In2O3-Based Thermoelectric Materials: The State of the Art and the Role of Surface State in the Improvement of the Efficiency of Thermoelectric Conversion
Abstract
:1. Introduction
2. How to Improve the Conversion Efficiency and Why Metal Oxides Can Be Used for This?
2.1. Increase in Operating Temperatures
2.2. Nanostructuring
3. n-Type Oxides Promising for Thermoelectric Applications
4. In2O3 and Its Advantages for Thermoelectric Applications
4.1. Electrophysical Properties
4.2. Thermal Conductivity
4.3. Thermoelectric Conversion Efficiency of In2O3-Based Oxides
4.3.1. Sintered Samples
4.3.2. Thin Nanostructured In2O3 Films
5. Summary
Acknowledgments
Conflicts of Interest
References
- Goldsmid, H.J. Electronic Refrigeration; Pion Limited: London, UK, 1986. [Google Scholar]
- Walia, S.; Balendhran, S.; Nili, H.; Zhuiykov, S.; Rosengarten, G.; Wang, Q.H.; Bhaskaran, M.; Sriram, S.; Strano, M.S.; Kalantar-zadeh, K. Transition metal oxides—Thermoelectric properties. Prog. Mater. Sci. 2013, 58, 1443–1489. [Google Scholar] [CrossRef]
- Date, A.; Date, A.; Dixon, C.; Akbarzadeh, A. Progress of thermoelectric power generation systems: Prospect for small to medium scale power generation. Renew. Sustain. Energy Rev. 2014, 33, 371–381. [Google Scholar] [CrossRef]
- Zheng, X.F.; Liu, C.X.; Yann, Y.Y.; Wang, Q. A review of thermoelectrics research—Recent developments and potentials for sustainable and renewable energy applications. Renew. Sustain. Energ. Rev. 2014, 32, 486–503. [Google Scholar] [CrossRef]
- Guan, M.; Wang, K.; Xu, D.; Liao, W.-H. Design and experimental investigation of a low-voltage thermoelectric energy harvesting system for wireless sensor nodes. Energy Convers. Manag. 2017, 138, 30–37. [Google Scholar] [CrossRef]
- Ong, K.S.; Naghavi, M.S.; Lim, C. Thermal and electrical performance of a hybrid design of a solar thermoelectric system. Energy Convers. Manag. 2017, 133, 31–40. [Google Scholar] [CrossRef]
- Riffat, S.B.; Ma, X. Thermoelectrics: a review of present and potential applications. Appl. Therm. Eng. 2003, 23, 913–935. [Google Scholar] [CrossRef]
- Rowe, D.W. Thermoelectrics Handbook: Micro to Nano; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric system. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Stabler, F.R. Automotive applications of thermoelectric materials. J. Electron. Mater. 2009, 38, 1245–1251. [Google Scholar] [CrossRef]
- Zhao, D.; Tan, G. A review of thermoelectric cooling: Materials, modeling and applications. Appl. Therm. Eng. 2014, 66, 15–24. [Google Scholar] [CrossRef]
- Kütt, L.; Millar, J.; Karttunen, A.; Lehtonen, M.; Karppinen, M. Thermoelectric applications for energy harvesting in domestic applications and micro-production units. Part I: Thermoelectric concepts, domestic boilers and biomass stoves. Renew. Sust. Energy Rev. 2017, in press. [Google Scholar]
- Siddique, A.R.M.; Mahmud, S.; Van Heyst, B. A review of the state of the science on wearable thermoelectric power generators (TEGs) and their existing challenges. Renew. Sustain. Energy Rev. 2017, 73, 730–744. [Google Scholar] [CrossRef]
- Koumoto, K.; Wang, Y.; Zhang, Z.; Kosuga, A.; Funahashi, R. Oxide thermoelectric materials: A nanostructuring approach. Annu. Rev. Mater. Res. 2010, 40, 363–394. [Google Scholar] [CrossRef]
- Mahan, G.D. Figure-of-merit for thermoelectrics. J. Appl. Phys. 1989, 65, 1578. [Google Scholar] [CrossRef]
- Nolas, G.S.; Sharp, J.; Goldsmid, H.J. Thermoelectrics Basic Principles and New Materials Developments; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Maignan, A.; Hébert, S.; Pelloquin, D.; Martin, C.; Pralong, V.; Caignaert, V.; Klein, Y. Thermoelectric oxides: Important role of the transition metal spin states. In Proceedings of the IEEE International Conference on Thermoelectrics, Vienna, Austria, 6–10 August 2006; p. 17. [Google Scholar]
- Ohta, H.; Sugiura, K.; Koumoto, K. Recent progress in oxide thermoelectric materials: p-Type Ca3Co4O9 and n-type SrTiO3. Inorg. Chem. 2008, 47, 8429–8436. [Google Scholar] [CrossRef] [PubMed]
- Bierwagen, O.; Speck, J.S. High electron mobility In2O3 (001) and (111) thin films with nondegenerate electron concentration. Appl. Phys. Lett. 2010, 97, 072103. [Google Scholar] [CrossRef]
- Preissler, N.; Bierwagen, O.; Ramu, A.T.; Speck, J.S. Electrical transport, electrothermal transport, and effective electron mass in single-crystalline In2O3 films. Phys. Rev. B 2013, 88, 085305. [Google Scholar] [CrossRef]
- Zheng, J.C. Recent advances on thermoelectric materials. Front. Physiol. China 2008, 3, 269–279. [Google Scholar] [CrossRef]
- Minnnich, A.J.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Bulk nanostructured thermoelectric materials: Current research and future prospects. Energy Environ. Sci. 2009, 2, 466–479. [Google Scholar] [CrossRef]
- Liu, W.; Yan, X.; Chen, G.; Ren, Z. Recent advances in thermoelectric nanocomposites. Nano Energy 2012, 1, 42–56. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K.K. Recent advances in thermoelectric materials. Prog. Mater. Sci. 2016, 83, 330–382. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Macklin, W.J.; Moseley, P.T. On the use of oxides for thermoelectric refrigeration. Mater. Sci. Eng. B 1990, 7, 111–117. [Google Scholar] [CrossRef]
- Ohtaki, M.; Ogura, D.; Eguchi, K.; Arai, H. High-temperature thermoelectric properties of In2O3-based mixed oxides and their applicability to thermoelectric power generation. J. Mater. Chem. 1994, 4, 653–656. [Google Scholar] [CrossRef]
- Koumoto, K.; Funahashi, R.; Guilmeau, E.; Miyazaki, Y.; Weidenkaff, A.; Wang, Y.; Wa, C. Thermoelectric ceramics for energy harvesting. J. Am. Ceram. Soc. 2013, 96, 1–23. [Google Scholar] [CrossRef]
- He, J.; Liu, Y. Oxide thermoelectrics: The challenges, progress, and outlook. J. Mater. Res. 2011, 26, 1762–1772. [Google Scholar] [CrossRef]
