Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein
Abstract
:1. Introduction
2. Results and Discussion
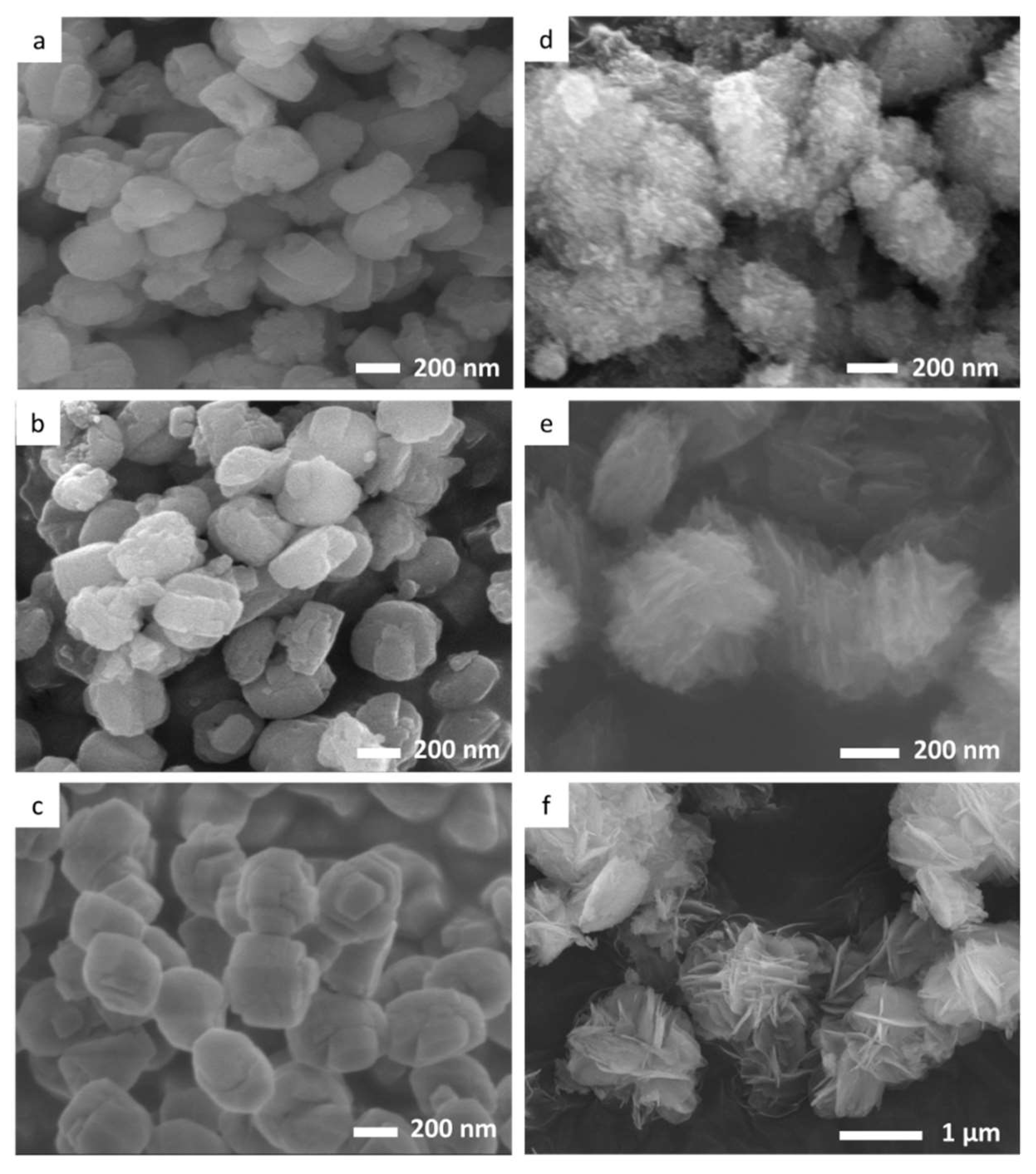
