Preparation of Three-Dimensional Photonic Crystals of Zirconia by Electrodeposition in a Colloidal Crystals Template
Abstract
:1. Introduction
2. Results and Discussion
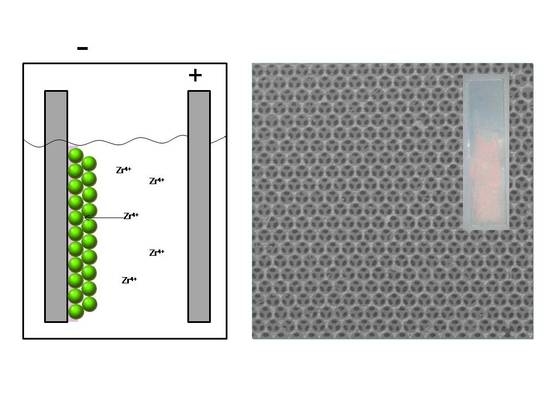
2.1. Electrodeposition of Zirconia
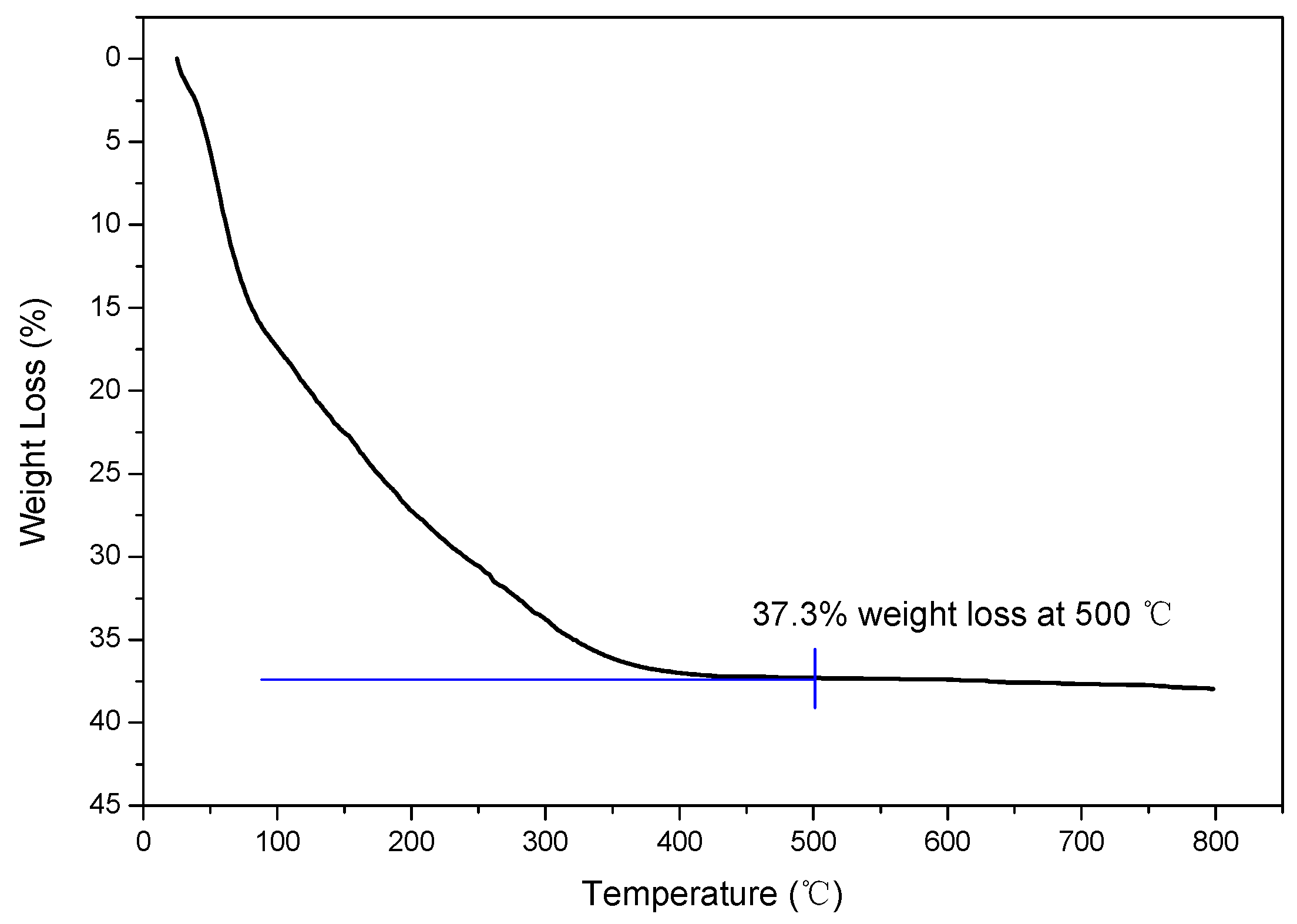
2.2. Thermogravimetric (TG) Analysis
2.3. X-Ray Diffraction(XRD) Analysis
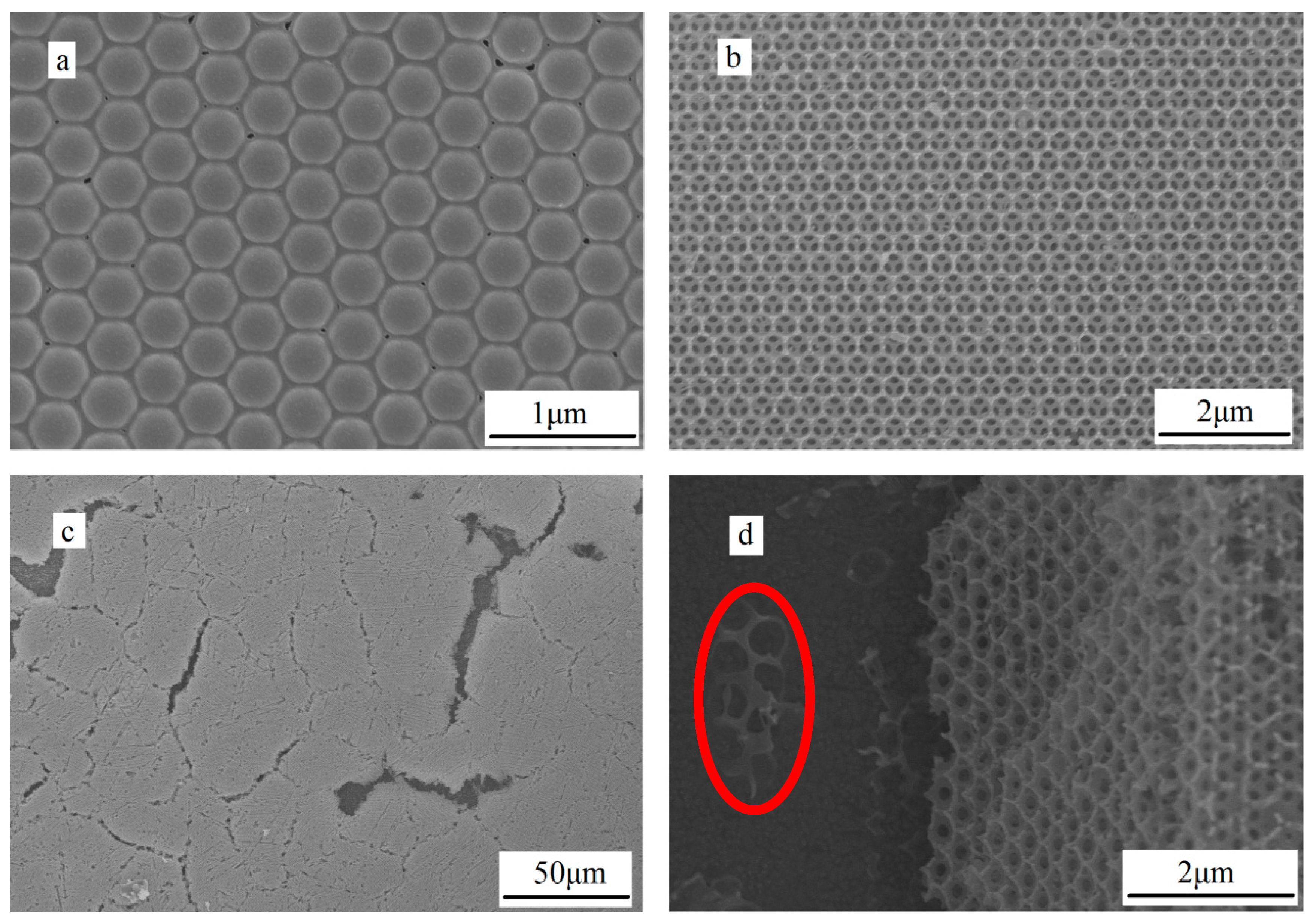
2.4. The Microstructure Analysis of Photonic Crystal Layer
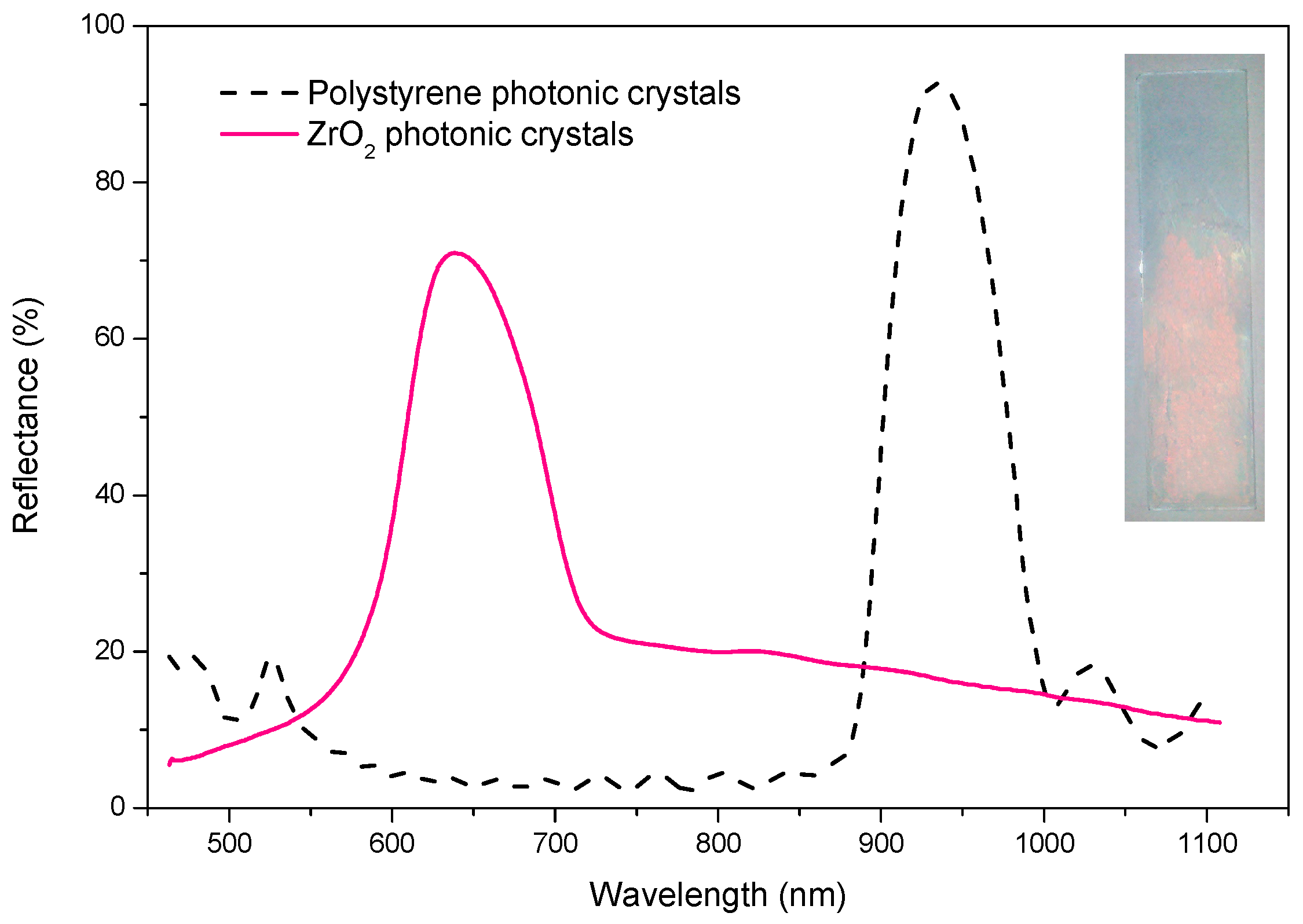
2.5. Spectral Analysis of Zirconia 3DPCs
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [PubMed]
- Mekis, A.; Chen, J.C.; Kurland, I.; Fan, S.H.; Villeneuve, P.R.; Joannopoulos, J.D. High transmission through sharp bends in photonic crystal waveguides. Phys. Rev. Lett. 1996, 77, 3787–3790. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chow, E.; Hietala, V.; Villeneuve, P.R.; Joannopoulos, J.D. Experimental demonstration of guiding and bending of electromagnetic waves in a photonic crystal. Science 1998, 282, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Yablonovitch, E. Photonic band-gap structures. J. Opt. Soc. Am. B: Opt. Phys. 1993, 10, 283–295. [Google Scholar] [CrossRef]
- Lai, C.-F.; Wang, Y.-C. Colloidal photonic crystals containing silver nanoparticles with tunable structural colors. Crystals 2016, 6, 61. [Google Scholar] [CrossRef]
- Blanco, A.; Chomski, E.; Grabtchak, S.; Ibisate, M.; John, S.; Leonard, S.W.; Lopez, C.; Meseguer, F.; Miguez, H.; Mondia, J.P.; et al. Large-scale synthesis of a silicon photonic crystal with a complete three-dimensional bandgap near 1.5 micrometres. Nature 2000, 405, 437–440. [Google Scholar] [PubMed]