- Zhu, Q.; Hopper, E.M.; Ingram, B.J.; Mason, T.O. Combined Jonker and Ioffe analysis of oxide conductors and semiconductors. J. Am. Ceram. Soc. 2011, 94, 187–193. [Google Scholar] [CrossRef]
- Nag, A.; Shubha, V. Oxide thermoelectric materials: A structure–property relationship. J. Electron. Mater. 2014, 43, 962–977. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Lan, J.; Zeng, C.; Liu, Y.; Zhan, B.; Butt, S.; Lin, Y.H.; Nan, C.W. High performance oxides-based thermoelectric materials. JOM 2015, 67, 211–221. [Google Scholar] [CrossRef]
- Yin, Y.; Tudu, B.; Tiwari, A. Recent advances in oxide thermoelectric materials and modules. Vacuum 2017, 146, 356–374. [Google Scholar] [CrossRef]
- Zhao, L.-D.; He, J.; Berardan, D.; Lin, Y.; Li, J.-F.; Nan, C.-W.; Dragoe, N. BiCuSeO oxyselenides: New promising thermoelectric materials. Energy Environ. Sci. 2014, 7, 2900–2924. [Google Scholar] [CrossRef]
- Mahan, G. Benedicks effect: Nonlocal electron transport in metals. Phys. Rev. B 1991, 43, 3945–3951. [Google Scholar] [CrossRef]
- Anatychuk, L.I.; Bulat, L.P. Thermoelectric phenomena under large temperature gradients. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 3–8. [Google Scholar]
- Lan, J.; Lin, Y.H.; Liu, Y.; Xu, S.; Nan, C.-W. High thermoelectric performance of nanostructured In2O3-based ceramics. J. Am. Ceram. Soc. 2012, 95, 2465–2469. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.-H.; Xu, W.; Cheng, B.; Lan, J.; Chen, D.; Zhu, H.; Nan, C.-W. High-temperature transport property of In2−xCexO3 (0 ≤ x ≤ 0.10) fine grained ceramics. J. Am. Ceram. Soc. 2012, 95, 2568–2572. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Liu, D.-B.; Yu, M.; Lin, Y.-H.; Nan, C.-W. Enhanced thermoelectric properties of Ga-doped In2O3 ceramics via synergistic band gap engineering and phonon suppression. Phys. Chem. Chem. Phys. 2015, 17, 11229–11233. [Google Scholar] [CrossRef] [PubMed]
- Minnich, A.; Lee, H.; Wang, X.; Joshi, G.; Dresselhaus, M.; Ren, Z.; Chen, G.; Vashaee, D. Modeling study of thermoelectric SiGe nanocomposites. Phys. Rev. B 2009, 80, 155327. [Google Scholar] [CrossRef]
- Liu, W.; Tan, X.; Yin, K.; Liu, H.; Tang, X.; Shi, J.; Zhang, Q.; Uher, C. Convergence of conduction bands as a means of enhancing thermoelectric performance of n-type Mg2Si1−xSnx solid solutions. Phys. Rev. Lett. 2012, 108, 166601. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Liang, D.; Feng, X.; Zhao, W.; Chen, Y.; He, Y. Thermoelectric properties of MnSi1.7 films with addition of aluminum and carbon. Mod. Phys. Lett. B 2007, 21, 1447–1460. [Google Scholar] [CrossRef]
- Fergus, J.W. Oxide materials for high temperature thermoelectric energy conversion. J. Eur. Ceram. Soc. 2012, 32, 525–540. [Google Scholar] [CrossRef]
- Fujita, K.; Mochida, T.; Nakamura, K. High-temperature thermoelectric properties of NaxCoO2−δ single crystals. Jpn. J. Appl. Phys. 2001, 40, 4644–4647. [Google Scholar] [CrossRef]
- Terasaki, I. Physics of the thermoelectric oxide NaCo2O4: A guide to new thermoelectric oxides. In Proceedings of the 21st International IEEE Conference on Thermoelectronics, Long Beach, CA, USA, 25–29 August 2002; pp. 185–191. [Google Scholar]
- Li, S.; Funahashi, R.; Matsubara, I.; Ueno, K.; Yamada, H. High temperature thermoelectric properties of oxide Ca9Co12O28. J. Mater. Chem. 1999, 9, 1659–1660. [Google Scholar] [CrossRef]
- Funahashi, R.; Matsuhara, I. Thermoelectric properties of Pb- and Ca-doped (Bi2Sr2O4), CoO2 whiskers. Appl. Phys. Lett. 2001, 79, 362–364. [Google Scholar] [CrossRef]
- Hebert, S.; Lambert, S.; Pelloquin, D.; Maignan, A. Large thermopower in a metallic cobaltite: The layered Tl-Sr-Co-O misfit. Phys. Rev. B 2001, 64, 172101. [Google Scholar] [CrossRef]
- Pelloquin, D.; Maignan, A.; Hehert, S.; Martin, C.; Hervieu, M.; Wang, L.B.; Raveau, B. New misfit cobaltites [Pb0.7A0.4Sr1.9O3][CoO2]1.8 (A = Hg, Co) with large thermopower. Chem. Mater. 2002, 14, 3100–3105. [Google Scholar] [CrossRef]
- Nagira, T.; Ito, M.; Katsuyama, S.; Majima, K.; Nagai, H. Thermoelectric properties of (Na1−yMy)xCo2O4 (M=K, Sr, Y, Nd, Sm and Yb; y = 0.01 ~ 0.35). J. Alloys Comp. 2003, 348, 263–269. [Google Scholar] [CrossRef]
- Funahashi, R.; Matsuhara, I.; Ikuta, H.; Takeuchi, T.; Mizutani, U.; Sodeoka, S. An oxide single crystal with high thermoelectric performance in air. Jpn. J. Appl. Phys. Part 2 2000, 39, L1127–L1129. [Google Scholar] [CrossRef]
- Motohashi, T.; Naujalis, E.; Ueda, R.; Isawa, K.; Karppinen, M.; Yamauchi, H. Simultaneously enhanced thermoelectric power and reduced resistivity of NaxCo2O4, by controlling Na nonstoichiometry. Appl. Phys. Lett. 2001, 79, 1480–1482. [Google Scholar] [CrossRef]
- Terasaki, I.; Ishii, Y.; Tanaka, D.; Takahata, K.; Iguchi, Y. Thermoelectric properties of NaCo2–x-CuxO4 improved by the substitution of Cu for CO. Jpn. J. Appl. Phys. 2001, 40, L65–L67. [Google Scholar] [CrossRef]
- Terasaki, I. Thermoelectric materials in layered transition-metal oxides. In Proceedings of the IEEE International Conference on Thermoelectrics, Clemson, SC, USA, 19–23 June 2005; pp. 289–294. [Google Scholar]
- Lan, J.L.; Liu, Y.C.; Zhan, B.; Lin, Y.H.; Zhang, B.; Yuan, X.; Zhang, W.; Xu, W.; Nan, C.W. Enhanced thermoelectric properties of Pb-doped BiCuSeO ceramics. Adv. Mater. 2013, 25, 5086–5090. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Li, J.; He, J.; Pei, Y.-L.; Berardan, D.; Wu, H.; Dragoe, N.; Cai, W.; Zhao, L.D. Texturation boosts the thermoelectric performance of BiCuSeO oxyselenides. Energy Environ. Sci. 2013, 6, 2916–2920. [Google Scholar] [CrossRef]
- Barreteau, C.; Berardan, D.; Zhao, L.; Dragoe, N. Influence of Te substitution on the structural and electronic properties of thermoelectric BiCuSeO. J. Mater. Chem. A 2013, 1, 2921–2926. [Google Scholar] [CrossRef]
