Synthesis, Characterization and Crystal Structures of 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide and 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide
Abstract
:1. Introduction
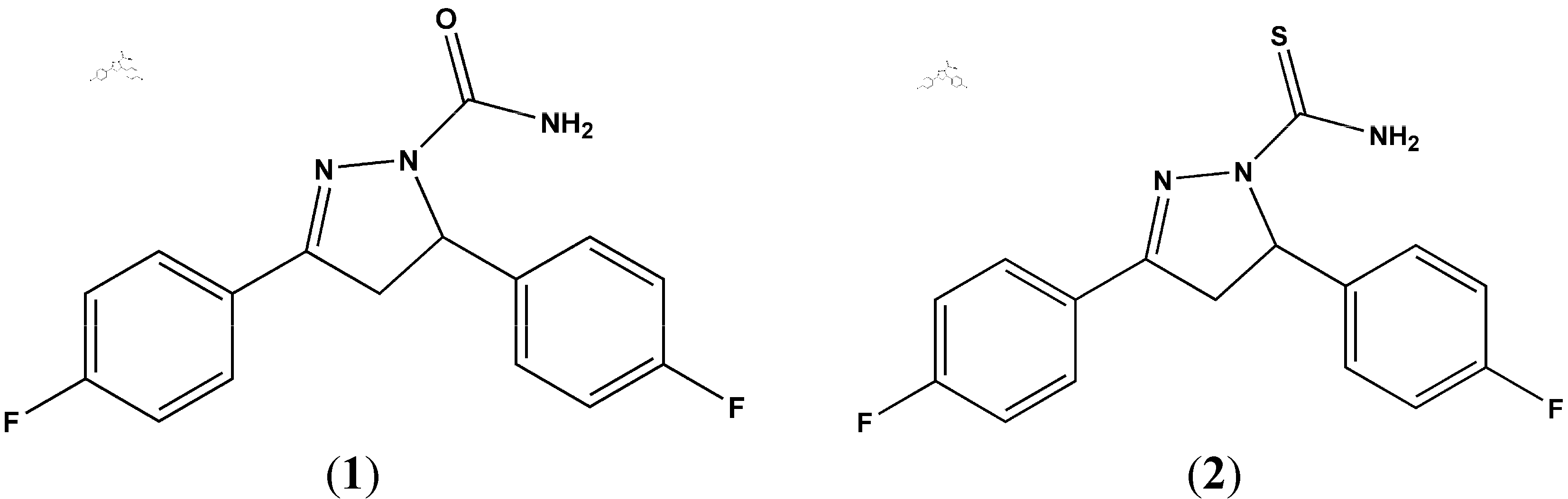
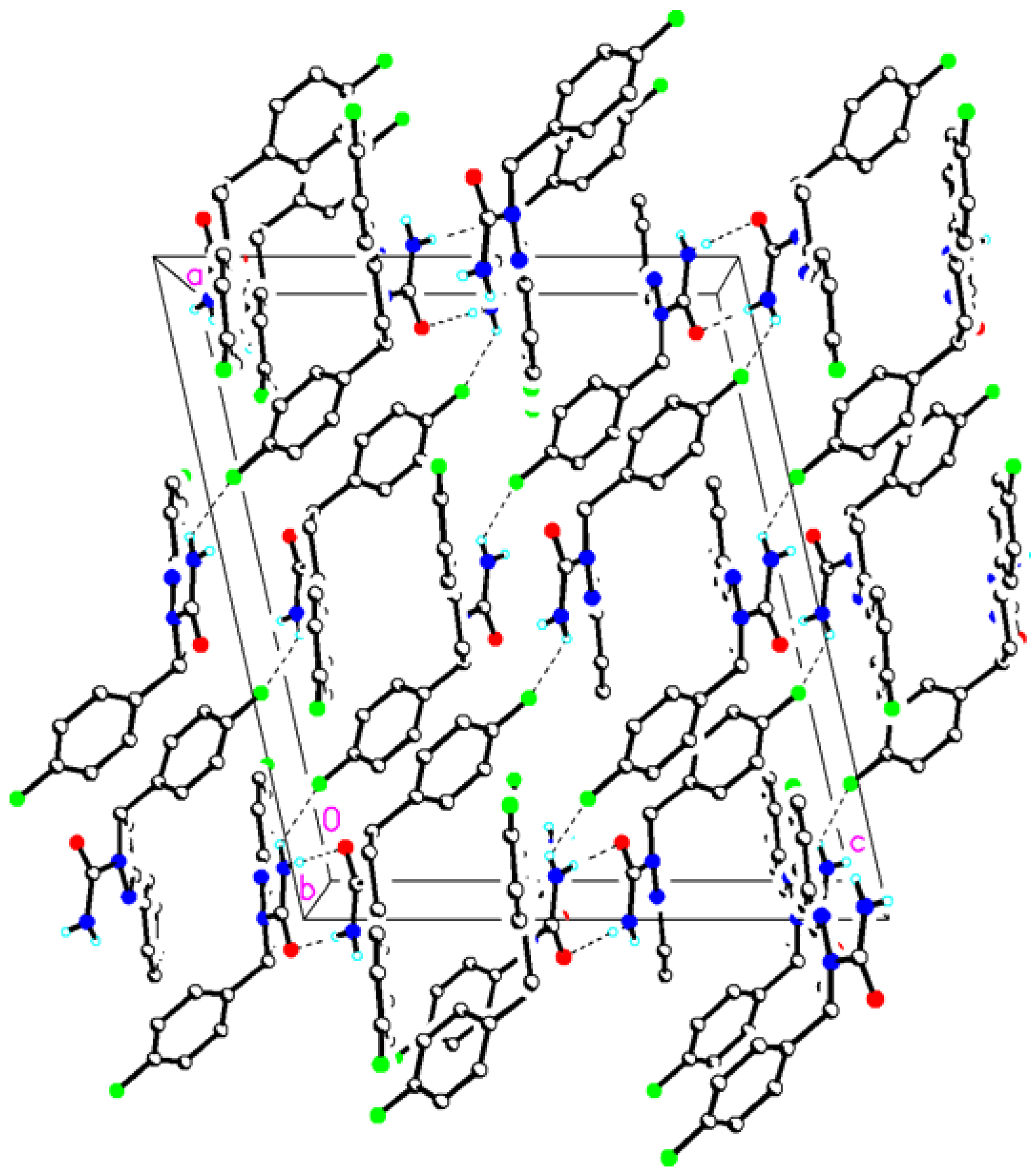
2. Results and Discussion
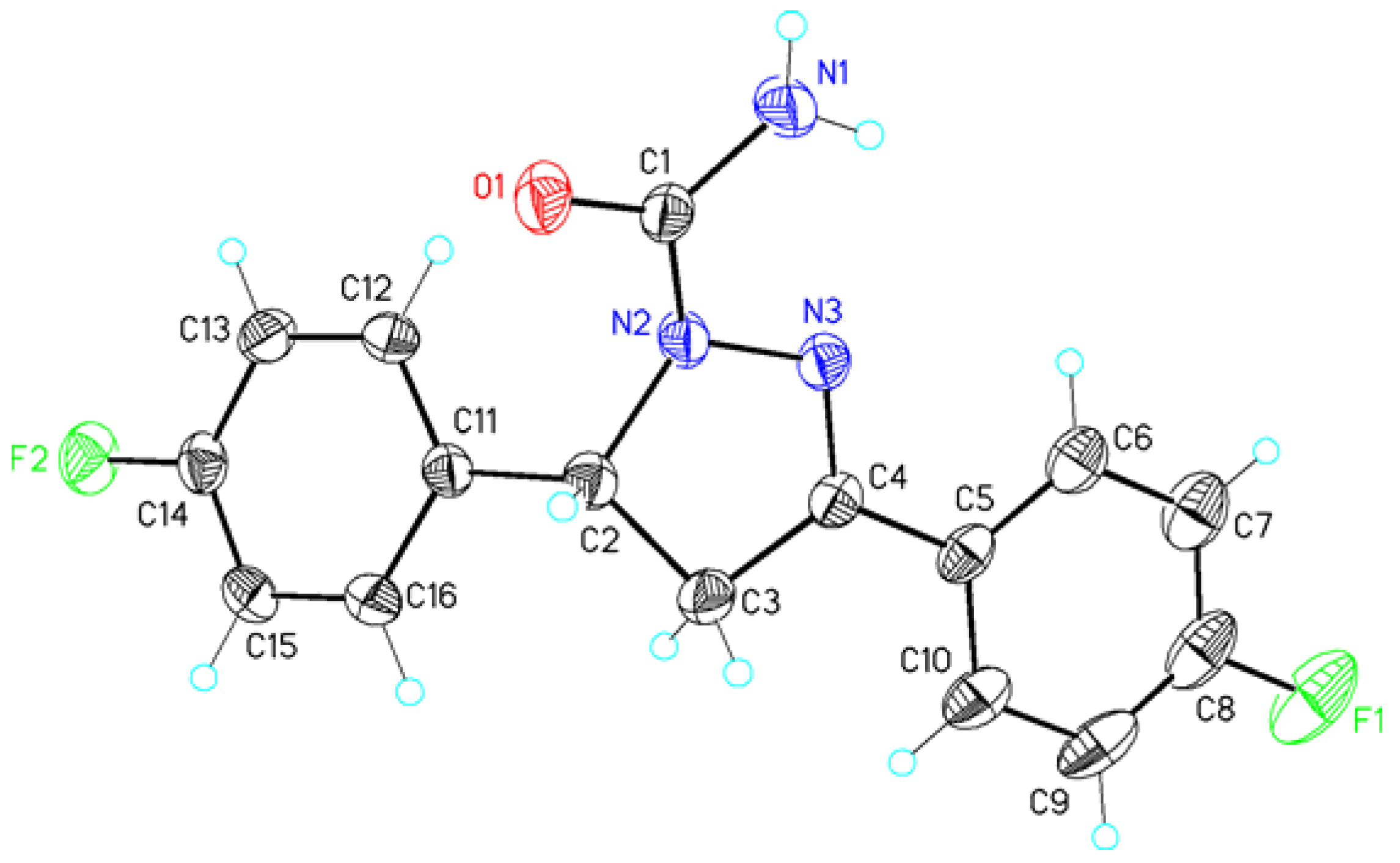
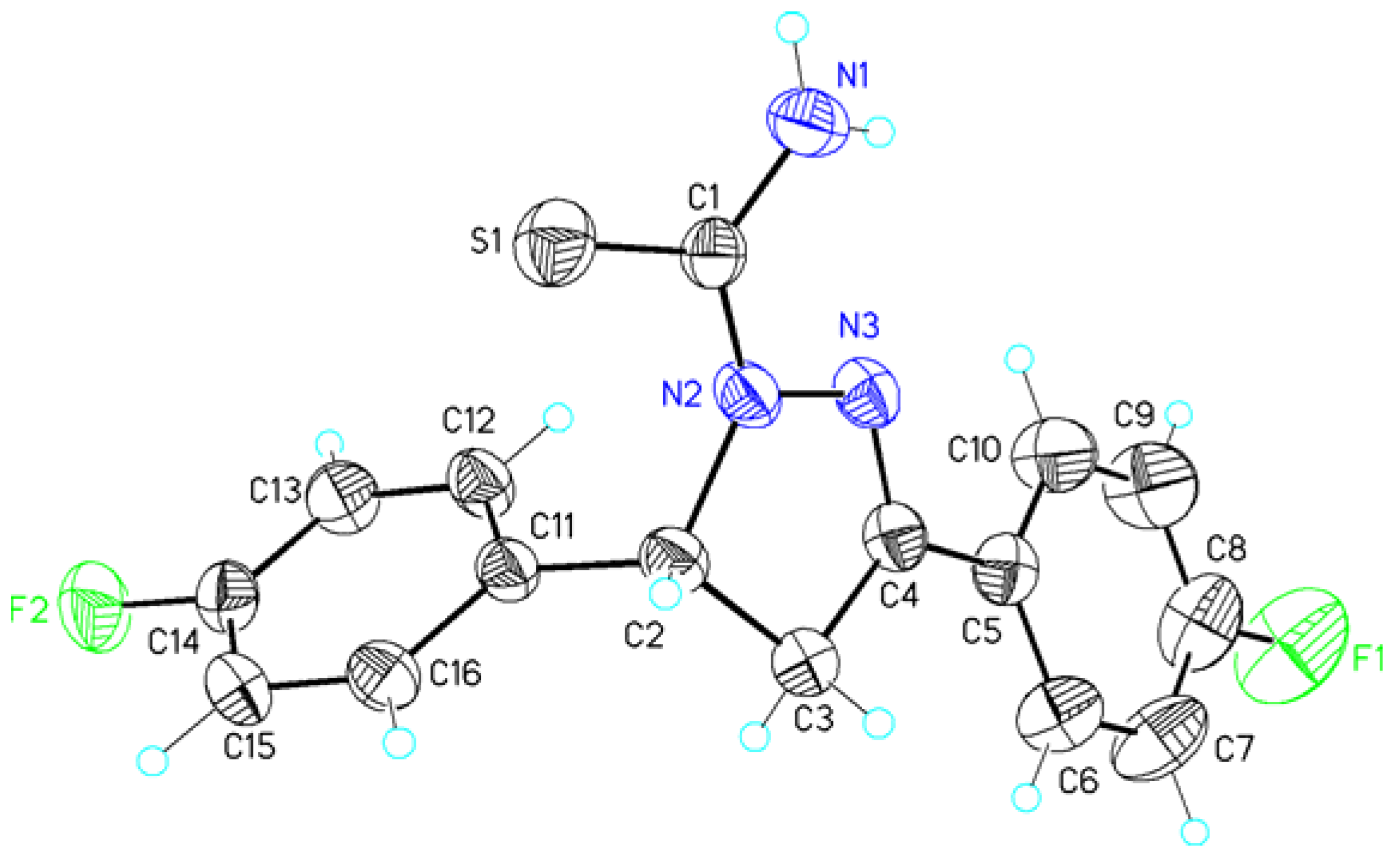
| Atoms 1 | Distance 1 | Atoms 2 | Distance 2 |
|---|---|---|---|
| C1—O1 | 1.2300(19) | C1—S1 | 1.6818(16) |
| C1—N1 | 1.340(2) | C1—N1 | 1.331(2) |
| N2—N3 | 1.3929(17) | N2—N3 | 1.3924(17) |
| N2—C2 | 1.4754(19) | N2—C2 | 1.4761(19) |
| N3—C4 | 1.2804(19) | N3—C4 | 1.283(2) |
| C2—C3 | 1.544(2) | C2—C3 | 1.538(2) |
| C3—C4 | 1.498(2) | C3—C4 | 1.501(2) |
| C8—F1 | 1.358(2) | C8—F1 | 1.356(2) |
| C14—F2 | 1.3658(18) | C14—F2 | 1.3665(19) |
| D–H...A | d (D–H) | d (H...A) | d (D...A) | < (DHA) |
|---|---|---|---|---|
| N1–H1NA...O1 #1 | 0.88(2) | 2.06(2) | 2.928(2) | 171(2) |
| N1–H1NB...F2 #2 | 0.88(2) | 2.38(2) | 3.129(2) | 143(2) |
| C13–H13A...F1 #3 | 0.93 | 2.54 | 3.386(2) | 151 |
| D–H...A | d (D–H) | d (H...A) | d (D...A) | < (DHA) |
|---|---|---|---|---|
| N1–H1NB...F2#1 | 0.87(2) | 2.41(2) | 3.258(2) | 164(2) |
| N1–H1NA...S1#2 | 0.84(2) | 2.85(2) | 3.525(2) | 138(2) |

3. Experimental Section
