Synthesis, Structures and Properties of Molecular Conductors Based on Bis-Fused Donors Composed of (Thio)Pyran-4-ylidene-1,3-dithiole and Tetraselenafulvalene
Abstract
:1. Introduction
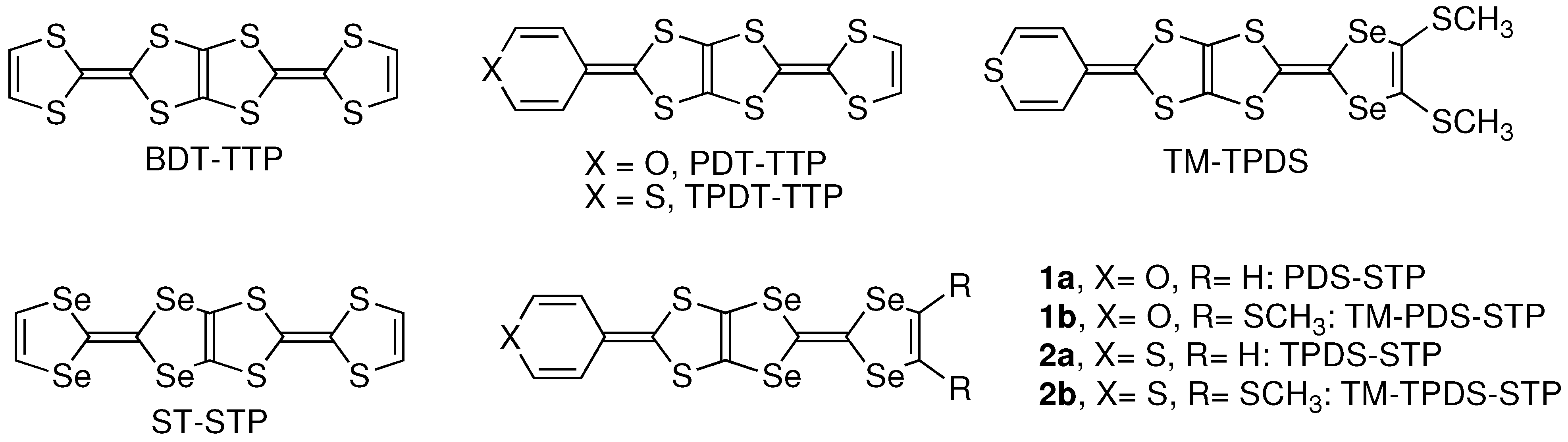
2. Results and Discussion
2.1. Synthesis
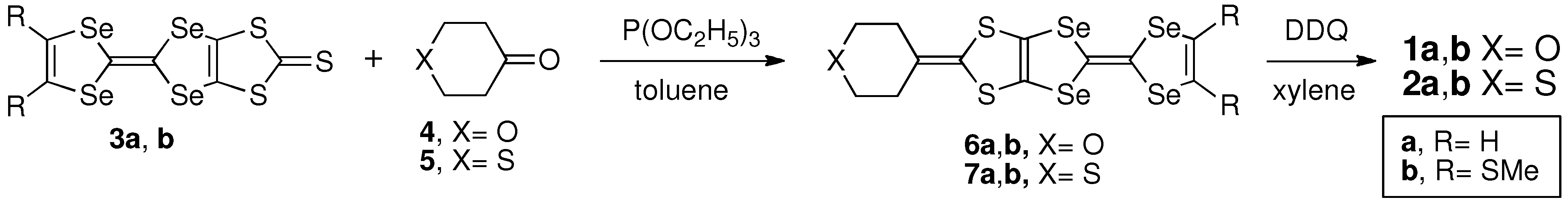
2.2. Electrochemical Properties
| Donor | E1 | E2 | E3 | E4 | ΔE (= E2 − E1) |
|---|---|---|---|---|---|
| PDS-STP 1a | −0.06 | +0.26 | +0.45 | +0.67 | 0.32 |
| TM-PDS-STP 1b | −0.05 | +0.30 | +0.47 | +0.67 | 0.35 |
| TPDS-STP 2a | −0.10 | +0.22 | +0.52 | +0.72 | 0.32 |
| TM-TPDS-STP 2b | −0.09 | +0.23 | +0.53 | +0.73 | 0.32 |
| ST-STP | 0.00 | +0.30 | +0.56 | +0.74 | 0.30 |
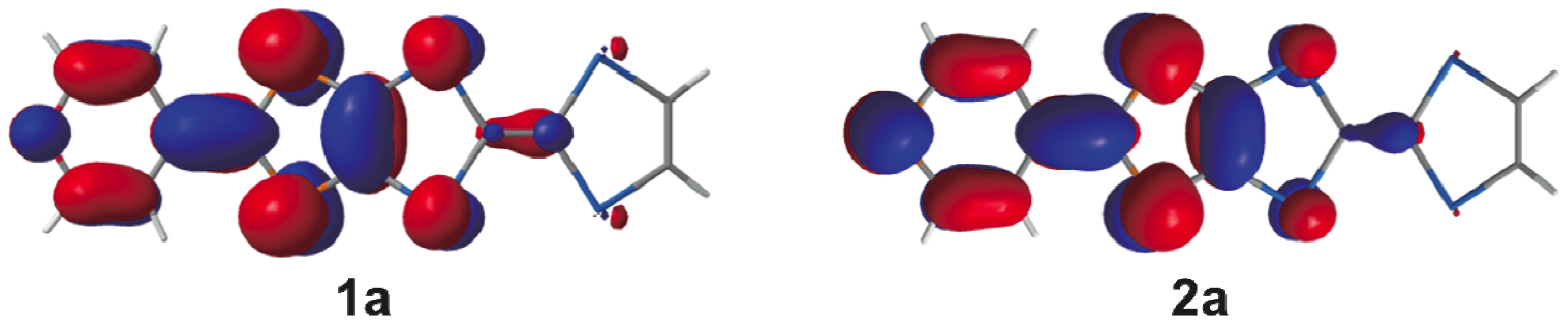
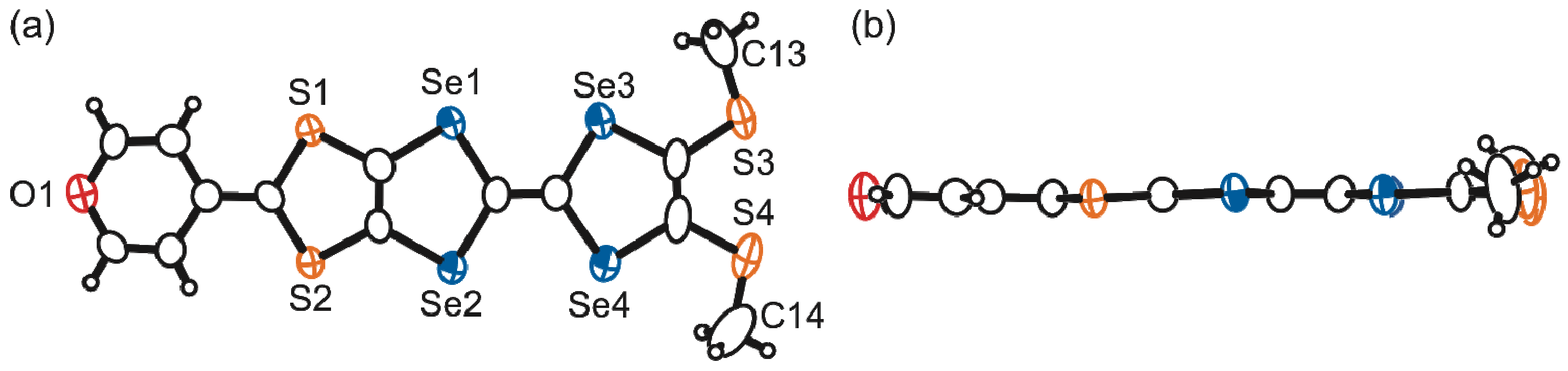
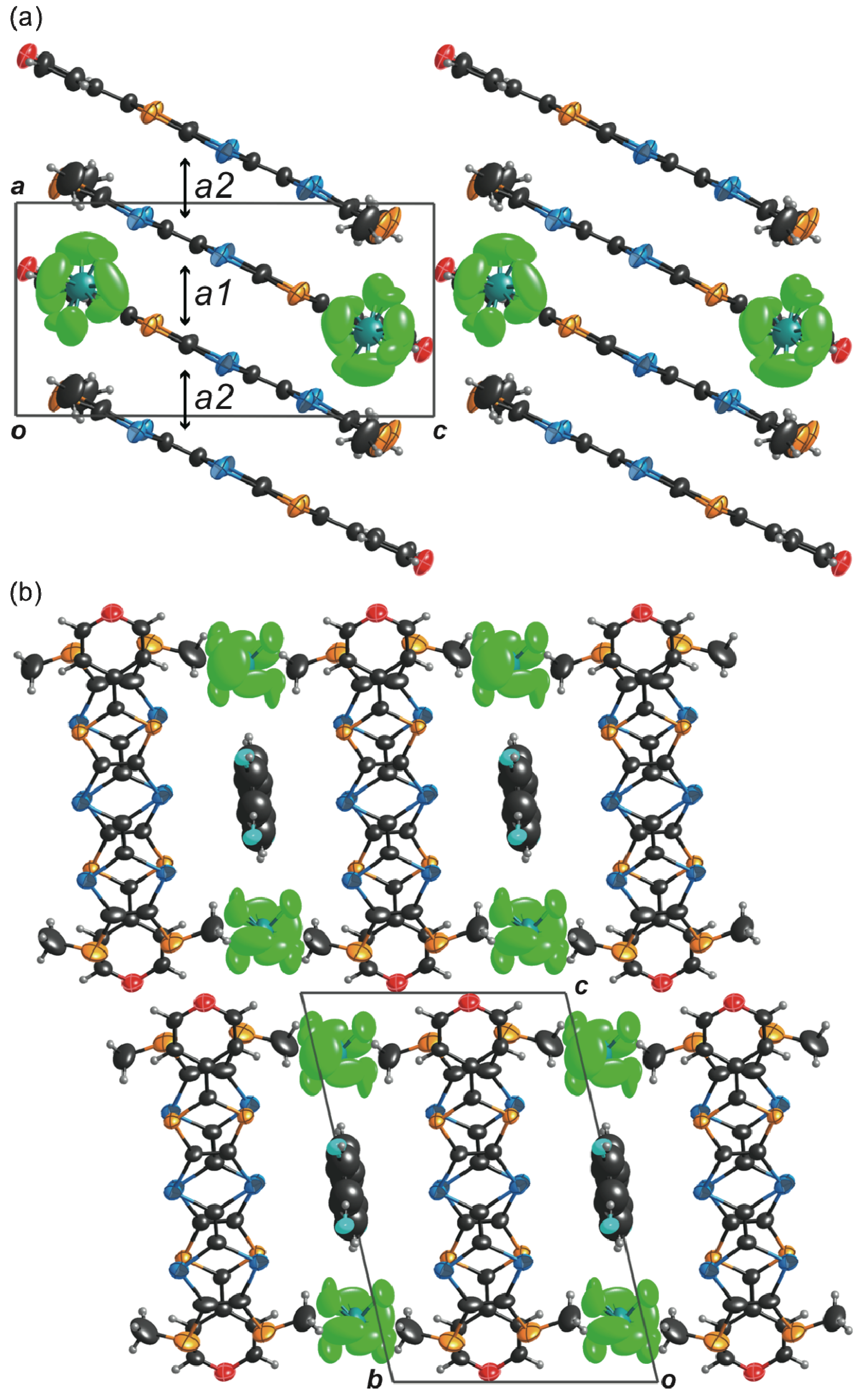
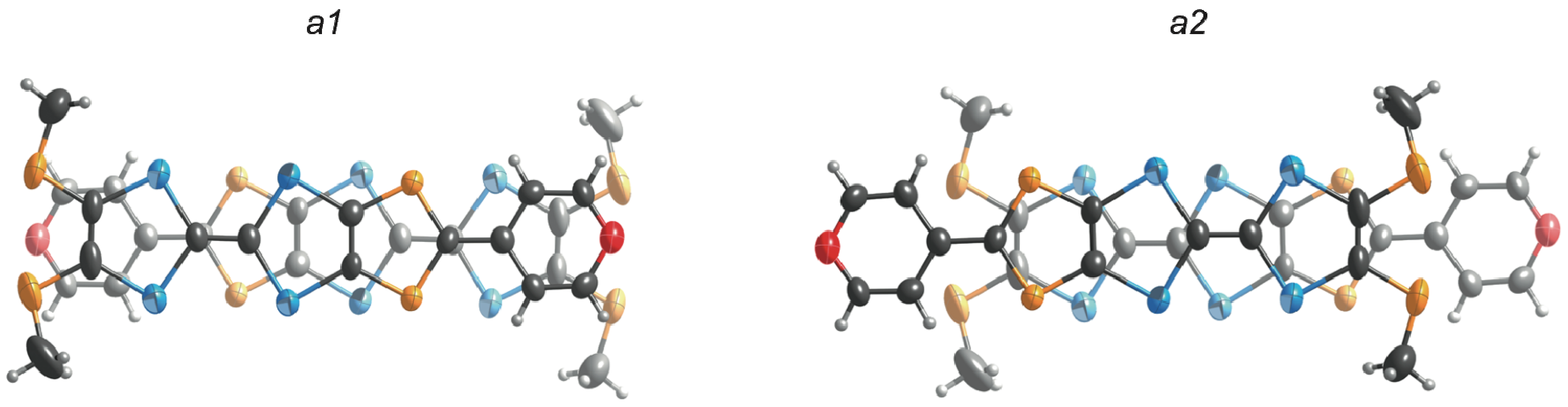
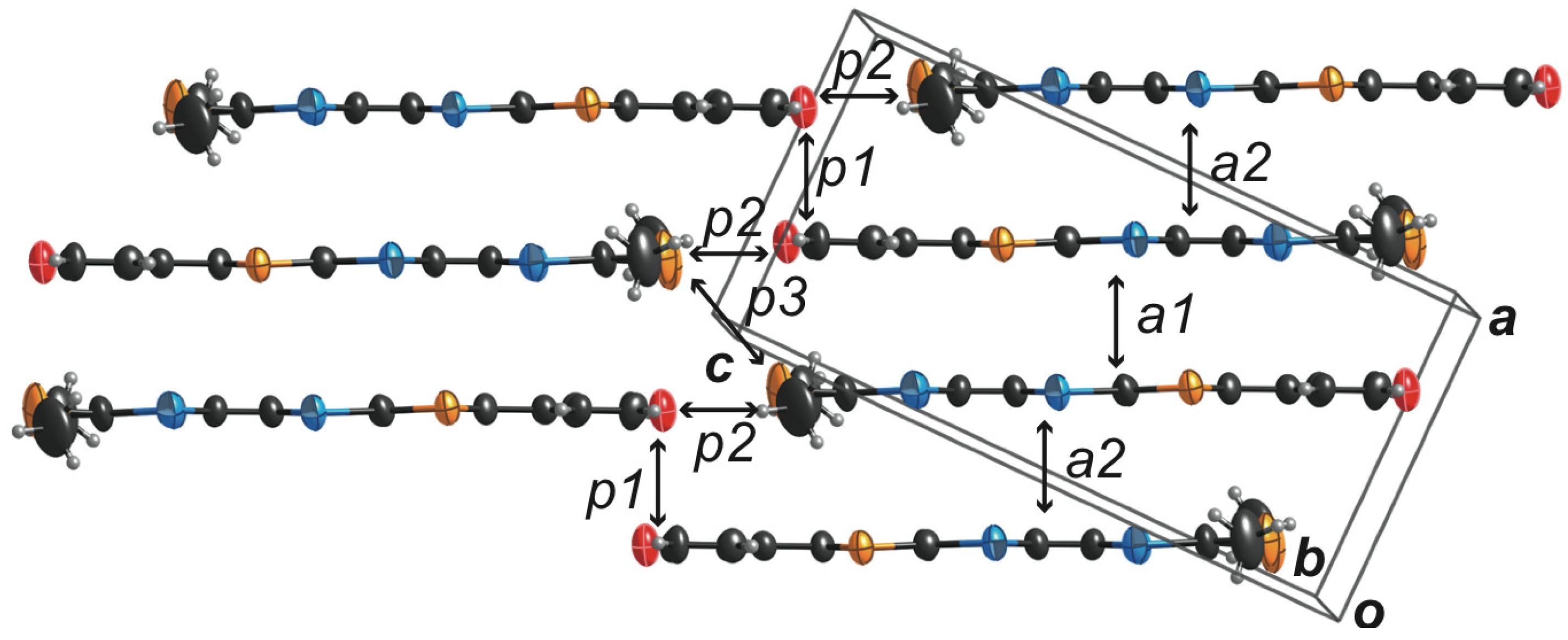