- Sun Lee, D.; An, T.-H.; Jeong, M.; Choi, H.-S.; Lim, Y.S.; Seo, W.-S.; Park, C.-H.; Park, C.; Park, H.-H. Density of state effective mass and related charge transport properties in K-doped BiCuOSe. Appl. Phys. Lett. 2013, 103, 232110. [Google Scholar] [CrossRef]
- Li, F.; Wei, T.-R.; Kang, F.; Li, J.-F. Enhanced thermoelectric performance of Ca-doped BiCuSeO in a wide temperature range. J. Mater. Chem. A 2013, 1, 11942–11949. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Y.; Zhao, L.-D.; An, P.; Lin, Y.-H.; Marcelli, A.; Wu, Z. Evidence of an interlayer charge transfer route in BiCu1-xSeO. J. Mater. Chem. A 2013, 1, 12154–12158. [Google Scholar] [CrossRef]
- MacDonald, D.K.C.; Roy, S.K. Vibrational anharmonicity and lattice thermal properties. II. Phys. Rev. 1955, 97, 673–676. [Google Scholar] [CrossRef]
- Roufosse, M.; Klemens, P. Thermal conductivity of complex dielectric crystals. Phys. Rev. B 1973, 7, 5379–5386. [Google Scholar] [CrossRef]
- Sanditov, D.; Belomestnykh, V. Relation between the parameters of the elasticity theory and averaged bulk modulus of solids. Tech. Phys. 2011, 56, 1619–1623. [Google Scholar] [CrossRef]
- Li, J.F.; Liu, W.S.; Zhao, L.D.; Zhou, M. High-performance nanostructured thermoelectric materials. NPG Asia Mater. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Kusainova, A.; Berdonosov, P.; Akselrud, L.; Kholodkovskaya, L.; Dolgikh, V.; Popovkin, B.J. New Layered Compounds with the General Composition (MO) (CuSe), Where M = Bi, Nd, Gd, Dy, and BiOCuS: Syntheses and Crystal Structure. Solid State Chem. 1994, 112, 189–191. [Google Scholar]
- Wiebe, C.; Greedan, J.; Gardner, J.; Zeng, Z.; Greenblatt, M. Charge and magnetic ordering in the electron-doped magnetoresistive materials CaMnO3−δ (δ = 0.06, 0.11). Phys. Rev. B 2001, 64, 064421. [Google Scholar] [CrossRef]
- Kieslich, G.; Cerretti, G.; Veremchuk, I.; Hermann, R.P.; Panthofer, M.; Grin, J.; Tremel, W. A chemists view: Metal oxides with adaptive structures for thermoelectric applications. Phys. Status Solidi A 2016, 213, 808–823. [Google Scholar] [CrossRef]
- Koumoto, K.; Terasaki, I.; Funahashi, R. Complex oxide materials for potential thermoelectric applications. Mater. Res. Soc. Bull. 2006, 31, 206–210. [Google Scholar] [CrossRef]
- Berardan, D.; Guilmeau, E.; Maignan, A.; Raveau, B. In2O3:Ge, a promising n-type thermoelectric oxide composite. Solid State Commun. 2008, 146, 97–101. [Google Scholar] [CrossRef]
- Guilmeau, E.; Bérardan, D.; Simon, Ch.; Maignan, A.; Raveau, B.; Ovono Ovono, D.; Delorme, F. Tuning the transport and thermoelectric properties of In2O3 bulk ceramics through doping at in-site. J. Appl. Phys. 2009, 106, 053715. [Google Scholar] [CrossRef]
- Hicks, L.D.; Dresselhaus, M.S. Effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. B 1993, 47, 12727–12731. [Google Scholar] [CrossRef]
- Simkin, N.V.; Mahan, G.D. Minimum thermal conductivity of superlattices. Phys. Rev. Lett. 2000, 84, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Dames, C.; Dresselhaus, M.S.; Chen, G. Phonon thermal conductivity of superlattice nanowires for thermoelectric applications. MRS Proc. 2003, 793, 15–20. [Google Scholar] [CrossRef]
- Vashaee, D.; Shakouri, A. Improved thermoelectric power factor in metal-based superlattices. Phys. Rev. Lett. 2004, 92, 106103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Han, G.; Yang, L.; Cheng, L.; Zou, J. Nanostructured thermoelectric materials: Current research and future challenge. Prog. Nat. Sci. Mater. Int. 2012, 22, 535–549. [Google Scholar] [CrossRef]
- Kanatzidis, M.G. Nanostructured thermoelectric: The new paradigm? Chem. Mater. 2010, 22, 648. [Google Scholar] [CrossRef]
- Vineis, C.J.; Shakouri, A.; Marjumdar, A.; Kanatzidis, M.G. Nanostructured thermoelectrics: Big efficiency gains from small features. Adv. Mater. 2010, 22, 3970. [Google Scholar] [CrossRef] [PubMed]
- GroB, E.; Riffel, M.; Stohrer, U. Thermoelectric generators made of FeSi2 and HMS: Fabrication and measurement. J. Mater. Res. 1995, 10, 34–40. [Google Scholar] [CrossRef]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmSbTe2+m: Bulk thermoelectric materials with high figure of merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.P.; Gogna, P. New directions for low-dimensional thermoelectric materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Lan, J.; Lin, Y.H.; Fang, H.; Mei, A.; Nan, C.W.; Liu, Y.; Xu, S.; Peters, M. High-temperature thermoelectric behaviors of fine-grained Gd-doped CaMnO3 ceramics. J. Am. Ceram. Soc. 2010, 93, 2121–2124. [Google Scholar] [CrossRef]
- Ohta, H. Thermoelectrics based on strontium titanate. Mater. Today 2007, 10, 44–49. [Google Scholar] [CrossRef]
- Flahaut, D.; Mihara, T.; Funahashi, R.; Nabeshima, N.; Lee, K.; Ohta, H.; Koumoto, K. Thermoelectrical properties of A-site substituted Ca1−xRexMnO3 system. J. Appl. Phys. 2006, 100, 084911. [Google Scholar] [CrossRef]
- Ohta, S.; Nomura, T.; Ohta, H.; Koumoto, K. High-temperature carrier transport and thermoelectric properties of heavily La- or Nb-doped SrTiO3 single crystals. J. Appl. Phys. 2005, 97, 034106. [Google Scholar] [CrossRef]
- Migiakis, P.; Androulakis, J.; Giapintzakis, J. Thermoelectric properties of LaNi1−xCoxO3 solid solution. J. Appl. Phys. 2005, 94, 7616–7620. [Google Scholar] [CrossRef]
- Ohta, S.; Nomura, T.; Ohta, H.; Hirano, M.; Hosono, H.; Koumoto, K. Large thermoelectric performance of heavily Nb-doped SrTiO3 epitaxial film at high temperature. Appl. Phys. Lett. 2005, 87, 092108. [Google Scholar] [CrossRef]