3.1. General

3.2. Synthesis of 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide (1)
3.3. Synthesis of 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (2)
3.4. Data Collection and Refinement
4. Conclusions
Acknowledgements
References
- Fustero, S.; Simon-Fuentes, A.; Sanz-Cervera, J.F. Recent advances in the synthesis of pyrazoles. A review. Org. Prep. Proced. Int. 2009, 41, 253–290. [Google Scholar] [CrossRef]
- Rajendra, P.Y.; Lakshmana, R.A.; Prasoona, L.; Murali, K.; Ravi, K.P. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2''-hydroxy naphthalen-1''-yl)-1,5-diphenyl-2-pyrazolines. Bioorg. Med. Chem. Lett. 2005, 15, 5030–5034. [Google Scholar] [CrossRef]
- Amir, M.; Kumar, H.; Khan, S.A. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef]
- Hes, R.V.; Wellinga, K.; Grosscurt, A.C. 1-Phenylcarbamoyl-2-pyrazolines, a new class of insecticides. 2. Synthesis and insecticidal properties of 3,5-diphenyl-1-phenylcarbamoyl-2-pyrazolines. J. Agric. Food Chem. 1978, 26, 915–918. [Google Scholar] [CrossRef]
- Grosscurt, A.C.; Hes, R.V.; Wellnga, K. 1-Phenylcarbamoyl-2-pyrazolines, a new class of insecticides. 3. Synthesis and insecticidal properties of 3,4-diphenyl-1-phenylcarbamoyl-2-pyrazolines. J. Agric. Food Chem. 1979, 27, 406–409. [Google Scholar] [CrossRef]