- Meng, X.; Al-Salman, R.; Zhao, J.; Borissenko, N.; Li, Y.; Endres, F. Electrodeposition of 3D ordered macroporous germanium from ionic liquids: A feasible method to make photonic crystals with a high dielectric constant. Angew. Chem. Int. Ed. 2009, 48, 2703–2707. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, L.; Ge, D.; Ding, Y.; Pan, L.; Zhao, J.; Li, Y. Magnetron sputtering sic films on nickel photonic crystals with high emissivity for high temperature applications. Appl. Surf. Sci. 2012, 259, 811–815. [Google Scholar] [CrossRef]
- Xin, W.; Zhao, J.; Ding, Y.; Li, Y. Fabrication of three-dimensionally ordered macroporous Ta2O5 films through aqueous organic gel process. Appl. Surf. Sci. 2011, 257, 10725–10728. [Google Scholar]
- Huang, K.M.; Ho, C.L.; Chang, H.J.; Wu, M.C. Fabrication of inverted zinc oxide photonic crystal using sol-gel solution by spin coating method. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Hao, J.; Zhang, K.; Zhao, J.; Su, B.-L.; Li, Y. Improved electrochromic performance and lithium diffusion coefficient in three-dimensionally ordered macroporous V2O5 films. J. Mater. Chem. C 2014, 2, 3651–3658. [Google Scholar] [CrossRef]
- Kapitonov, A.M.; Gaponenko, N.V.; Bogomolov, V.N.; Prokofiev, A.V.; Samoilovich, S.M.; Gaponenko, S.V. SiO2/TiO2 submicron 3D lattice: A new step towards visible-range photonic crystals. Nanoscale Res. Lett. 1997, 54–57. [Google Scholar] [CrossRef]