- Ohta, H.; Kim, S.; Mune, Y.; Mizoguchi, T.; Nomura, K.; Ohta, S.; Nomura, T.; Nakanishi, Y.; Ikuhara, Y.; Hirano, M.; et al. Giant thermoelectric Seebeck coefficient of a two-dimensional electron gas in SrTiO3. Nat. Mater. 2007, 6, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Han, L.; Ba, Y.; Wang, Y.; Wan, C.; Fujinami, K.; Koumoto, K. Effects of YSZ additions on thermoelectric properties of Nb-doped strontium titanate. J. Electron. Mater. 2010, 39, 1777. [Google Scholar] [CrossRef]
- Wang, N.; Han, L.; He, H.; Ba, Y.; Koumoto, K. Effects of mesoporous silica addition on thermoelectric properties of Nb-doped SrTiO3. J. Alloys Compd. 2010, 497, 308. [Google Scholar] [CrossRef]
- Wang, N.; He, H.; Ba, Y.; Wan, C.; Koumoto, K. Thermoelectric properties of Nb-doped SrTiO3 ceramics enhanced by potassium titanate nanowires addition. J. Ceram. Soc. Jpn. 2010, 118, 1098. [Google Scholar] [CrossRef]
- Wang, Y.; Fujinami, K.; Zhang, R.; Wan, C.; Ba, Y.; Koumoto, K. Interfacial thermal resistance and thermal conductivity in nanograined SrTiO3. Appl. Phys. Express 2010, 3, 031101. [Google Scholar] [CrossRef]
- Abutaha, A.I.; Kumar, S.; Li, K.; Dehkordi, A.; Tritt, T.M.; Alshareef, H. Enhanced thermoelectric figure-of-merit in thermally robust, nanostructured superlattices based on SrTiO3. Chem. Mater. 2015, 27, 2165–2171. [Google Scholar] [CrossRef]
- Pickett, W.E. Electronic structure of the high-temperature oxide superconductors. Rev. Mod. Phys. 1989, 61, 433–512. [Google Scholar] [CrossRef]
- Imada, M.; Fujimori, A.; Tokura, Y. Metal-insulator transitions. Rev. Mod. Phys. 1998, 70, 1039–1263. [Google Scholar] [CrossRef]
- Terasaki, I.; Iwakawa, M.; Nakano, T.; Tsukuda, A.; Kobayashi, W. Novel thermoelectric properties of complex transition-metal oxides. Dalton Trans. 2010, 39, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, I. High-temperature oxide thermoelectrics. J. Appl. Phys. 2011, 110, 053705. [Google Scholar] [CrossRef]
- Okinaka, N.; Akiyama, T. Thermoelectric properties of non-stoichiometric titanium oxides for wasre heat recovery in steel works. ISIJ Int. 2010, 50, 1296–1299. [Google Scholar] [CrossRef]
- Kurita, D.; Ohta, S.; Sugiura, K.; Ohta, H.; Koumoto, K. Carrier generation and transport properties of heavily Nb-doped anatase TiO2 epitaxial films at high temperatures. J. Appl. Phys. 2006, 100, 096105. [Google Scholar] [CrossRef]
- Sheppard, L.R.; Bak, T.; Nowotny, J. Electrical properties of niobium-doped titanium dioxide. 1. Defect disorder. J. Phys. Chem. B 2006, 110, 22447–22454. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, L.R.; Bak, T.; Nowotny, J. Electrical properties of niobium-doped titanium dioxide. 3. Thermoelectric power. J. Phys. Chem. C 2008, 112, 611–617. [Google Scholar] [CrossRef]
- Miao, L.; Tanemura, S.; Huang, R.; Liu, C.Y.; Huang, C.M.; Xu, G. Large Seebeck coefficients of protonated titanate nanotubes for high-temperature thermoelectric conversion. ACS Appl. Mater. Interfaces 2010, 2, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Tsuyumoto, I.; Hosono, T.; Murata, M. Thermoelectric power in nonstoichiometric orthorhombic titanium oxides. J. Am. Ceram. Soc. 2006, 89, 2301–2303. [Google Scholar] [CrossRef]
- Tsubota, T.; Ohtaki, M.; Eguchi, K.; Arai, H. Thermoelectric properties of Al-doped ZnO as a promising oxide material for high-temperature thermoelectric conversion. J. Mater. Chem. 1997, 7, 85–90. [Google Scholar] [CrossRef]
- Ohtaki, M.; Tsubota, T.; Eguchi, K.; Arai, H. High-temperature thermoelectric properties of (Zn1−xAlx)O. J. Appl. Phys. 1996, 79, 1816–1818. [Google Scholar] [CrossRef]
- Ong, K.P.; Singh, D.J.; Wu, P. Analysis of the thermoelectric properties of n-type ZnO. Phys. Rev. B 2011, 83, 115110. [Google Scholar] [CrossRef]
- Katsuyama, S.; Takagi, Y.; Ito, M.; Majima, K.; Nagai, H.; Sakai, H.; Yoshimura, K.; Kosuge, K. Thermoelectric properties of (Zn1−yMgy)1–xAlxO ceramics prepared by the polymerized complex method. J. Appl. Phys. 2002, 92, 1391. [Google Scholar] [CrossRef]
- Liang, X. Thermoelectric transport properties of Fe-enriched ZnO with high-temperature nanostructure refinement. ACS Appl. Mater. Interfaces 2015, 7, 7927–7937. [Google Scholar] [CrossRef] [PubMed]
- Liang, X. Thermoelectric transport properties of naturally nanostructured Ga–ZnO ceramics: Effect of point defect and interfaces. J. Eur. Ceram. Soc. 2016, 36, 1643–1650. [Google Scholar] [CrossRef]
- Guilmeau, E.; Díaz-Chao, P.; Lebedev, I.O.; Recnik, A.; Schafer, M.C.; Delorme, F.; Giovannelli, F.; Kosir, M.; Bernik, S. Inversion boundaries and phonon scattering in Ga:ZnO thermoelectric compounds. Inorg. Chem. 2017, 56, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Michiue, Y.; Nishijima, H.; Suzuki, Y.; Mori, T. Synthesis and thermoelectric properties of composite oxides in the pseudobinary system ZnO-Ga2O3. Solid State Sci. 2017, 65, 29–32. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, J.-H.; Cho, S.-J.; Park, J.-S. Studies of thermoelectric transport properties of atomic layer deposited gallium-doped ZnO. Ceram. Int. 2017, 43, 7784–7788. [Google Scholar] [CrossRef]
- Masuda, Y.; Ohta, M.; Seo, W.-S.; Pitschke, W.; Koumoto, K. Structure and thermoelectric transport properties of isoelectronically substituted (ZnO)5In2O3. J. Solid State Chem. 2000, 150, 221–227. [Google Scholar] [CrossRef]
- Isobe, S.; Tani, T.; Masuda, Y.; Seo, W.-S.; Koumoto, K. Thermoelectric performance of Yttrium-substituted (ZnO)5In2O3 improved through ceramic texturing. Jpn. J. Appl. Phys. 2002, 41, 731–732. [Google Scholar] [CrossRef]
- Ohta, H.; Seo, W.-S.; Koumoto, K. Thermoelectric properties of homologous compounds in the ZnO-In2O3 system. J. Am. Ceram. Soc. 1996, 79, 2193–2196. [Google Scholar] [CrossRef]