- Sarojini, B.K.; Vidyagayatri, M.; Darshanraj, C.G.; Bharath, B.R.; Manjunatha, H. DPPH scavenging assay of novel 1,3-disubstituted-1H-pyrazol-5-ols and their in silico studies on some proteins involved in Alzheimer’s disease signaling cascade. Lett. Drug Des. Discov. 2010, 7, 214–224. [Google Scholar]
- Amir, M.; Kumar, S. Synthesis, anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of 3,5-dimethylpyrazoles, 3-methyl pyrazol-5-ones and 3,5-disubstituted pyrazolines. Indian J. Chem. 2005, 44, 2532–2537. [Google Scholar] [CrossRef]
- Klimova, E.I.; Marcos, M.; Klimova, T.B.; Cecilio, A.T.; Ruben, A.T.; Lena, R.R. The structure of bicyclic ferrocenylmethylene substituted 2-pyrazolines and their reactions with azodicarboxylic acid N-phenylimide. J. Organomet. Chem. 1999, 585, 106–114. [Google Scholar] [CrossRef]
- Kettmann, V.; Svetlík, J. 4,5-Dihydro-3-methyl-5-(4-methylphenyl)-1H-pyrazole-1-carboxamide. Acta Cryst. 2003, C59, o419–o421. [Google Scholar]
- Fun, H.K.; Suwunwong, T.; Chantrapromma, S. 3-(4-Bromophenyl)-5-[4-(dimethylamino)phenyl]-4,5-dihydro-1H-pyrazole-1-carbothioamide. Acta Cryst. 2011, E67, o701–o702. [Google Scholar]
- Samshuddin, S.; Narayana, B.; Shetty, D.N.; Raghavendra, R. An efficient synthesis of 2,4,6-triarylpyridines and their biological evaluation. Der Pharma Chemica 2011, 3, 232–240. [Google Scholar]