2.3. Molecular and Crystal Structures of a Neutral Molecule TM-PDS-STP (1b)

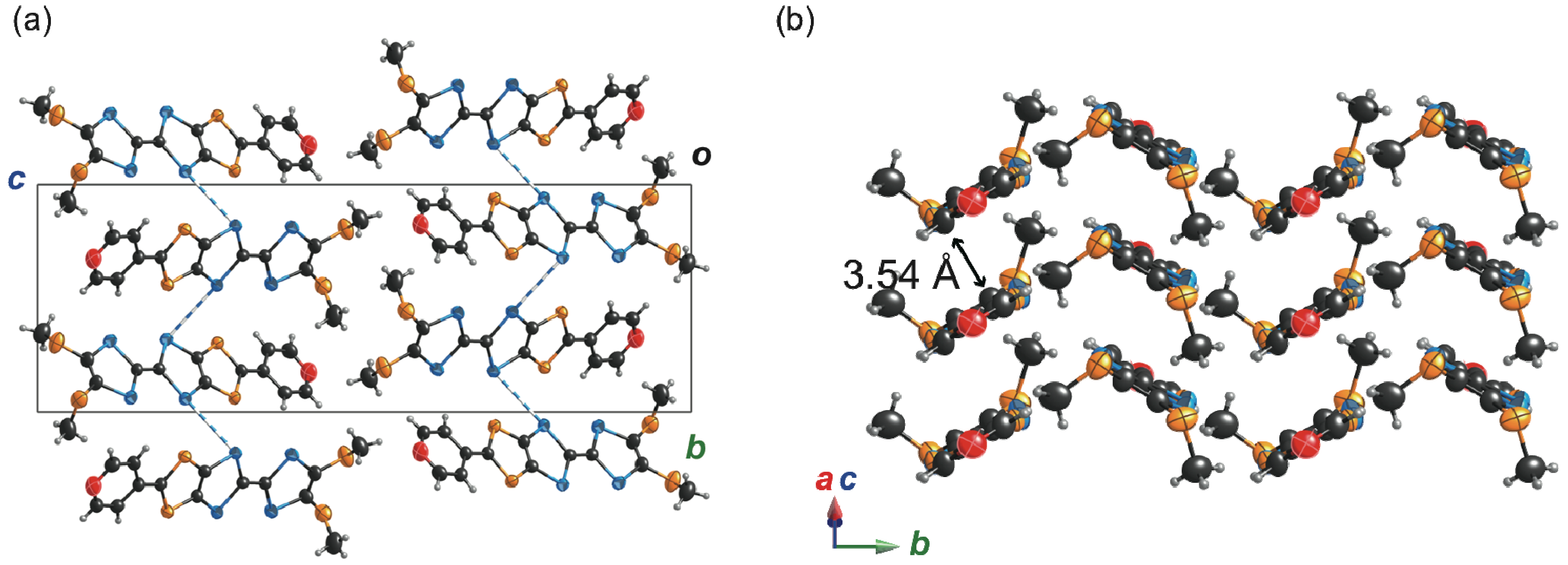
2.4. Preparation, Crystal and Band Structures of Radical Cation Salts
2.4.1. (TM-PDS-STP)PF6(PhCl)0.5
2.4.2. (TM-TPDS-STP)PF6(PhCl)
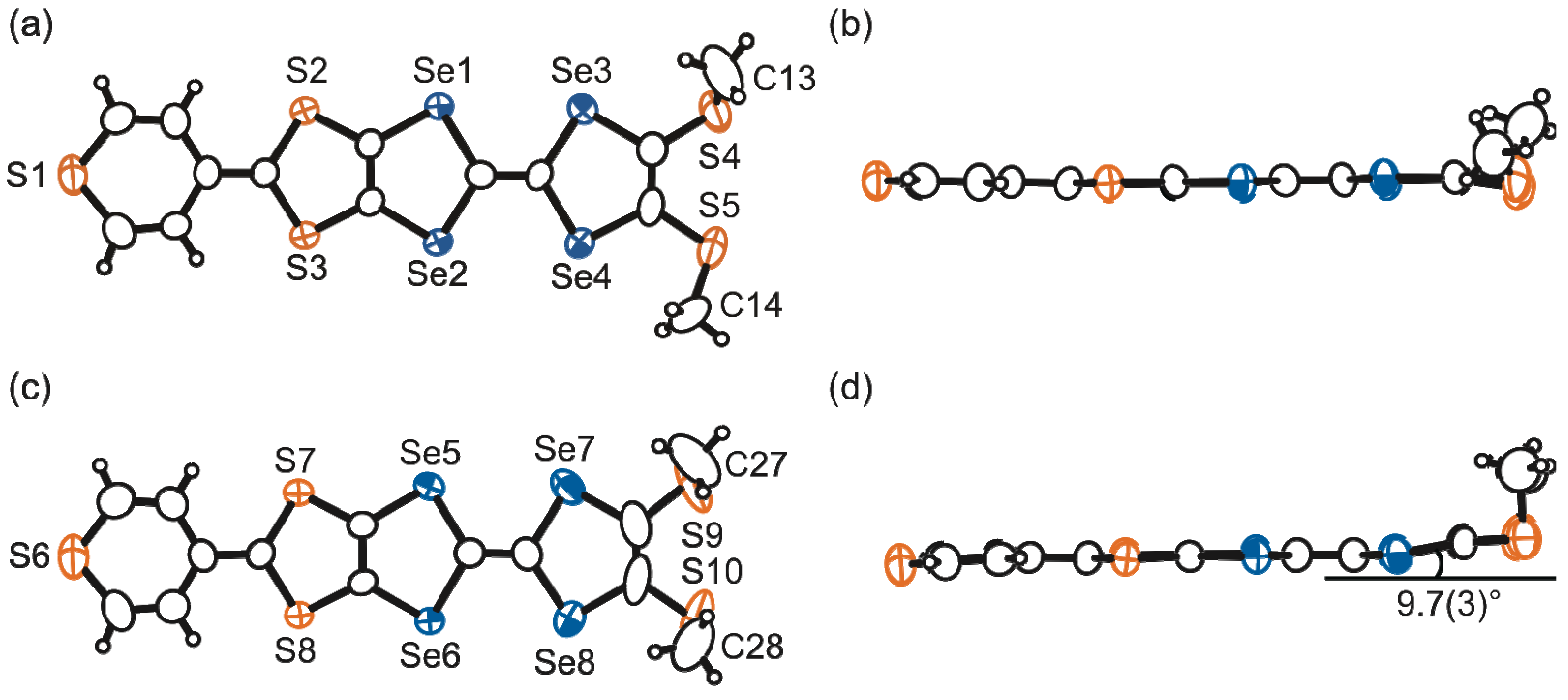
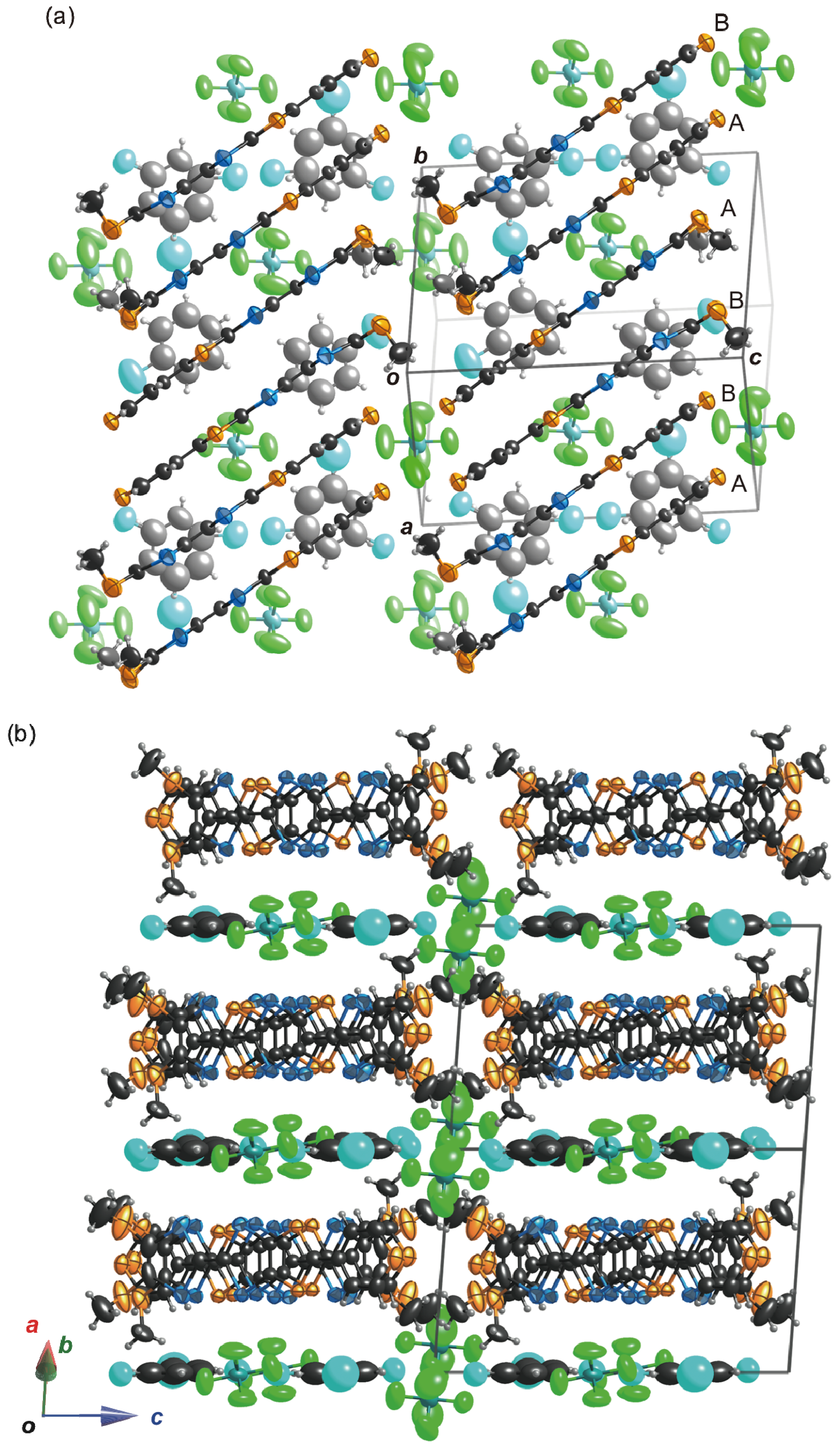


2.5. Electrical Properties of the Conducting Materials
| Donor | Acceptor (Solvent) | D:A (:Solvent) | γ | σrt (S cm−1)a | Ea (eV) |
|---|---|---|---|---|---|
| PDS-STP 1a | TCNQ | 5:4 | 0.63 | 7.1 × 100 | 0.20 |
| I3− | 5:4 | 0.8 | 1.6 × 100 | 0.040 | |
| TM-PDS-STP 1b | TCNQ | 1:1 | 0.72 | 3.9 × 10–1 | 0.030 |
| I3− | 5:4 | 0.8 | 3.2 × 10–1 | 0.082 | |
| PF6− (C6H5Cl) | 1:1:0.5 | 1.0 | 1.8 × 10−3 b | 0.22 | |
| TPDS-STP 2a | TCNQ | 5:4 | 0.73 | 1.8 × 101 | 0.033 |
| I3− | 2:1 | 0.5 | 1.9 × 101 | 0.024 | |
| TM-TPDS-STP 2b | TCNQ | 3:2 | 0.45 | 9.1 × 100 | 0.017 |