- Liang, X.; Baram, M.; Clarke, D.R. Thermal (Kapitza) resistance of interfaces in compositional dependent ZnO-In2O3 superlattices. Appl. Phys. Lett. 2013, 102, 223903. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Clarke, D.R. Relation between thermolectric properties and phase equilibria in the ZnO–In2O3 binary system. Acta Mater. 2014, 63, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Bernik, S.; Kosir, M.; Guilmeau, E. Microstructure and thermoelectric characteristics of (ZnO)kIn2O3–based ceramics (k = 5 and 11). Zastita Mater. 2016, 57, 318–325. [Google Scholar] [CrossRef]
- Kosir, M.; Podlogar, M.; Daneu, N.; Recnik, A.; Guilmeau, E.; Bernik, S. Phase formation, microstructure development and thermoelectric properties of (ZnO)kIn2O3 ceramics. J. Eur. Ceram. Soc. 2017, 37, 2833–2842. [Google Scholar] [CrossRef]
- Moriga, T.; Edwards, D.D.; Mason, T.O.; Palmer, G.B.; Poeppelmeier, K.R.; Schindler, J.L.; Kannewurf, C.R.; Nakabayashi, I. Phase relationships and physical properties of homologous compounds in the Zinc oxide-Indium oxide system. J. Am. Ceram. Soc. 1998, 81, 1310–1316. [Google Scholar] [CrossRef]
- Kimizuka, N.; Isobe, M.; Nakamura, M. Syntheses and single-crystal data of homologous compounds, In2O3(ZnO)m (m = 3, 4, and 5), InGaO3(ZnO)3, and Ga2O3(ZnO)m (m = 7, 8, 9, and 16) in the In2O3–ZnGa2O4–ZnO system. J. Solid State Chem. 1995, 116, 170–178. [Google Scholar] [CrossRef]
- Kimizuka, N.; Yamazaki, S. Layered compounds in the In2O3–Ga2O3–ZnO system and related compounds in the ternary system. In Physics and Technology of Crystalline Oxide Semiconductor CAAC-IGZO: Fundamentals; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–49. [Google Scholar]
- Yoon, S.W. Ga ordering and electrical conductivity in nanotwin and superlattice-structured Ga-doped ZnO. Cryst. Growth Des. 2012, 12, 1167–1172. [Google Scholar] [CrossRef]
- Ohtaki, M.; Araki, K.; Yamamoto, K. High thermoelectric performance of dually doped ZnO ceramics. J. Electron. Mater. 2009, 38, 1234–1238. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, B.; Ye, D.; Liu, Y.; Li, S. Enhanced Al/Ni co-doping and power factor in textured ZnO thermoelectric ceramics prepared by hydrothermal synthesis and spark plasma sintering. J. Alloys Compd. 2016, 656, 784–792. [Google Scholar] [CrossRef]
- Jood, P.; Mehta, R.J.; Zhang, Y.L.; Peleckis, G.; Wang, X.L.; Siegel, R.W.; Borca-Tasciuc, T.; Dou, S.X.; Ramanath, G. Al-doped zinc oxide nanocomposites with enhanced thermoelectric properties. Nano Lett. 2011, 11, 4337–4342. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-H.; Xie, H.-Q.; Zhai, Y.-B. Enhanced thermoelectric figure of merit in nanostructured ZnO by nanojunction effect. Appl. Phys. Lett. 2013, 103, 243901. [Google Scholar] [CrossRef]
- Liang, X. Remarkable enhancement in the Kapitza resistance and electron potential barrier of chemically modified In2O3(ZnO)9 natural superlattice interfaces. Phys. Chem. Chem. Phys. 2015, 17, 29655–29660. [Google Scholar] [CrossRef] [PubMed]
- Kosir, M.; Ceh, M.; Ow-Yang, C.W.; Guilmeau, E.; Bernik, S. Structural features and thermoelectric properties of Al-doped (ZnO)5In2O3 homologous phases. J. Am. Ceram. Soc. 2017, 100, 3712–3721. [Google Scholar] [CrossRef]
- Schauble, N.; Suess, B.E.; Populoh, S.; Weidenkaff, A.; Aguirre, M.H. A morphology study on thermoelectric Al-substituted ZnO. AIP Conf. Proc. 2012, 1449, 421–424. [Google Scholar]
- Wang, C.; Wang, Y.; Zhang, G.; Peng, C.; Yang, Y. Theoretical investigation of the effects of doping on the electronic structure and thermoelectric properties of ZnO nanowires. Phys. Chem. Chem. Phys. 2014, 16, 3771–3776. [Google Scholar] [CrossRef] [PubMed]
- Brinzari, V.; Damaskin, I.; Trakhtenberg, L.; Cho, B.K.; Korotcenkov, G. Thermoelectrical properties of spray pyrolyzed indium oxide thin films doped by tin. Thin Solid Films 2014, 552, 225–231. [Google Scholar] [CrossRef]
- Brinzari, V.; Nika, D.L.; Damaskin, I.; Cho, B.K.; Korotcenkov, G. Electrical conductivity and Seebeck coefficient of nanoscaled indium-tin-oxide within the electron filtering model. Phys. E 2016, 81, 49–58. [Google Scholar] [CrossRef]
- Brinzari, V.I.; Cocemasov, A.I.; Nika, D.L.; Korotcenkov, G.S. Ultra-low thermal conductivity of nanogranular indium tin oxide films deposited by spray pyrolysis. Appl. Phys. Lett. 2017, 110, 071904. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Trakhtenberg, L.; Cho, B.K. In2O3-based thin films deposited by spray pyrolysis as promising thermoelectric material. Adv. Mater. Res. 2014, 1043, 40–44. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. In2O3-based multicomponent metal oxide films and their prospects for thermoelectric applications. Solid State Sci. 2016, 52, 141–148. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Nanoscaled In2O3:Sn films as material for thermoelectric applications: Achievements and limitations. Bull. Mater. Sci. 2016, 9, 1349–1354. [Google Scholar] [CrossRef]
- Rauf, I.A. Structure and properties of tin-doped indium oxide thin films prepared by reactive electron-beam evaporation with a zone-confining arrangement. J. Appl. Phys. 1996, 79, 4057. [Google Scholar] [CrossRef]
- Taga, N.; Shigesato, Y.; Kamei, M. Electrical properties and surface morphology of heteroepitaxial-grown tin-doped indium oxide thin films deposited by molecular-beam epitaxy. J. Vac. Sci. Technol. A 2000, 18, 1663–1667. [Google Scholar] [CrossRef]
- Gregory, O.; Luo, Q.; Crisman, E. High temperature stability of indium tin oxide films. Thin Solid Films 2002, 406, 286–293. [Google Scholar] [CrossRef]
- Cheng, B.; Fang, H.; Lan, J.; Liu, Y.; Lin, Y.-H.; Nan, C.-W. Thermoelectric performance of Zn and GeCo-doped In2O3 fine-grained ceramics by the spark plasma sintering. J. Am. Ceram. Soc. 2011, 94, 2279–2281. [Google Scholar] [CrossRef]
- Cahill, D.G.; Watson, S.K.; Pohl, R.O. Lower limit to the thermal conductivity of disordered crystals. Phys. Rev. B 1992, 46, 6131–6140. [Google Scholar] [CrossRef]