- Samshuddin, S.; Butcher, R.J.; Akkurt, M.; Narayana, B.; Yathirajan, H.S.; Sarojini, B.K. 1,3-Bis(4-fluorophenyl)-N,N'-(propane-1,3-diylidene) dihydroxylamine. Acta Cryst. 2011, E67, o1954–o1955. [Google Scholar]
- Jasinski, J.P.; Guild, C.J.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. 2,3-Dibromo-1,3-bis(4-fluorophenyl)propan-1-one. Acta Cryst. 2010, E66, o2018. [Google Scholar]
- Jasinski, J.P.; Guild, C.J.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. 3,5-Bis(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole. Acta Cryst. 2010, E66, o1948–o1949. [Google Scholar]
- Fun, H.K.; Hemamalini, M.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. 1-[3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]ethanone. Acta Cryst. 2010, E66, o582–o583. [Google Scholar]
- Fun, H.K.; Hemamalini, M.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. Methyl-4,6-bis(4-fluorophenyl)-2-oxocyclohex-3-ene-1-carboxylate. Acta Cryst. 2010, E66, o864–o865. [Google Scholar]
- Baktir, Z.; Akkurt, M.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. 2,4-Bis(4-fluorophenyl)-2,3-dihydro-1H-1,5-benzodiazepine. Acta Cryst. 2011, E67, o1262. [Google Scholar]
- Baktir, Z.; Akkurt, M.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde. Acta Cryst. 2011, E67, o1292–o1293. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part I. bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar]
- Oxford Diffraction. In CrysAlis PRO and CrysAlis RED; Oxford Diffraction Ltd.: Oxfordshire, UK, 2010.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jasinski, J.P.; Golen, J.A.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. Synthesis, Characterization and Crystal Structures of 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide and 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide. Crystals 2012, 2, 1108-1115. https://doi.org/10.3390/cryst2031108
Jasinski JP, Golen JA, Samshuddin S, Narayana B, Yathirajan HS. Synthesis, Characterization and Crystal Structures of 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide and 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide. Crystals. 2012; 2(3):1108-1115. https://doi.org/10.3390/cryst2031108
Chicago/Turabian StyleJasinski, Jerry P., James A. Golen, Seranthimata Samshuddin, Badiadka Narayana, and Hemmige S. Yathirajan. 2012. "Synthesis, Characterization and Crystal Structures of 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide and 3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide" Crystals 2, no. 3: 1108-1115. https://doi.org/10.3390/cryst2031108




