a new ligand generated from unusual in situ aza-Michael addition reaction. Cryst. Eng. Commun. 2014, 16, 3015–3019. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Qin, C.; Wang, X.-L.; Shao, K.-Z.; Su, Z.-M. A highly electrical conducting, 3D supermolecular Ag(I) coordination polymer material with luminescent properties. Inorg. Chem. Commun. 2016, 70, 31–34. [Google Scholar] [CrossRef]
- Bahemmat, S.; Ghassemzadeh, M.; Neumüller, B. A new silver(I) coordination polymer containing 3-methylthio-(1H)-1,2,4-triazole as precursor for preparation of silver nanorods. Inorg. Chim. Acta, 2015, 435, 159–166. [Google Scholar] [CrossRef]
- Semitut, E.; Komarov, V.; Sukhikh, T.; Filatov, E.; Potapov, A. Synthesis, crystal structure and thermal stability of 1d linear silver(i) coordination polymers with 1,1,2,2-tetra(pyrazol-1-yl)ethane. Crystals 2016, 6, 138. [Google Scholar] [CrossRef]
- Braunstein, P.; Naud, F. Hemilability of hybrid ligands and the coordination chemistry of oxazoline-based systems. Angew. Chem. Int. Ed. 2001, 40, 680–699. [Google Scholar] [CrossRef]
- Deria, P.; Mondloch, J.E.; Karagiaridi, O.; Bury, W.; Hupp, J.T.; Farha, O.K. Beyond post-synthesis modification: Evolution of metal–organic frameworks via building block replacement. Chem. Soc. Rev. 2014, 43, 5896–5912. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-Q.; Fang, C.-J.; He, Z.; Gao, E.-Q.; Yan, C.-H.; Hor, T.S.A. Chelating schiff base assisted azide-bridged Mn(II), Ni(II) and Cu(II) magnetic coordination polymers. Dalton Trans. 2012, 41, 13379–13387. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-Q.; Leelasubcharoen, S.; Chen, X.; Koh, L.L.; Zuo, J.-L.; Hor, T.S.A. Crystallographic elucidation of chiral and helical Cu(II) polymers assembled from a heterodifunctional 1,2,3-triazole ligand. Cryst. Growth Des. 2010, 10, 1715–1720. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Kwang, J.Y.; Koh, L.L.; Young, D.J.; Hor, T.S.A. Functionalized 1,2,3-triazoles as building blocks for photoluminescent POLOs (polymers of oligomers) of copper(I). Dalton Trans. 2010, 39, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Z.; Bai, S.-Q.; Hor, T.S.A. “Click-and-click”—hybridised 1,2,3-triazoles supported Cu(I) coordination polymers for azide–alkyne cycloaddition. Dalton Trans. 2013, 42, 9437–9443. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-Q.; Jiang, L.; Zuo, J.-L.; Hor, T.S.A. Hybrid NS ligands supported Cu(I)/(II) complexes for azide–alkyne cycloaddition reactions. Dalton Trans. 2013, 42, 11319–11326. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-Q.; Jiang, L.; Sun, B.; Young, D.J.; Hor, T.S.A. Five Cu(I) and Zn(II) clusters and coordination polymers of 2-pyridyl-1,2,3-triazoles: Synthesis, structures and luminescence properties. Cryst. Eng. Commun. 2015, 17, 3305–3311. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Ke, K.L.; Young, D.J.; Hor, T.S.A. Structure and photoluminescence of cubane-like [Cu4I4] cluster-based 1D coordination polymer assembled with bis(triazole)pyridine ligand. J. Organomet. Chem. 2017, 137–141. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Young, D.J.; Hor, T.S.A. Hydrogen-Bonding interactions in luminescent quinoline-triazoles with dominant 1d crystals. Molecules 2017, 22, 1600. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-Q.; Jiang, L.; Tan, A.L.; Yeo, S.C.; Young, D.J.; Hor, T.S.A. Assembly of photoluminescent [CunIn] (n = 4, 6 and 8) clusters by clickable hybrid [N,S] ligands. Inorg. Chem. Front. 2015, 2, 1011–1018. [Google Scholar] [CrossRef]
- Bruker AXS Inc. SAINT Software Reference Manual; Version 6.0; Bruker AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G. M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
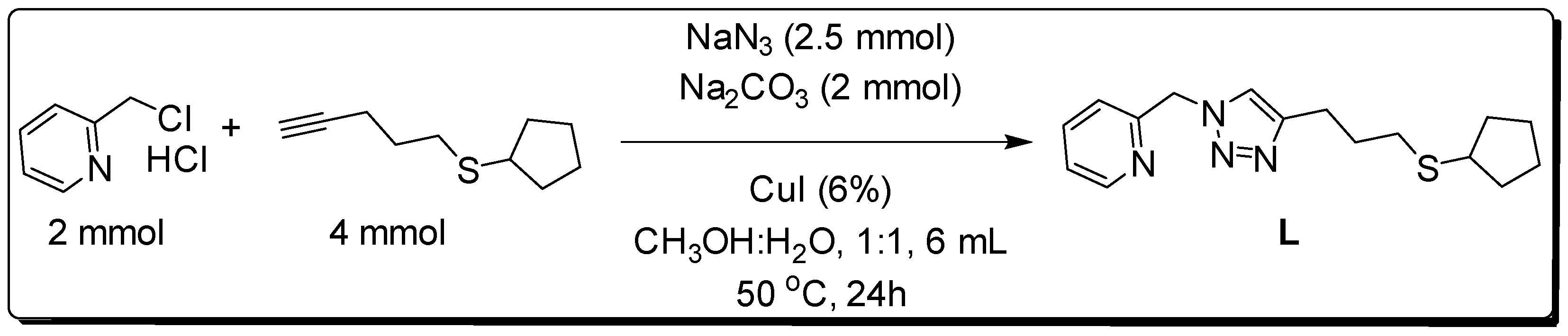


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.-Q.; Wong, I.H.K. A One-Dimensional Coordination Polymer Containing Cyclic [Ag4] Clusters Supported by a Hybrid Pyridine and Thioether Functionalized 1,2,3-Triazole. Crystals 2018, 8, 16. https://doi.org/10.3390/cryst8010016
Bai S-Q, Wong IHK. A One-Dimensional Coordination Polymer Containing Cyclic [Ag4] Clusters Supported by a Hybrid Pyridine and Thioether Functionalized 1,2,3-Triazole. Crystals. 2018; 8(1):16. https://doi.org/10.3390/cryst8010016
Chicago/Turabian StyleBai, Shi-Qiang, and Ivy Hoi Ka Wong. 2018. "A One-Dimensional Coordination Polymer Containing Cyclic [Ag4] Clusters Supported by a Hybrid Pyridine and Thioether Functionalized 1,2,3-Triazole" Crystals 8, no. 1: 16. https://doi.org/10.3390/cryst8010016