- Bhame, S.D.; Zhou, T.; Guilmeau, E.; Kinemuchi, Y.; Delorme, F.; Raveau, B. Synthesis and thermoelectric properties of oxygen deficient fluorite derivative Ga3-xIn5+xSn2O16. J. Appl. Phys. 2010, 108, 093708. [Google Scholar] [CrossRef]
- Hoel, C.A.; Mason, T.O.; Gaillard, J.-F.; Poeppelmeier, K.R. Transparent conducting oxides in the ZnO-In2O3-SnO2 system. Chem. Mater. 2010, 22, 3569–3579. [Google Scholar] [CrossRef]
- Zhu, B.B.; Zhang, T.; Tian, R.; Tan, T.T.; Donelson, R.S.; Li, S. Enhancement of thermoelectric properties in Sn doped (In0.95Lu0.05)2O3. J. Alloys Compd. 2015, 644, 119–123. [Google Scholar] [CrossRef]
- Van Hest, M.F.A.M.; Dabney, M.S.; Perkins, J.D.; Ginley, D.S.; Taylor, M.P. Titanium-doped Indium oxide: A high-mobility transparent conductor. Appl. Phys. Lett. 2005, 87, 032111. [Google Scholar] [CrossRef]
- Koida, T.; Kondo, M. Comparative studies of transparent conductive Ti-, Zr-, and Sn-doped In2O3 using a combinatorial approach. J. Appl. Phys. 2007, 101, 063713. [Google Scholar] [CrossRef]
- Van Hest, M.F.A.M.; Dabney, M.S.; Perkins, J.D.; Ginley, D.S. High mobility molybdenum doped indium oxide. Thin Solid Films 2006, 496, 70–74. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Miao, W.; Huang, L.; Zhang, Z. Transparent conductive oxide thin films of tungsten-doped indium oxide. Thin Solid Films 2006, 515, 2471–2474. [Google Scholar] [CrossRef]
- Calnan, S.; Tiwari, A.N. High mobility transparent conducting oxides for thin film solar cells. Thin Solid Films 2010, 518, 1839–1849. [Google Scholar] [CrossRef]
- Koida, T.; Fujiwara, H.; Kondo, M. Structural and electrical properties of hydrogen-doped In2O3 films fabricated by solid-phase crystallization. J. Non-Cryst. Solids 2008, 354, 2805–2808. [Google Scholar] [CrossRef]
- Bierwagen, O.; Speck, J.S. Plasma-assisted molecular beam epitaxy of Sn-doped In2O3:Sn incorporation, structural changes, doping limits, and compensation. Phys. Status Solidi A 2014, 211, 48–53. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. The role of grain size on the thermal stability of nanostructured metal oxides used in gas sensor applications and approaches for grain-size stabilization. Prog. Cryst. Growth Charact. Mater. 2012, 58, 167–208. [Google Scholar] [CrossRef]
- Giaquinta, D.M.; Davis, W.M.; Zur Loye, H.C. Structure of indium iron oxide. Acta Crystallogr. C 1994, 50, 5–7. [Google Scholar] [CrossRef]
- Yoo, Y.K.; Xue, Q.; Lee, H.-C.; Cheng, S.; Xiang, X.-D.; Dionne, G.F.; Xu, S.; He, J.; Chu, Y.S.; Preite, S.D.; et al. Bulk synthesis and high–temperature ferromagnetism of (In1−xFex)2O3−σ with Cu co–doping. Appl. Phys. Lett. 2005, 86, 042506. [Google Scholar] [CrossRef]
- Ratko, A.; Babushkin, O.; Baran, A.; Baran, S. Sorption and gas sensitive properties of In2O3 based ceramics doped with Ga2O3. J. Eur. Ceram. Soc. 1998, 18, 2227–2232. [Google Scholar] [CrossRef]
- Frank, G.; Brock, L.; Bausen, H.D. The solubilities of Sn in In2O3 and of In in SnO2 crystals grown from Sn–In melts. J. Cryst. Growth 1976, 36, 179–180. [Google Scholar] [CrossRef]
- Ohya, Y.; Ito, T.; Kaneko, M.; Ban, T.; Takahashi, Y. Solid solubility of SnO2 in In2O3. J. Ceram. Soc. Jpn. 2000, 108, 803–806. [Google Scholar] [CrossRef]
- Gonzalez, G.B.; Mason, T.O.; Okasinski, J.S.; Buslaps, T.; Honkimaki, V. Determination of the solubility of tin in indium oxide using in situ and ex situ X-ray diffraction. J. Am. Ceram. Soc. 2012, 95, 809–815. [Google Scholar] [CrossRef]
- Kaleemulla, S.; Madhusudhana Rao, N.; Girish Joshi, M.; Sivasankar Reddy, A.; Uthanna, S.; Sreedhara Reddy, P. Electrical and optical properties of In2O3:Mo thin films prepared at various Mo–doping levels. J. Alloys Compd. 2010, 504, 351–356. [Google Scholar] [CrossRef]
- Sunde, T.O.L.; Lindgren, M.; Mason, T.O.; Einarsrud, M.-A.; Grande, T. Solid solubility of rare earth elements (Nd, Eu, Tb) in In2−xSnxO3–effect on electrical conductivity and optical properties. Dalton Trans. 2014, 43, 9620–9632. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, S.; Gokulakrishnan, V.; Ramamurthi, K.; Elangovan, E.; Martins, R.; Fortunato, E.; Ganesan, R. High near–infrared transparent molybdenum–doped indium oxide thin films for nanocrystalline silicon solar cell applications. Solar Energy Mater. Sol. Cells 2009, 93, 92–97. [Google Scholar] [CrossRef]
- Park, D.H.; Son, K.Y.; Lee, J.H.; Kim, J.J.; Lee, J.S. Effect of ZnO addition in In2O3 ceramics: Defect chemistry and sintering behavior. Solid State Ion. 2004, 172, 431–434. [Google Scholar] [CrossRef]
- Godzhieva, O.V.; Porotnikov, N.V.; Pikhidchuk, V.P. Physical–chemical study of triple oxides formed in In2O3–CaO–CuO system. Zhur. Neorg. Khim. 1992, 37, 1184–1188. [Google Scholar]
- Horyn, R.; Bukowska, E.; Sikora, A. Studies of BaO–In2O3–CuO ternary system. Part I: Phase equilibria in the isothermal cross–section of 930 °C. J. Alloys Compd. 2000, 305, 103–108. [Google Scholar] [CrossRef]
- Lan, J.-L.; Liu, Y.; Lin, Y.-H.; Nan, C.-W.; Cai, Q.; Yang, C. Enhanced thermoelectric performance of In2O3-based ceramics via nanostructuring and point defect engineering. Sci. Rep. 2015, 5, 7783. [Google Scholar] [CrossRef] [PubMed]
- Bergman, D.J.; Levy, O. Thermoelectric properties of a composite medium. J. Appl. Phys. 1991, 70, 6821. [Google Scholar] [CrossRef]
- Medlin, D.L.; Snyder, G.J. Interfaces in bulk thermoelectric materials: A review for current opinion in colloid and interface science. Curr. Opin. Colloid Interface Sci. 2009, 14, 226. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zide, J.; Gossard, A.; Klenov, D.; Stemmer, S.; Shakouri, A.; Majumdar, A. Thermal conductivity reduction and thermoelectric figure of merit increase by embedding nanoparticles in crystalline semiconductors. Phys. Rev. Lett. 2006, 96, 045901. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Scullin, M.L.; Huijben, M.; Ramesh, R.; Majumdar, A. Thermal conductivity reduction in oxygen-deficient strontium titanates. Appl. Phys. Lett. 2008, 92, 191911. [Google Scholar] [CrossRef]