| I3− | 5:4 | 0.8 | 3.1 × 100 | 0.070 | |
| PF6− (C6H5Cl) | 1:1:1 | 1.0 | 3.7 × 10−2 b | 0.16 | |
| AsF6− | 8.6 × 10−2 b | 0.13 |
3. Experimental Section
3.1. General
3.2. Synthesis
3.3. General Procedure for Preparation of TCNQ Complexes and I3− Salts
3.4. General Procedure for Preparation of Cation Radical Salts
3.5. X-ray Crystallographic Analysis
| Compound | TM-PDS-STP | (TM-PDS-STP)PF6(C6H5Cl)0.5 | (TM-TPDS-STP)PF6(C6H5Cl) |
| Formula | C14H10OS4Se4 | C16H16F6O2PS4Se4 | C20H15ClF6PS5Se4 |
| Formula weight | 638.30 | 829.34 | 911.89 |
| Crystal system | Monoclinic | Triclinic | Triclinic |
| Space group | P21/n | Pī | Pī |
| a (Å) | 5.230(1) | 7.917(3) | 12.666(3) |
| b (Å) | 11.006(2) | 10.582(3) | 14.299(4) |
| c (Å) | 31.718(6) | 15.979(5) | 16.289(5) |
| α (°) | 90 | 76.67(2) | 85.39(2) |
| β (°) | 93.903(6) | 89.92(2) | 88.219(19) |
| γ (°) | 90 | 89.12(2) | 79.79(2) |