- Zakhidov, A.A.; Baughman, R.H.; Iqbal, Z.; Cui, C.X.; Khayrullin, I.; Dantas, S.O.; Marti, I.; Ralchenko, V.G. Carbon structures with three-dimensional periodicity at optical wavelengths. Science 1998, 282, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Yang, L.; Tong, Z.; Ding, Y.; Xin, W.; Zhao, J.; Li, Y. Ion diffusion and optical switching performance of 3D ordered nanostructured polyaniline films for advanced electrochemical/electrochromic devices. Electrochim. Acta 2013, 104, 191–197. [Google Scholar] [CrossRef]
- Zhao, J.P.; Li, Y.; Xin, W.H.; Li, X. Preparation and characterization of three-dimensionally ordered macroporous yttria-stabilized zirconia by aqueous organic gel route. J. Solid State Chem. 2008, 181, 239–244. [Google Scholar] [CrossRef]
- Lashtabeg, A.; Drennan, J.; Knibbe, R.; Bradley, J.L.; Lu, G.Q. Synthesis and characterisation of macroporous yttria stabilised zirconia (YSZ) using polystyrene spheres as templates. Microporous Mesoporous Mater. 2009, 117, 395–401. [Google Scholar] [CrossRef]
- Lashtabeg, A.; Bradley, J.L.; Vives, G.; Drennan, J. The effects of templating synthesis procedures on the microstructure of yttria stabilised zirconia (YSZ) and NiO/YSZ templated thin films. Ceram. Int. 2010, 36, 653–659. [Google Scholar] [CrossRef]
- Xiaolong, X.; Hui, Z.; Shengnan, L.; Fan, L.; Jinxiu, W. Preparation of three-dimensional ordered macroporous zirconia and characteristics of its pore walls. J. Chin. Ceram. Soc. 2013, 36, 705–709. [Google Scholar]
- Shen, Y.; Wu, Q.Z.; Li, Y.G. Three dimensional ordered macroporous ZrO2 prepared by directly calcining ZrOCl2. Acta. Phys. Chim. Sin. 2006, 22, 1121–1125. [Google Scholar]