- Zide, J.M.O.; Vashaee, D.; Bian, Z.X.; Zeng, G.H.; Bowers, J.E.; Shakouri, A. Demonstration of electron filtering to increase the Seebeck coefficient in In0.53Ga0.47As/In0.53Ga0.28Al0.19As superlattices. Phys. Rev. B 2006, 74, 205335. [Google Scholar] [CrossRef]
- Tritt, T.M. Thermal Conductivity: Theory, Properties, and Applications; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Zhou, T.; Bhame, S.D.; Guilmeau, E.; Marinel, S.; Raveau, B. Enhanced densification and thermoelectric performance of In4Sn3O12 by reactive sintering in the In-Sn-Ga-O system. J. Am. Ceram. Soc. 2011, 94, 3733–3737. [Google Scholar] [CrossRef]
- Edwards, D.D.; Mason, T.O.; Sinlker, W.; Marks, L.D.; Goutenoire, F.; Poeppelmeier, K.R. A structural investigation of Ga3−xIn5+xSn2O16. J. Solid State Chem. 1998, 140, 242–250. [Google Scholar] [CrossRef]
- Weidenkaff, A.; Robert, R.; Aguirre, M.H.; Bocher, L.; Schlapbach, L. Nanostructured thermoelectric oxides with low thermal conductivity. Phys. Status Solidi Rapid Res. Lett. 2007, 1, 247–249. [Google Scholar] [CrossRef]
- Ashida, T.; Miyamura, A.; Oka, N.; Sato, Y.; Yagi, T.; Taketoshi, N.; Baba, T.; Shigesato, Y. Thermal transport properties of polycrystalline tin-doped indium oxide film. J. Appl. Phys. 2009, 105, 073709. [Google Scholar] [CrossRef]
- Ohtaki, M. Recent aspects of oxide thermoelectric materials for power generation from mi-to-high temperature heat source. J. Ceram. Soc. Jpn. 2011, 119, 770–775. [Google Scholar] [CrossRef]
- Zebarjadi, M.; Esfarjani, K.; Bian, Z.; Shakouri, A. Low-temperature thermoelectric power factor enhancement by controlling nanoparticle size distribution. Nano Lett. 2011, 11, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.H.; Choi, C.J.; Jang, S.P.; Choi, S. A review of thermal conductivity data, mechanisms and models for nanofluids. Int. J. Micro-Nano Scale Transp. 2010, 1, 269–322. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly anisotropic thermal conductivity of free-standing reduced graphene oxide films annealed at high temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Nika, D.L.; Pokatilov, E.P.; Balandin, A.A.; Fomin, V.M.; Rastelli, A.; Schmidt, O.G. Reduction of lattice thermal conductivity in one-dimensional quantum-dot superlattices due to phonon filtering. Phys. Rev. B 2011, 84, 165415. [Google Scholar] [CrossRef]
- Nika, D.L.; Cocemasov, A.I.; Isacova, C.I.; Balandin, A.A.; Fomin, V.M.; Schmidt, O.G. Suppression of phonon heat conduction in cross-section-modulated nanowires. Phys. Rev. B 2012, 85, 205439. [Google Scholar] [CrossRef]
- Nika, D.L.; Cocemasov, A.I.; Crismari, D.V.; Balandin, A.A. Thermal conductivity inhibition in phonon engineered core-shell cross-section modulated Si/Ge nanowires. Appl. Phys. Lett. 2013, 102, 213109. [Google Scholar] [CrossRef]
- Kazeoka, M.; Hiramatsu, H.; Seo, W.-S.; Koumoto, K. Improvement in thermoelectric properties of (ZnO)5In2O3 through partial substitution of yttrium for indium. J. Mater. Res. 1998, 13, 523–526. [Google Scholar] [CrossRef]
- Park, K.; Kim, K.K.; Lee, N.; Kim, J.; Seo, W.-S.; Yang, B. High-temperature thermoelectric properties of Sb-containing (ZnO)m(In1-xSbx)2O3 (0 ≤ x <0.1). J. Korean Phys. Soc. 2006, 49, 1548–1552. [Google Scholar]
- Berardan, D.; Guilmeau, E.; Maignan, A.; Raveau, B. Enhancement of the thermoelectric performances of In2O3 by the coupled substitution of M2+/Sn4+ for In3+. J. Appl. Phys. 2008, 104, 064918. [Google Scholar] [CrossRef]
- Combe, E.; Chubileau, C.; Berardan, D.; Guilmeau, E.; Maignan, A.; Raveau, B. Citrate gel process and thermoelectric properties of Ge-doped In2O3 bulk ceramics. Powder Technol. 2011, 208, 503–508. [Google Scholar] [CrossRef]
- Seo, D.K.; Shin, S.; Cho, H.H.; Kong, B.H.; Whang, D.M.; Cho, H.K. Drastic improvement of oxide thermoelectric performance using thermal and plasma treatments of the InGaZnO thin films grown by sputtering. Acta Mater. 2011, 59, 6743–6750. [Google Scholar] [CrossRef]
- Løveng Sunde, T.O.; Einarsrud, M.-A.; Grande, T. Solid state sintering of nano-crystalline indium tin oxide. J. Eur. Ceram. Soc. 2013, 33, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Mori, N.; Ueno, J.; Uesugi, Y.; Miki, K. Thermoelectric properties in transparent-conductive Cerium-doped In2O3 films. e-J. Surf. Sci. Nanotechnol. 2012, 10, 471–475. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.-H.; Lan, J.; Zhang, B.-P.; Xu, W.; Nan, C.-W.; Zhu, H. Thermoelectric performance of Zn and Nd Co-doped In2O3 ceramics. J. Electron. Mater. 2011, 40, 1083–1086. [Google Scholar] [CrossRef]
- Combe, E.; Guilmeau, E.; Savary, E.; Marinel, S.; Cloots, R.; Funahashi, R.; Boschini, F. Microwave sintering of Ge-doped In2O3 thermoelectric ceramics prepared by slip casting process. J. Eur. Ceram. Soc. 2015, 35, 145–151. [Google Scholar] [CrossRef]
- Amani, M.; Tougas, I.M.; Gregory, O.J.; Fralick, G.C. High-temperature thermoelectric properties of compounds in the system ZnxInyOx+1.5y. J. Electron. Mater. 2013, 42, 114–120. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.-H.; Lan, J.; Xu, W.; Zhang, B.-P.; Nan, C.-W.; Zhu, H. Effect of transition-metal cobalt doping on the thermoelectric performance of In2O3 ceramics. J. Am. Ceram. Soc. 2010, 93, 2938–2941. [Google Scholar] [CrossRef]
- Pitschke, W.; Werner, J.; Behr, G.; Koumoto, K. Structure and thermoelectric properties of Me-substituted In4Sn3O12, Me = Y and Ti. J. Solid State Chem. 2000, 153, 349–356. [Google Scholar] [CrossRef]
- Edwards, D.D.; Mason, T.O.; Goutenoire, F.; Poeppelmeier, K.R. A new transparent conducting oxide in the Ga2O3–In2O3–SnO2 system. Appl. Phys. Lett. 1997, 70, 1706–1708. [Google Scholar] [CrossRef]