| V (Å3) | 1821.4(6) | 1302.5(7) | 2894(1) |
| Z | 4 | 2 | 2 |
| λ (Å) | 0.71070 | 0.71070 | 0.71070 |
| Dcalc (mg m−3) | 2.328 | 2.115 | 2.093 |
| μ (mm−1) | 8.514 | 6.072 | 5.631 |
| Number of reflections collected | 16677 | 13884 | 31475 |
| Number of independent reflections | 4129 | 5841 | 12916 |
| Number of reflections with [ I > 2σ(I)] | 2787 | 2799 | 6484 |
| Number of parameters refined | 211 | 382 | 689 |
| R | 0.0601 | 0.0786 | 0.0749 |
3.6. Electronic Band Calculations
4. Conclusions
Acknowledgments
References and Notes
- For a recent review of TTP-based organic conductors, see Misaki, Y. Tetrathiapentalene-based organic conductors. Sci. Technol. Adv. Mater. 2009, 10, 024301:1–024301:22. [Google Scholar]
- Misaki, Y.; Fujiwara, H.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. Structure and conducting properties of BDT-TTP salts. Chem Lett. 1994, 23, 1653–1656. [Google Scholar]
- Misaki, Y.; Tanaka, K.; Taniguchi, M.; Yamabe, T.; Mori, T. Structures and electrical properties of (EO-TTP)2AsF6. Chem. Lett. 1999, 28, 1249–1250. [Google Scholar]
- Misaki, Y.; Fujiwara, H.; Maruyama, T.; Taniguchi, M.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. Novel oxygen containing π-electron donors for organic metals: 2-(1,3-dithiol-2-ylidene)-5-(pyran-4-ylidene)-1,3,4,6-tetrathiapentalenes. Chem. Mater. 1999, 11, 2360–2368. [Google Scholar]
- Misaki, Y.; Fujiwara, H.; Yamabe, T. Novel bis-fused p-electron donors for organic metals: 2-(1,3-dithiol-2-ylidene)-5-(thiopyran-4-ylidene)-1,3,4,6-tetrathiapentalene. J. Org. Chem. 1996, 61, 3650–3656. [Google Scholar]
- Misaki, Y.; Kaibuki, T.; Taniguchi, M.; Tanaka, K.; Kawamoto, T.; Mori, T.; Nakamura, T. A Novel organic conductor with three-dimensional donor array: (TM-TPDS)2AsF6. Chem. Lett. 2000, 29, 1274–1275. [Google Scholar]