- Davis, M.; Ramirez, D.A.; Hope-Weeks, L.J. Formation of three-dimensional ordered hierarchically porous metal oxides via a hybridized epoxide assisted/colloidal crystal templating approach. ACS Appl. Mater. Interfaces 2013, 5, 7786–7792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Li, W.; Ni, D.; Sun, K. High performance three-dimensionally ordered macroporous composite cathodes for intermediate temperature solid oxide fuel cells. Rsc Adv. 2012, 2, 802–804. [Google Scholar] [CrossRef]
- Liang, B.; Suzuki, T.; Hamamoto, K.; Yamaguchi, T.; Sumi, H.; Fujishiro, Y.; Ingram, B.J.; Carter, J.D. A reduced temperature solid oxide fuel cell with three-dimensionally ordered macroporous cathode. J. Power Sources 2012, 212, 86–92. [Google Scholar] [CrossRef]
- Zhang, X.; Su, H.; Yang, X. Catalytic performance of a three-dimensionally ordered macroporous Co/ZrO2 catalyst in fischer-tropsch synthesis. J. Mol. Catal. A Chem. 2012, 360, 16–25. [Google Scholar] [CrossRef]
- Misawa, H.; Nishijima, Y.; Ueno, K.; Juodkazis, S.; Mizeikis, V.; Maeda, M.; Minaki, M. Tunable single-mode photonic lasing from zirconia inverse opal photonic crystals. Opt. Express 2008, 16, 13676–13684. [Google Scholar] [CrossRef] [PubMed]
- Schroden, R.C.; Al-Daous, M.; Blanford, C.F.; Stein, A. Optical properties of inverse opal photonic crystals. Chem. Mater. 2002, 14, 3305–3315. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef]
- Zhigang, X.; Tameru, S.; Sankar, J. Synthesis of yttria stabilized zirconia thin films by electrolytic deposition. Ceram. Eng. Sci. Proc. 2004, 25, 339–344. [Google Scholar]
- Aghazadeh, M. Cathodic electrodeposition of ZrO2: Impact of current density on the crystal structure, composition and morphology. J. Electrochem. Soc. 2012, 159, E53–E58. [Google Scholar] [CrossRef]