- Brinzari, V.; Cho, B.K.; Kamei, M.; Korotcenkov, G. Photoemission surface characterization of (001) In2O3 thin film through the interactions with oxygen, water and carbon monoxide: Comparison with (111) orientation. Appl. Surf. Sci. 2015, 324, 123–133. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Interference effects between hydrogen and ozone in the response of SnO2-based gas sensors. Sens. Actuators B 2017, 243, 507–515. [Google Scholar] [CrossRef]
- Mizuno, M.; Miyamoto, T.; Ohnishi, T.; Hayashi, H. Effects of thin doping and oxygen vacancies on the electronic states of indium oxide. Jpn. J. Appl. Phys. 1997, 36, 3408–3413. [Google Scholar] [CrossRef]
- Bartolome, J.; Maestre, D.; Cremades, A.; Amatti, M.; Piqueras, J. Composition-dependent electronic properties of indium–zinc–oxide elongated microstructures. Acta Mater. 2013, 61, 1932–1943. [Google Scholar] [CrossRef]
- Vorobyeva, N.A.; Rumyantseva, M.N.; Forsh, P.A.; Gaskov, A.M. Conductivity of nanocrystalline ZnO(Ga). Semiconductors 2013, 47, 650–654. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. In2O3- and SnO2-based ozone sensors: Design and characterization. Crit. Rev. Solid State Mater. Sci. 2017, 1–50. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Stetter, J.R.; Blinov, I.; Blaja, V. The nature of processes controlling the kinetics of indium oxide-based thin film gas sensor response. Sens. Actuators B 2007, 128, 51–63. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Blinov, I.; Ivanov, M.; Stetter, J.R. Ozone sensors on the base of SnO2 films deposited by spray pyrolysis. Sens. Actuators B 2007, 120, 679–686. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Ivanov, M.; Cerneavschi, A.; Rodriguez, J.; Cirera, A.; Cornet, A.; Morante, J. Structural stability of indium oxide films deposited by spray pyrolysis during thermal annealing. Thin Solid Films 2005, 479, 38–51. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Bulk doping influence on the response of conductometric SnO2 gas sensors: Understanding through cathodoluminescence study. Sens. Actuators B 2014, 196, 80–98. [Google Scholar] [CrossRef]
- Harvey, S.P.; Poeppelmeier, K.R.; Mason, T.O. Subsolidus phase relationships in the ZnO-In2O3-SnO2 system. J. Am. Ceram. Soc. 2008, 91, 3683–3689. [Google Scholar] [CrossRef]
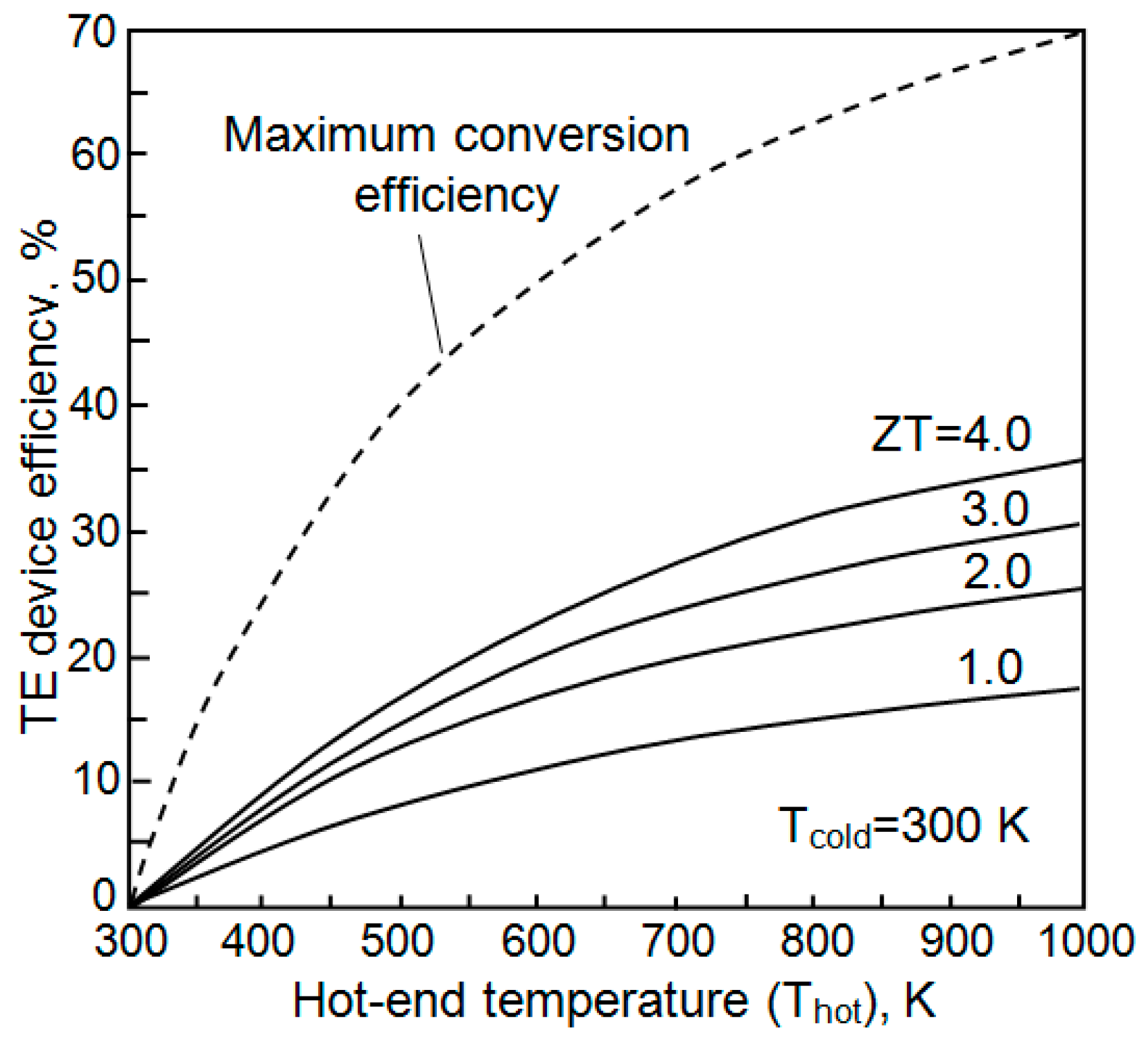


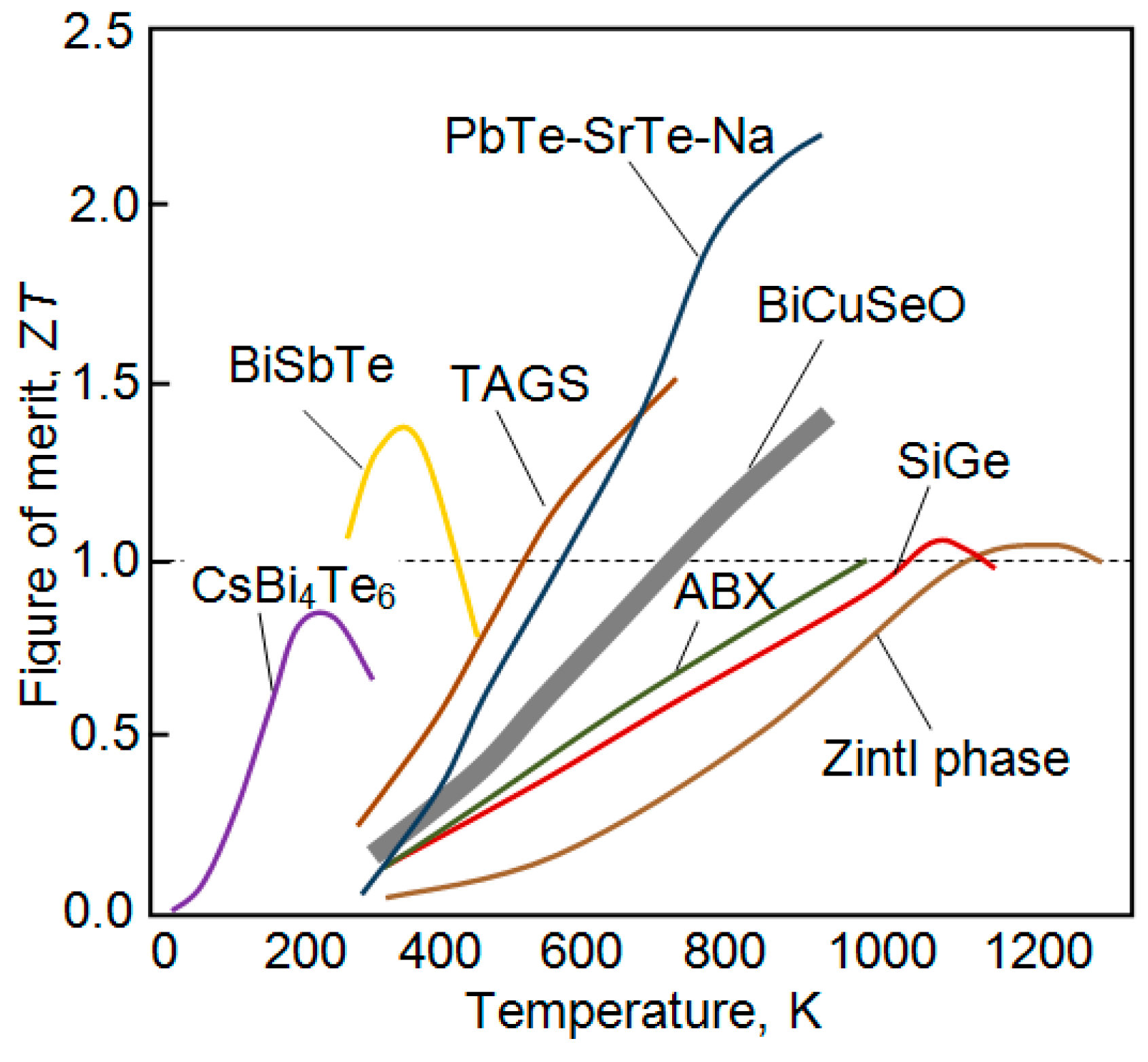
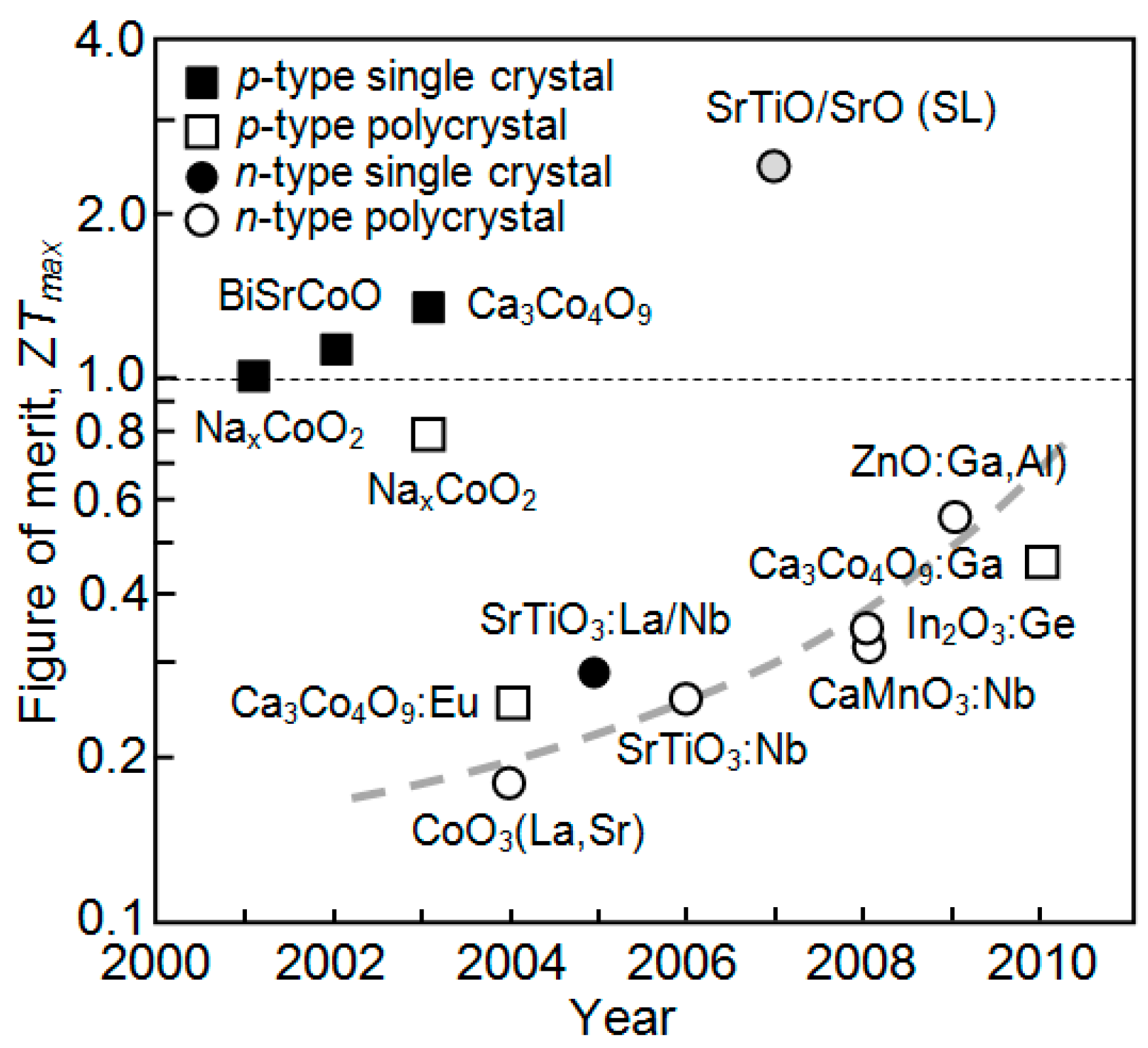


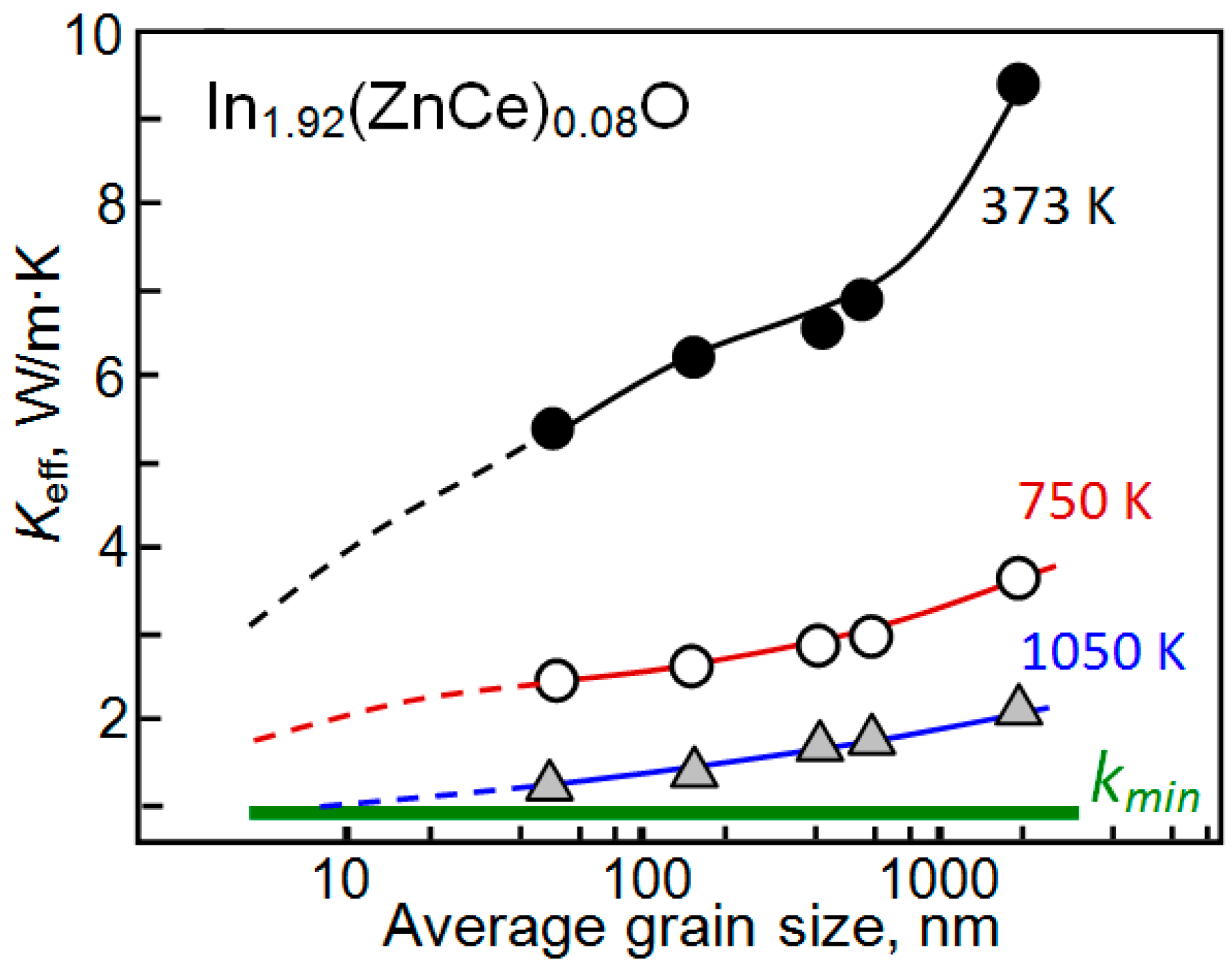
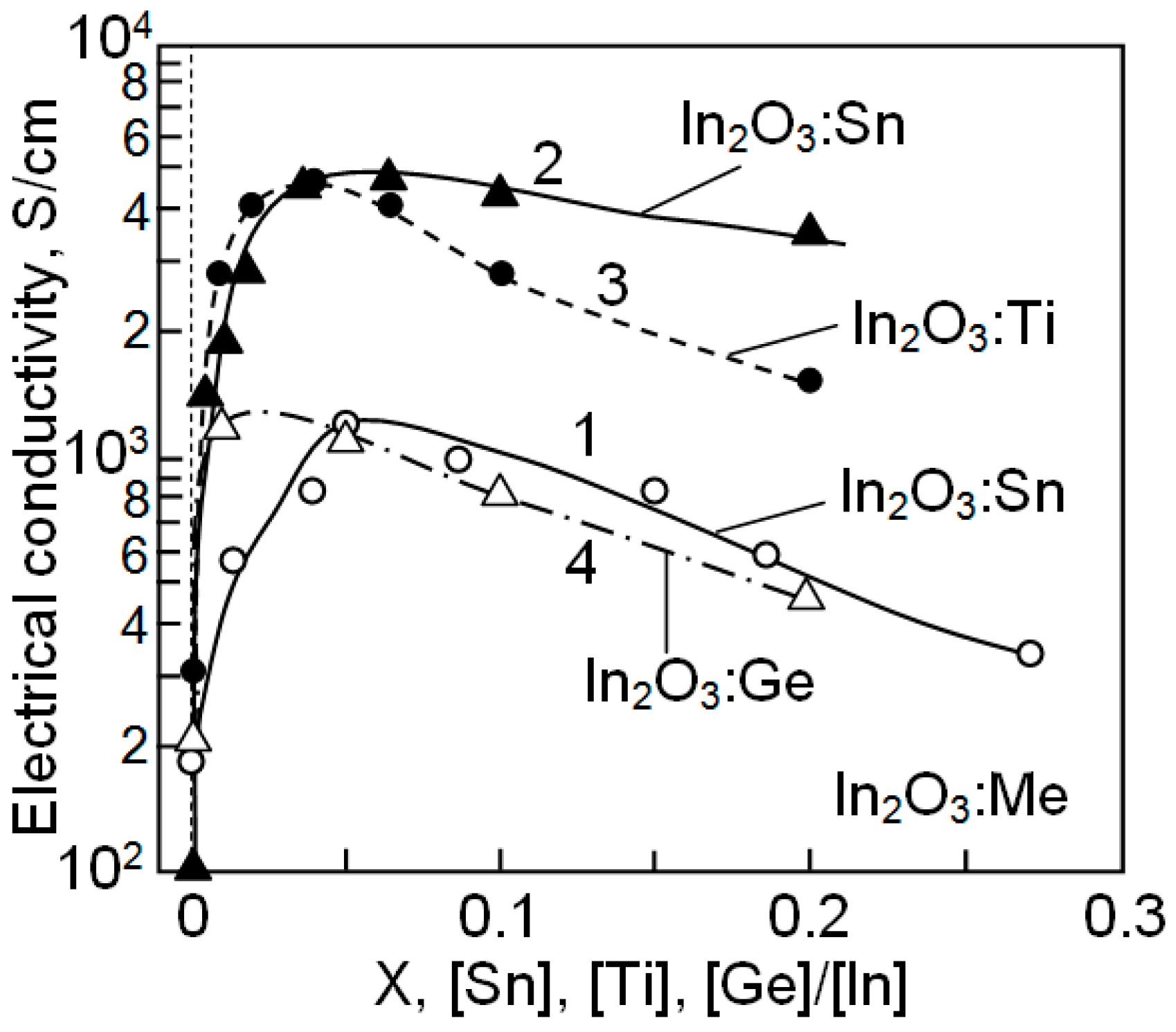
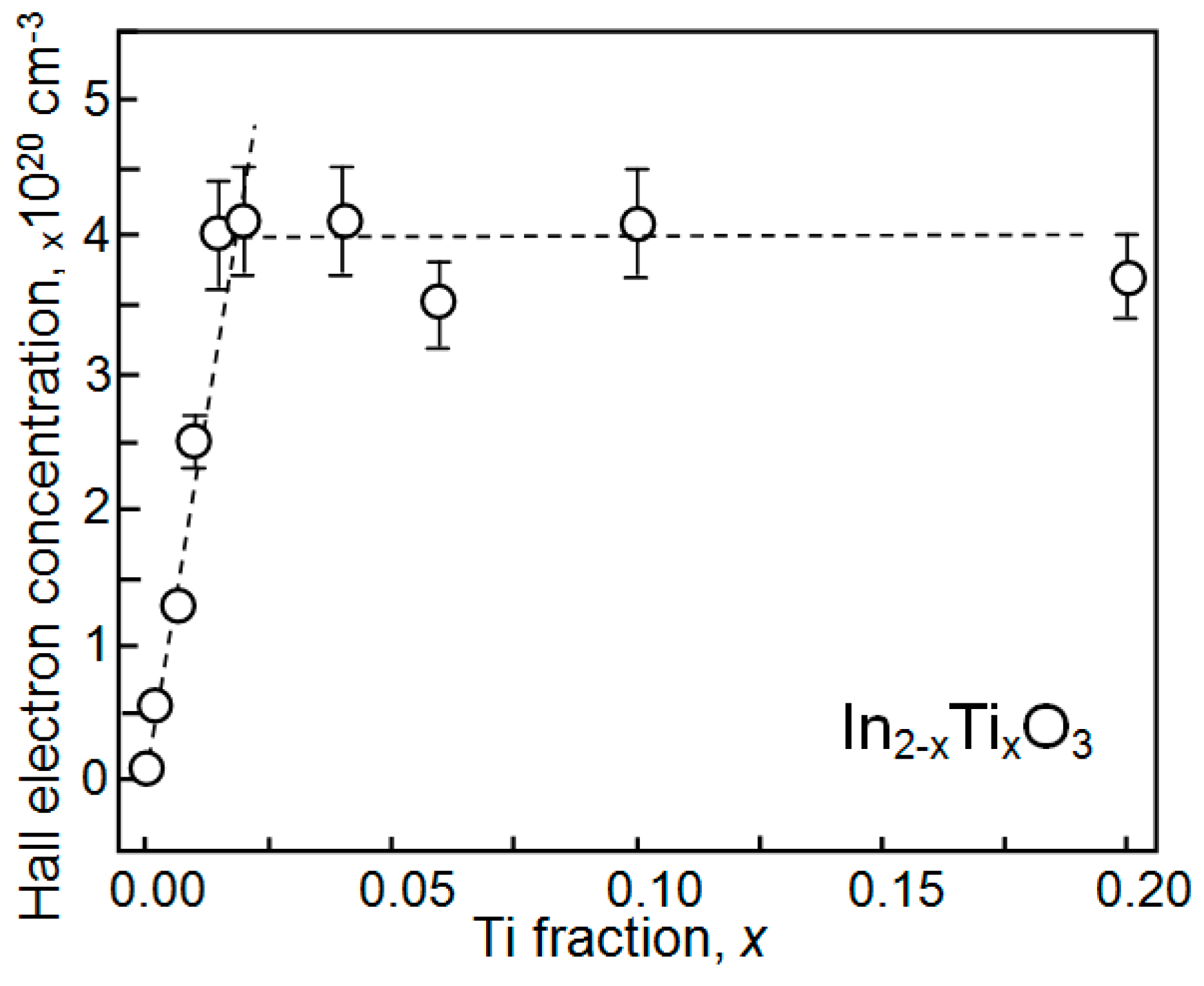

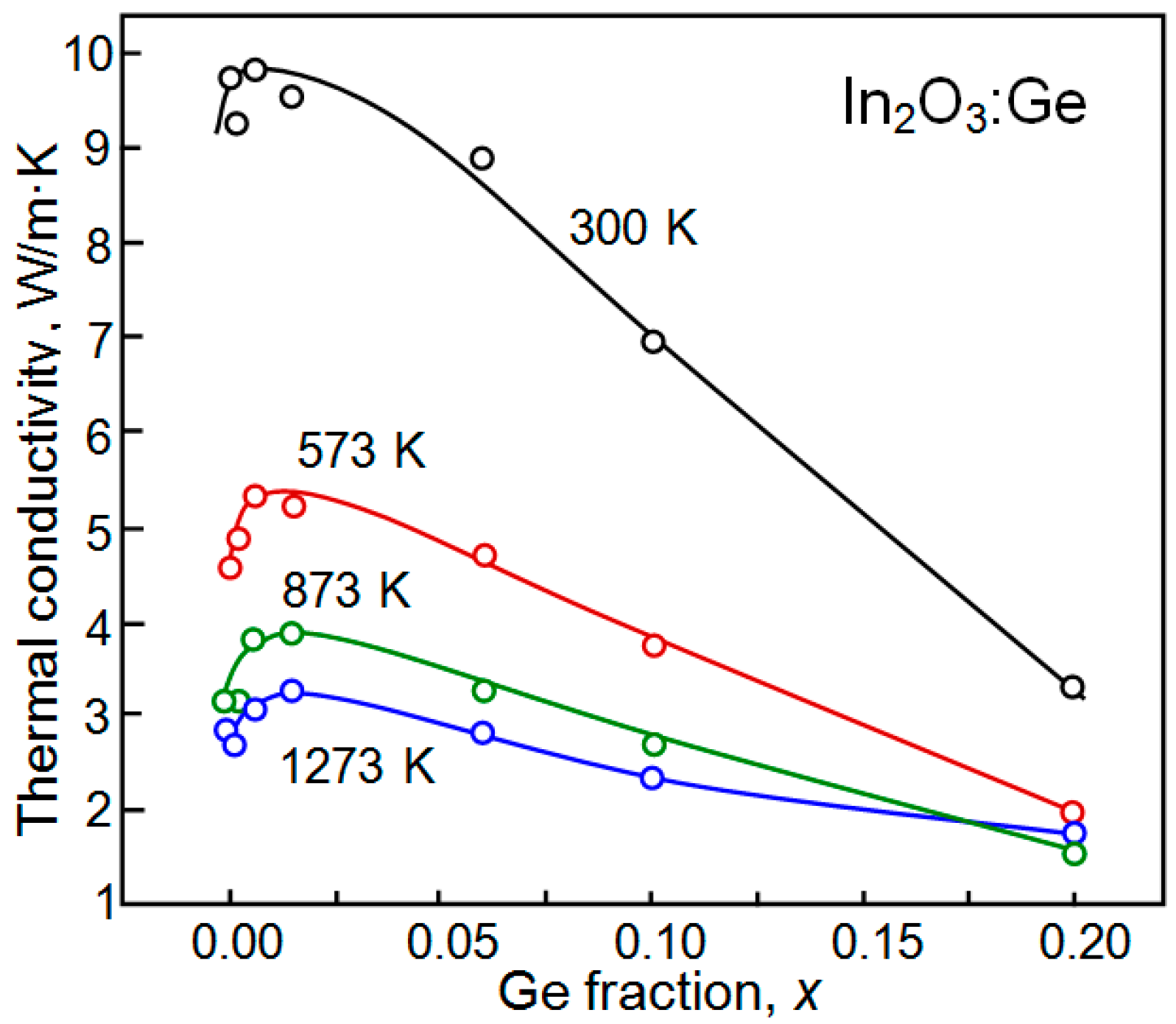


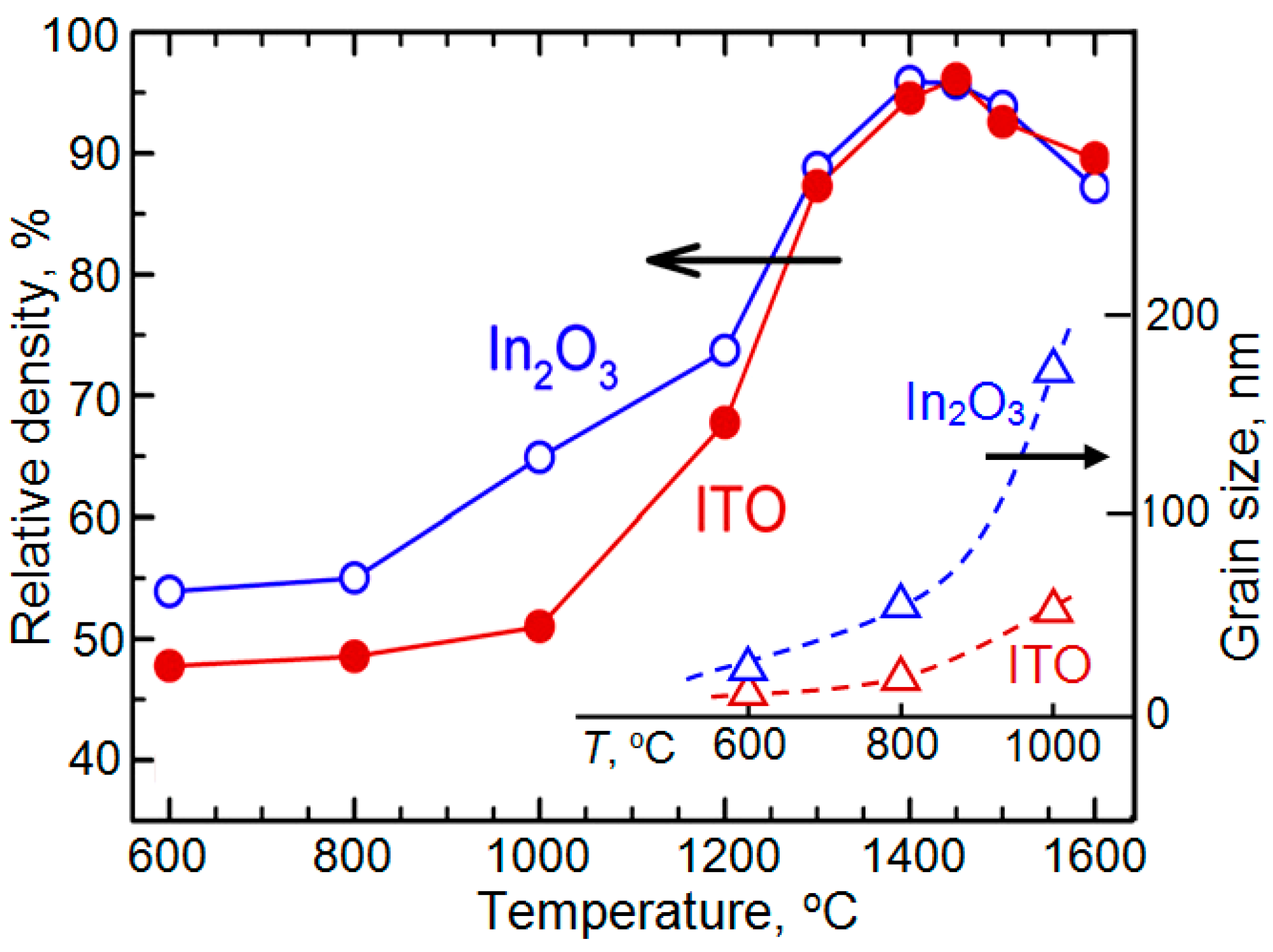
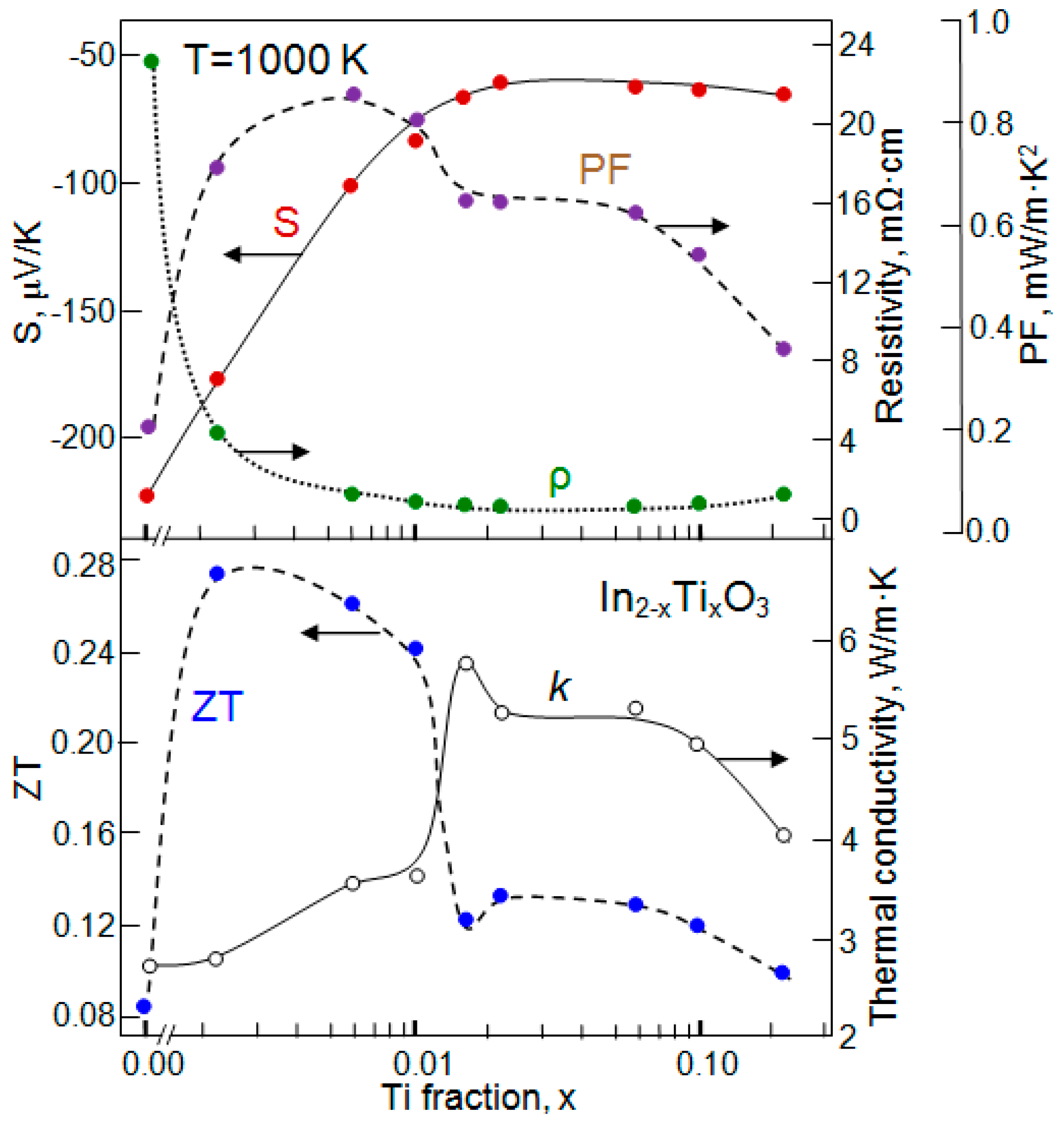

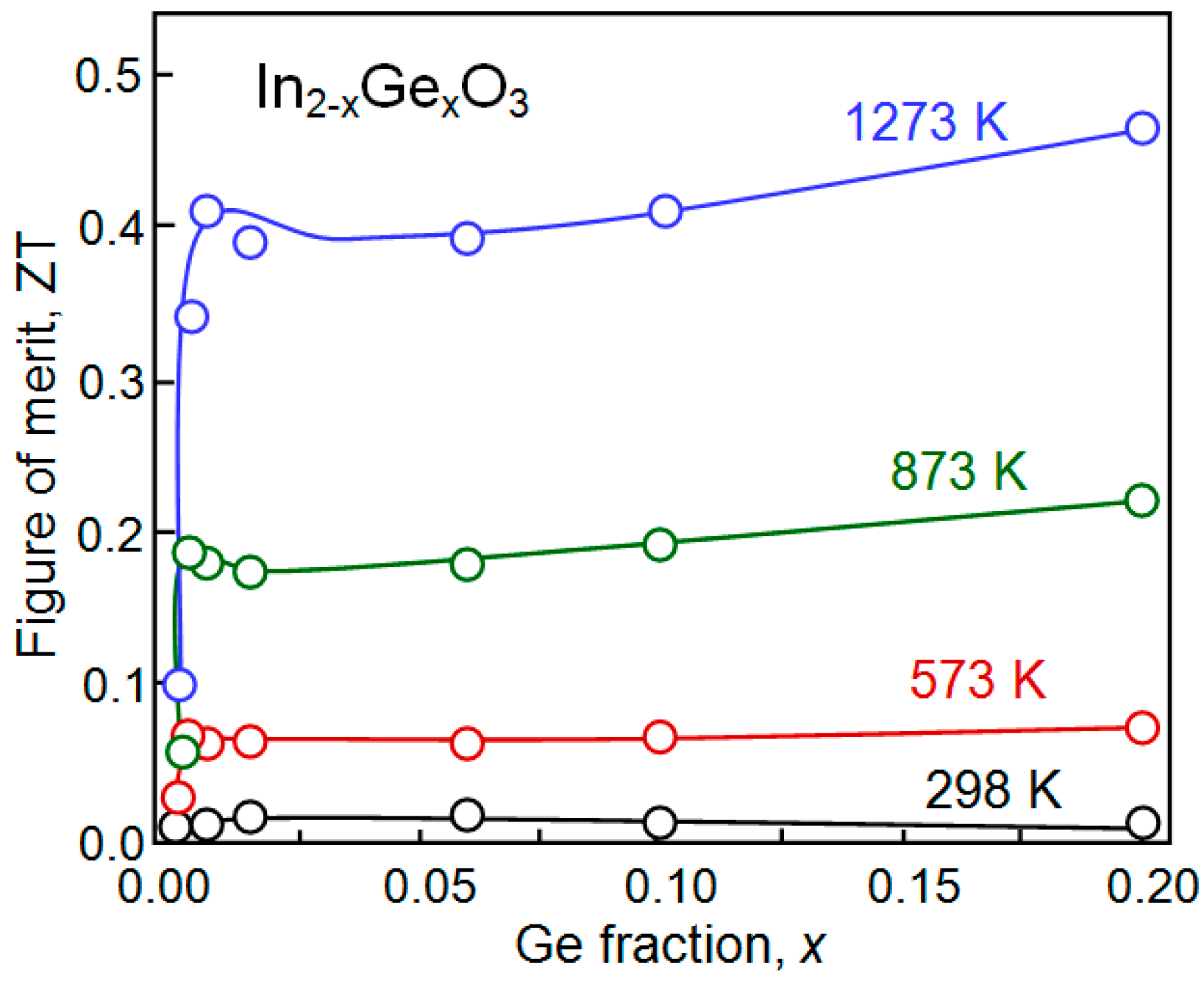

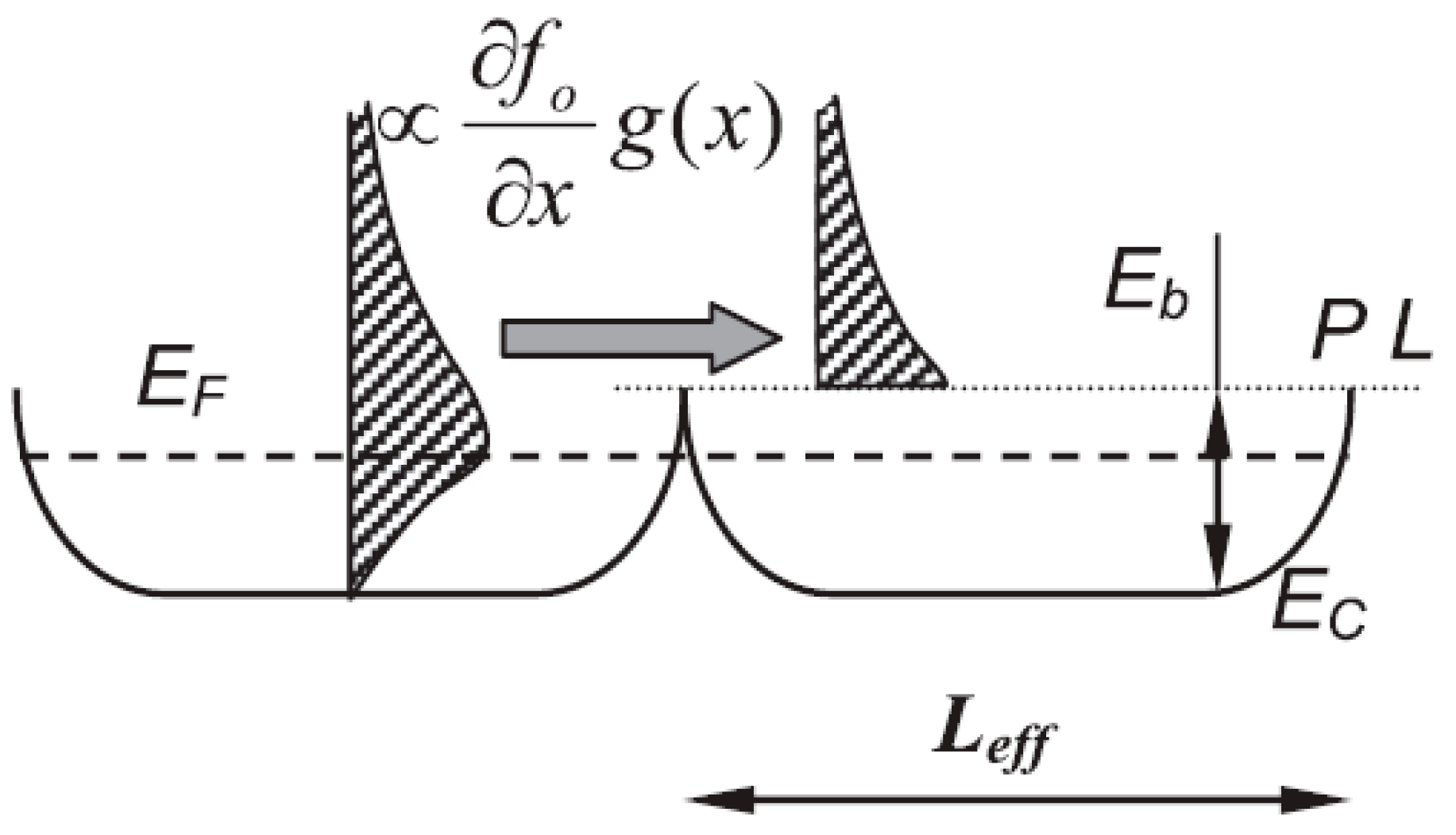
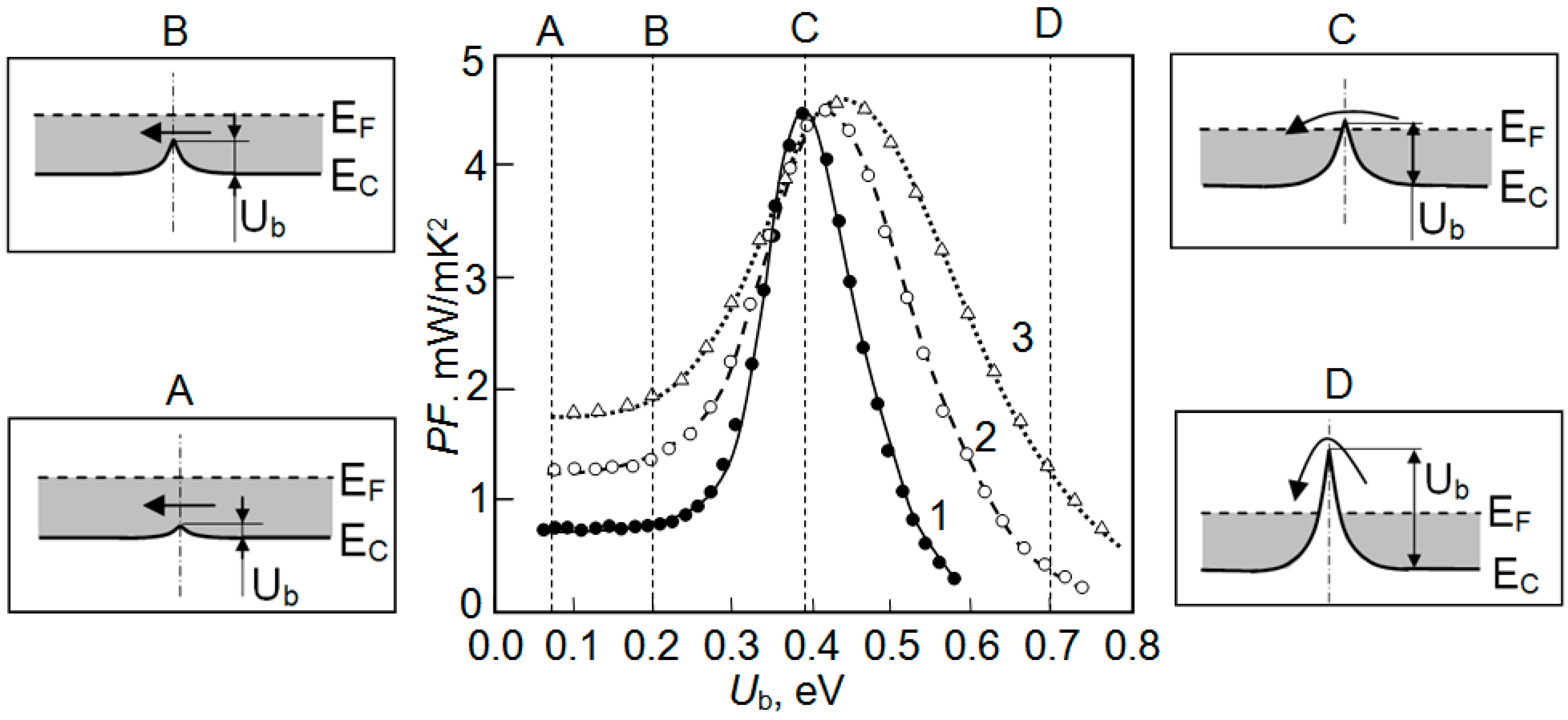
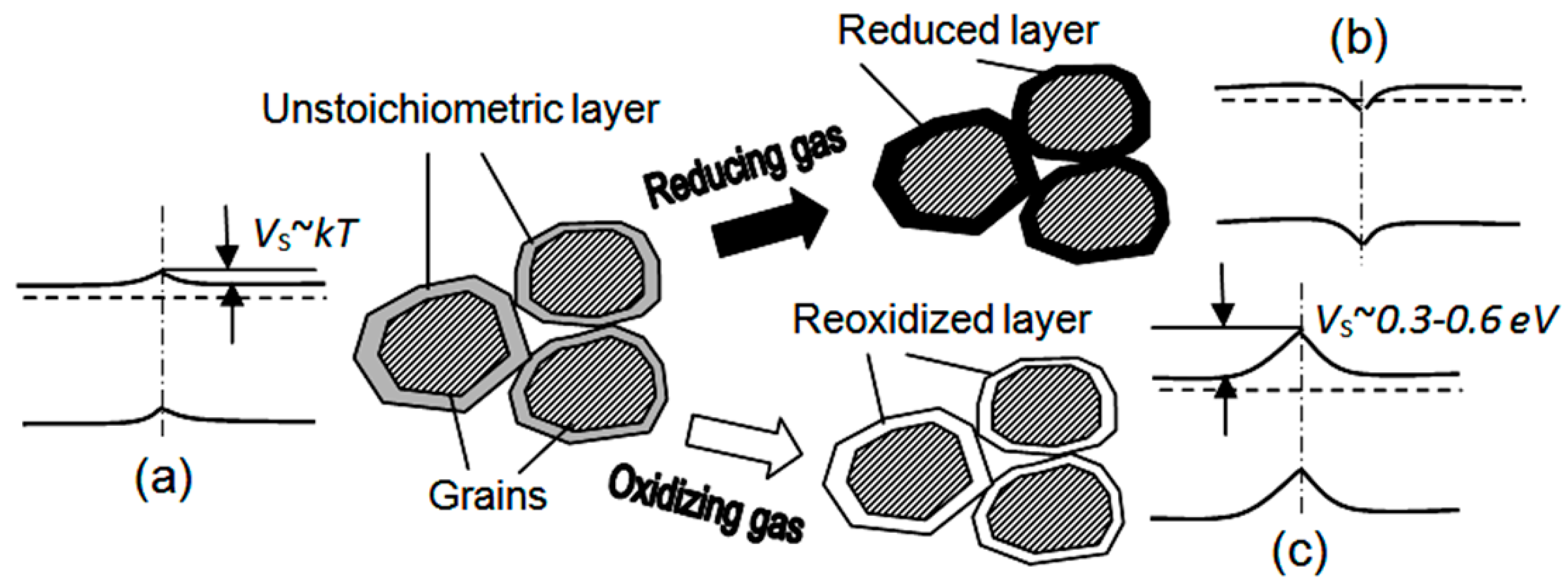
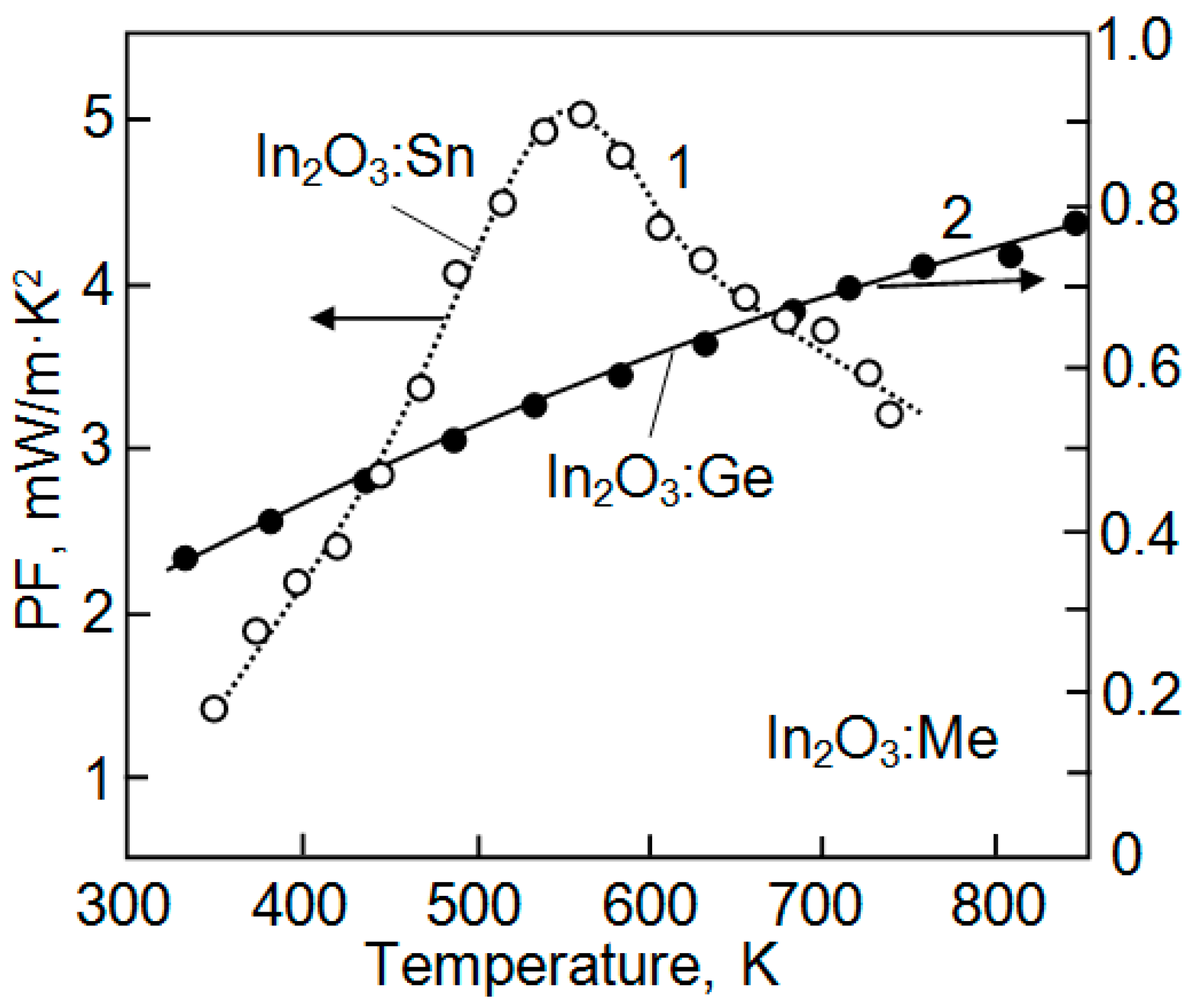

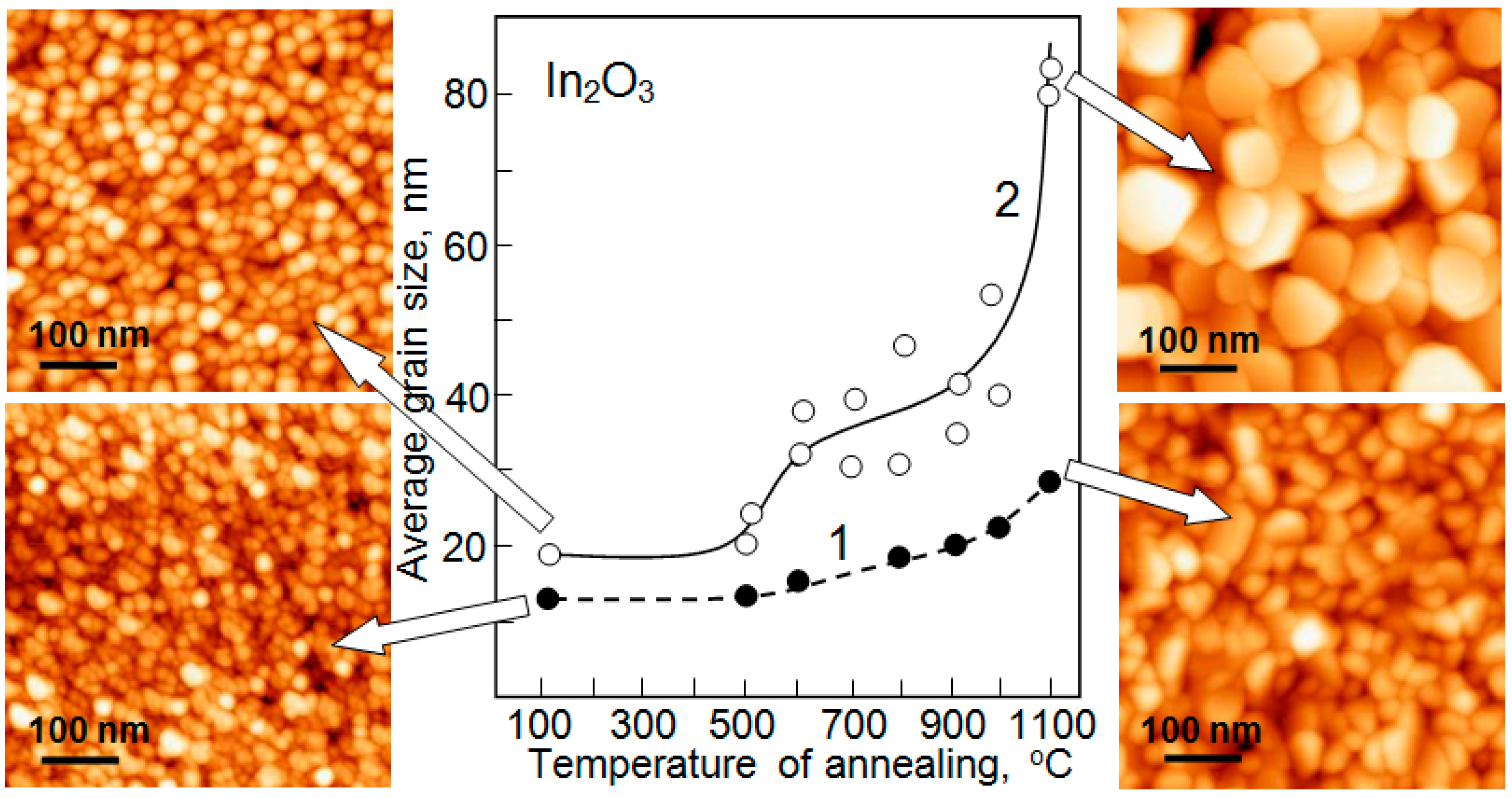
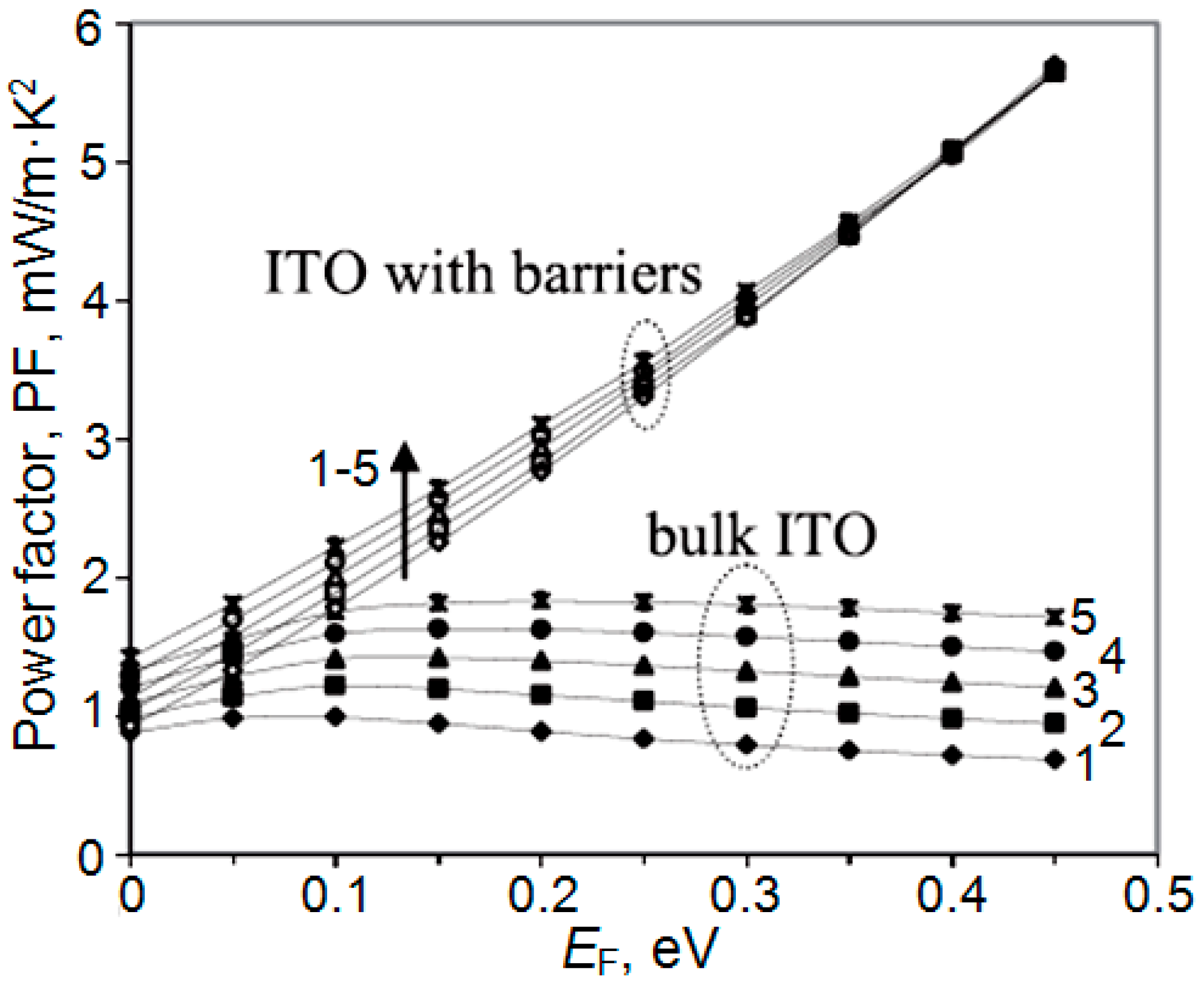
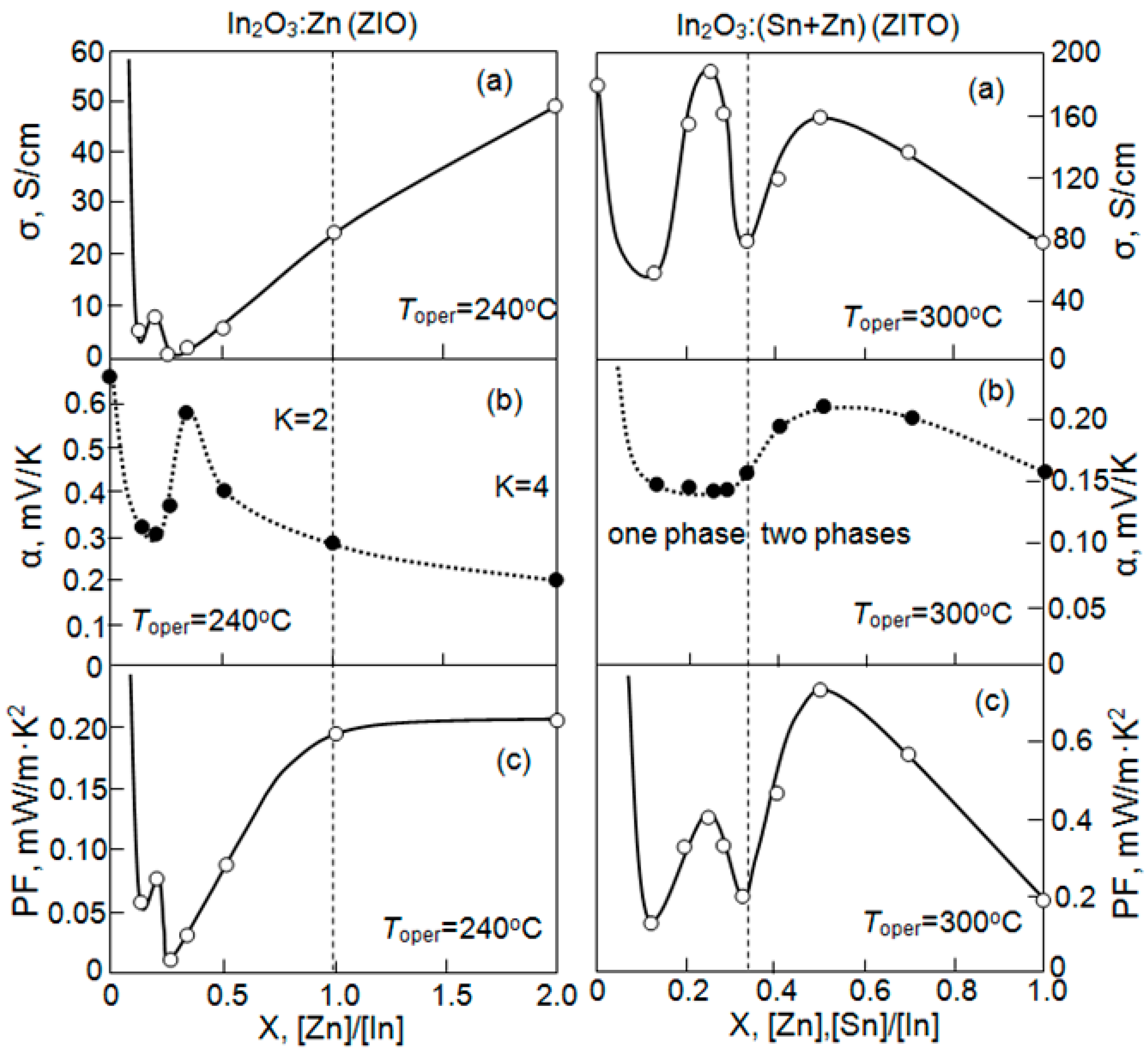
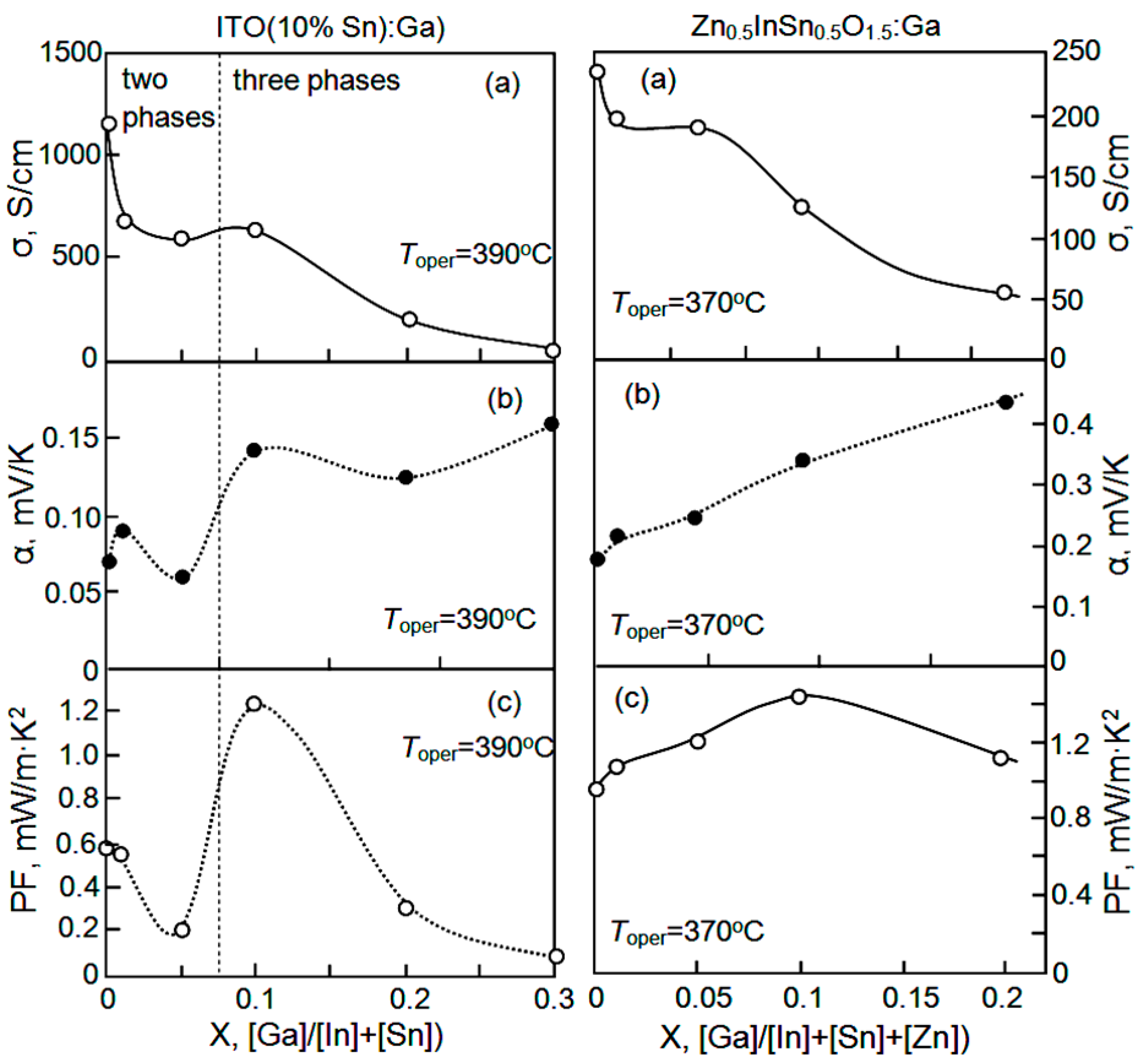
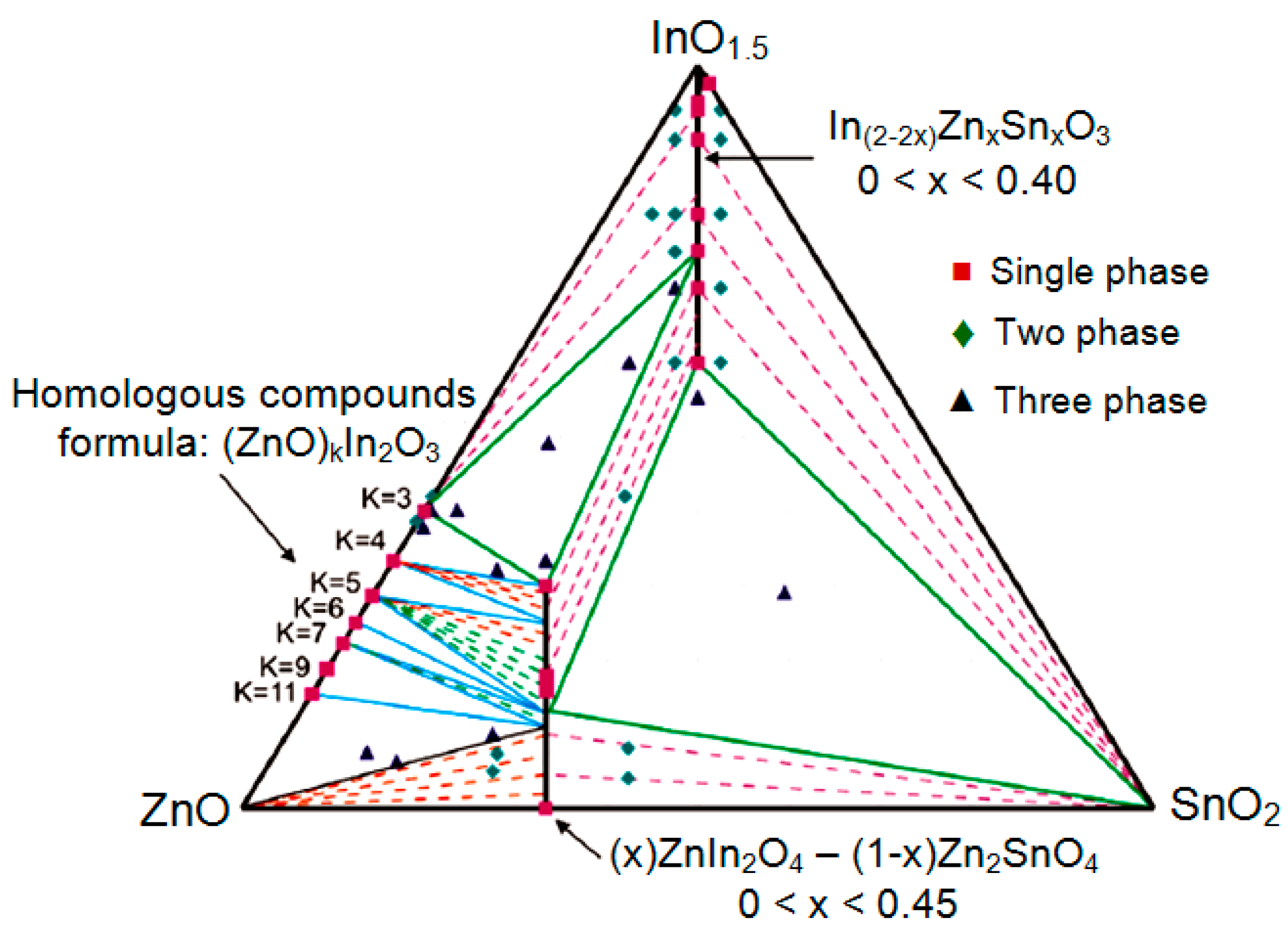
| Dopant | N (1020 cm−3) | μ (cm2/V·s) | ρ (10−4 Ω·cm) |
|---|---|---|---|
| Mo | 2–7.1 | 50–250 | 0.7–7.3 |
| Ti | 0.8–5.0 | 80–199 | 1.0–5.2 |
| Zr | 0.8–4.3 | 63–170 | 2.3–5.7 |
| W | 2.4–4.0 | 26–358 | 2.7–10 |
| Sn | 2–20 | 43–145 | 0.9–6 |
| Metal Oxide/Addition | Limit Solubility | Ref. |
|---|---|---|
| In2O3:Fe | ~20% | [154,155] |
| In2O3:Ga | 10%–12% | [156] |
| In2O3:Sn | 0.5%–8% | [157,158,159] |
| In2O3:Ti | 3%–5% | [71] |
| In2O3:Nb | 1%–3% | [160,161] |
| In2O3:Mo | 1%–2% | [162] |
| In2O3:Ge | 1%–1.5% | [70] |
| In2O3:Co | ~1% | [161] |
| In2O3:Zn | ~0.5% | [163] |
| In2O3:Cu | <<1% | [164,165] |
| Metal Oxide | σ, S/cm | S, μV/K | PF, mW/m·K2 | k, W/m·K | ZT | T, K | Ref. |
|---|---|---|---|---|---|---|---|
| In2O3 | 20–200 | −200–−340 | 0.2–0.23 | 2.5–3.3 | 0.09–0.13 | 1000 | [31,70] |
| In2O3:Ce (3–8%) | 120-180 | −160 | 2.3 | 0.21 | 1000 | [39,191] | |
| In2O3:(Zn,Ce) (8%) | 420–550 | −140–−150 | 0.81 | 1.5–2.7 | 0.32–0.4 | 1000 | [38,166] |
| In2O3:(Zn,Nb) (4%) | 140 | −190 | 0.52 | 2.1 | 0.25 | 1000 | [192] |
| In2O3:Ga (10%) | 400 | −160 | 2.3 | 0.37 | 1000 | [40] | |
| Ga3−xIn5+xSn2O16 | 200–700 | −90–−145 | 0.36–0.55 | 1.4–1.91 | 0.23–0.27 | 1000 | [71,143] |
| In2O3:Ge (1–10%) | 500–1000 | −60–−110 | 0.88 | 3.5–5.0 | 0.12–0.4 | 1000 | [70,193] |
| In2O3:Ti (2–6%) | ~900 | −105 | 0.85 | 1.7–3.5 | 0.26 | 1000 | [71] |
| In2O3:Sn (2–6%) | 600 | −90–−110 | 0.15–0.94 | 3.0–3.5 | 0.28–04 | 1000 | [28,71] |
| (In0.95Lu0.05)2O3:Sn (≤1%) | ~1000 | –75 | 0.5 | 2.5–3.5 | 0.13–014 | 1000 | [145] |
| In2O3:ZnO = 3:2 | ~70 | –290 | 0.57 | 3.5 | 0.26 | 1000 | [194] |
| In2O3:(Nb,Zn) (4%) | 130 | –190 | 0.53 | 2.1 | 0.25 | 1000 | [192] |
| In2O3:Co (2%) | 90 | –200 | 0.46 | 1.8 | 0.20 | 1000 | [195] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotcenkov, G.; Brinzari, V.; Ham, M.-H. In2O3-Based Thermoelectric Materials: The State of the Art and the Role of Surface State in the Improvement of the Efficiency of Thermoelectric Conversion. Crystals 2018, 8, 14. https://doi.org/10.3390/cryst8010014
Korotcenkov G, Brinzari V, Ham M-H. In2O3-Based Thermoelectric Materials: The State of the Art and the Role of Surface State in the Improvement of the Efficiency of Thermoelectric Conversion. Crystals. 2018; 8(1):14. https://doi.org/10.3390/cryst8010014
Chicago/Turabian StyleKorotcenkov, Ghenadii, Vladimir Brinzari, and Moon-Ho Ham. 2018. "In2O3-Based Thermoelectric Materials: The State of the Art and the Role of Surface State in the Improvement of the Efficiency of Thermoelectric Conversion" Crystals 8, no. 1: 14. https://doi.org/10.3390/cryst8010014