- Takahashi, K.; Nakayashiki, T.; Taniguchi, M.; Misaki, Y.; Tanaka, K. A novel organic conductor with two-dimensional molecular array by the "edge-to-edge" donor interaction. Chem. Lett. 2001, 30, 162–163. [Google Scholar]
- Ishizu, K.; Watanabe, M.; Tanahashi, T.; Misaki, Y.; Ashizawa, M.; Mori, T. Synthesis and properties of new TTP donors composed of TTF and TSF moieties. J. Phys. Confer. Ser. 2008, 132, 012021:1–012021:5. [Google Scholar]
- Ashizawa, M.; Ishizu, K.; Watanabe, M.; Tanahashi, T.; Shirahata, T.; Kawamoto, T.; Mori, T.; Misaki, Y. Novel bis-fused π-electron donor composed of tetrathiafulvalene and tetraselenafulvalene. Chem. Lett. 2010, 39, 1093–1095. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Anzai, H.; Delrieu, J.M.; Takasaki, S.; Nakatsuji, S.; Yamada, J. Crystal growth of organic charge-transfer complexes by electrocrystallization with controlled applied current. J. Cryst. Growth 1995, 154, 145–150. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.; Camalli, M.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.; Polidori, G.; Spagna, R. SIR97: a new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-97, Program for the Refinement of Crystal Structures; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent molecular orbital methods. XII. Further extensions of Gaussian—Type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2011. [Google Scholar]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The intermolecular interaction of tetrathiafulvalene and bis(ethylenedithio)tetrathiafulvalene in organic metals. Calculation of orbital overlaps and models of energy-band structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ishidzu, K.-i.; Ashizawa, M.; Watanabe, M.; Shirahata, T.; Misaki, Y. Synthesis, Structures and Properties of Molecular Conductors Based on Bis-Fused Donors Composed of (Thio)Pyran-4-ylidene-1,3-dithiole and Tetraselenafulvalene. Crystals 2012, 2, 1092-1107. https://doi.org/10.3390/cryst2031092
Ishidzu K-i, Ashizawa M, Watanabe M, Shirahata T, Misaki Y. Synthesis, Structures and Properties of Molecular Conductors Based on Bis-Fused Donors Composed of (Thio)Pyran-4-ylidene-1,3-dithiole and Tetraselenafulvalene. Crystals. 2012; 2(3):1092-1107. https://doi.org/10.3390/cryst2031092
Chicago/Turabian StyleIshidzu, Ken-ichi, Minoru Ashizawa, Masaki Watanabe, Takashi Shirahata, and Yohji Misaki. 2012. "Synthesis, Structures and Properties of Molecular Conductors Based on Bis-Fused Donors Composed of (Thio)Pyran-4-ylidene-1,3-dithiole and Tetraselenafulvalene" Crystals 2, no. 3: 1092-1107. https://doi.org/10.3390/cryst2031092