- Allard, M.; Sargent, E.H.; Kumacheva, E.; Kalinina, O. Characterization of internal order of colloidal crystals by optical diffraction. Opt. Quant. Electron. 2002, 34, 27–36. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Wang, M.Z.; Ge, X.W. Preparation of monodisperse polystyrene particles from emulsifier-free miniemulsion polymerization. Chem. Lett. 2008, 37, 1158–1159. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Y.; Xu, H.; Ding, Y.; Li, Y.; Zhao, J. Synthesis of silica particles with precisely tailored diameter. Chin. J. Chem.Phys. 2014, 27, 563–567. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Xu, H.; Sun, Y.; Zhao, J.; Li, Y. Preparation of Three-Dimensional Photonic Crystals of Zirconia by Electrodeposition in a Colloidal Crystals Template. Crystals 2016, 6, 76. https://doi.org/10.3390/cryst6070076
Pan L, Xu H, Sun Y, Zhao J, Li Y. Preparation of Three-Dimensional Photonic Crystals of Zirconia by Electrodeposition in a Colloidal Crystals Template. Crystals. 2016; 6(7):76. https://doi.org/10.3390/cryst6070076
Chicago/Turabian StylePan, Lei, Hongbo Xu, Yunyong Sun, Jiupeng Zhao, and Yao Li. 2016. "Preparation of Three-Dimensional Photonic Crystals of Zirconia by Electrodeposition in a Colloidal Crystals Template" Crystals 6, no. 7: 76. https://doi.org/10.3390/cryst6070076
APA StylePan, L., Xu, H., Sun, Y., Zhao, J., & Li, Y. (2016). Preparation of Three-Dimensional Photonic Crystals of Zirconia by Electrodeposition in a Colloidal Crystals Template. Crystals, 6(7), 76. https://doi.org/10.3390/cryst6070076