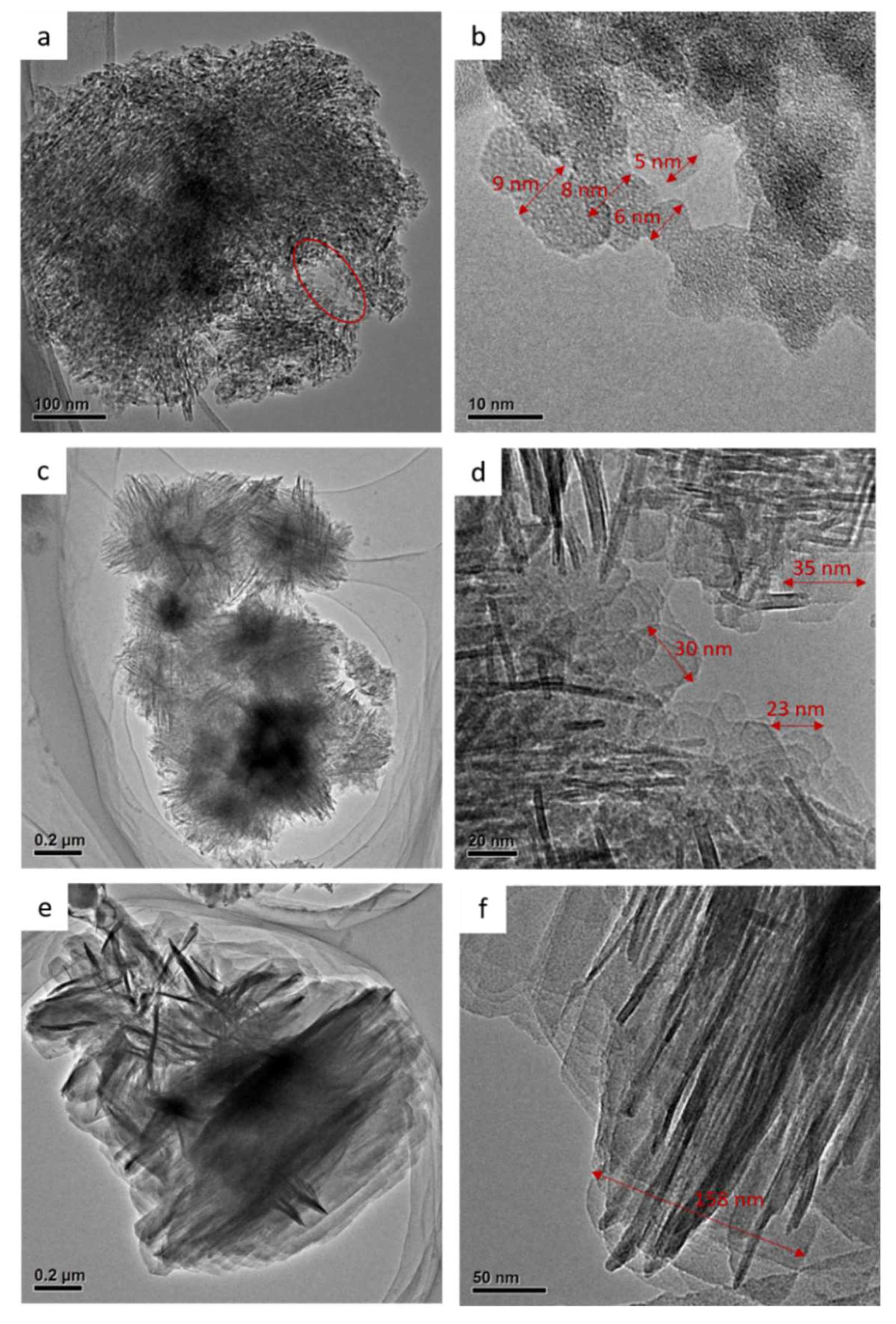
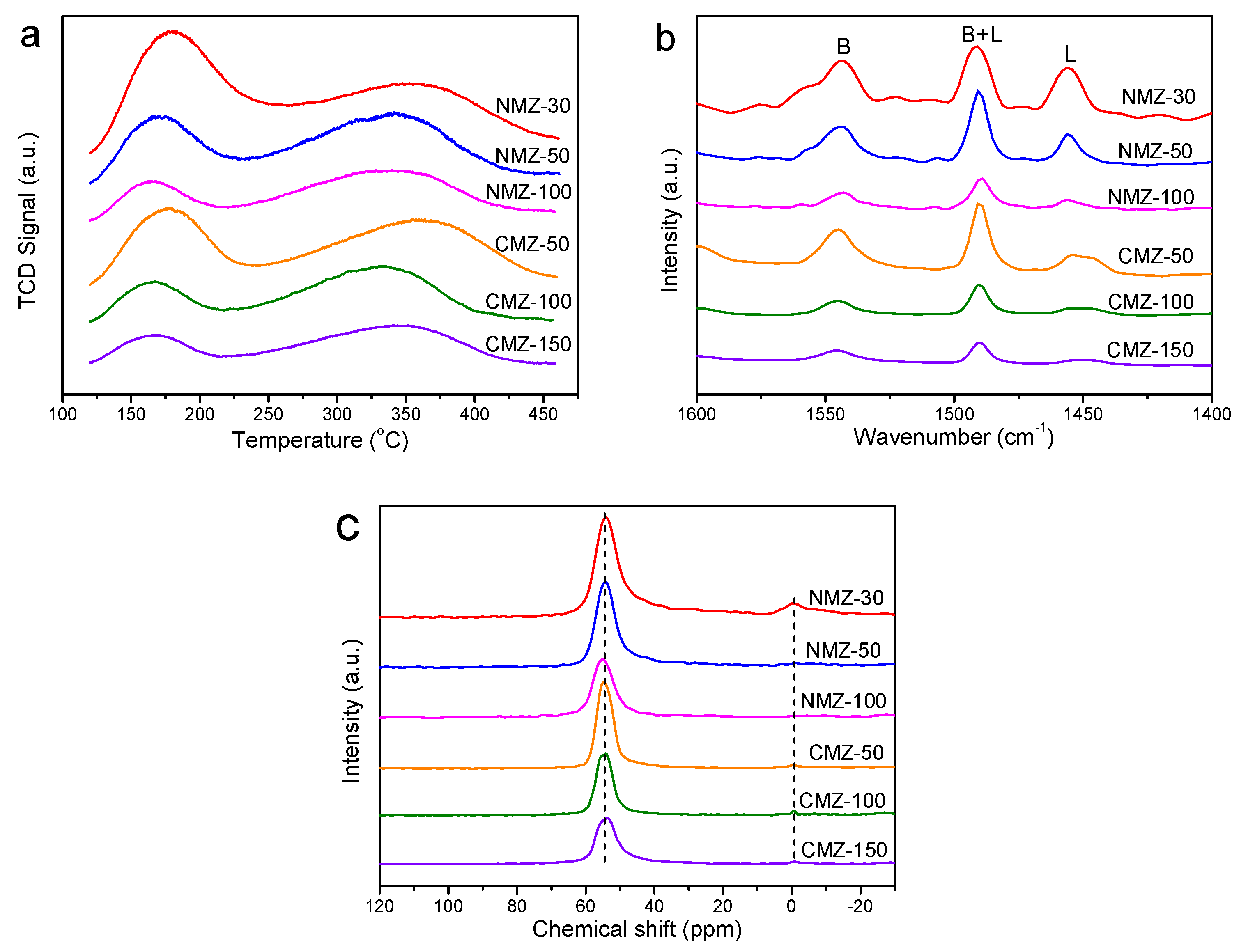
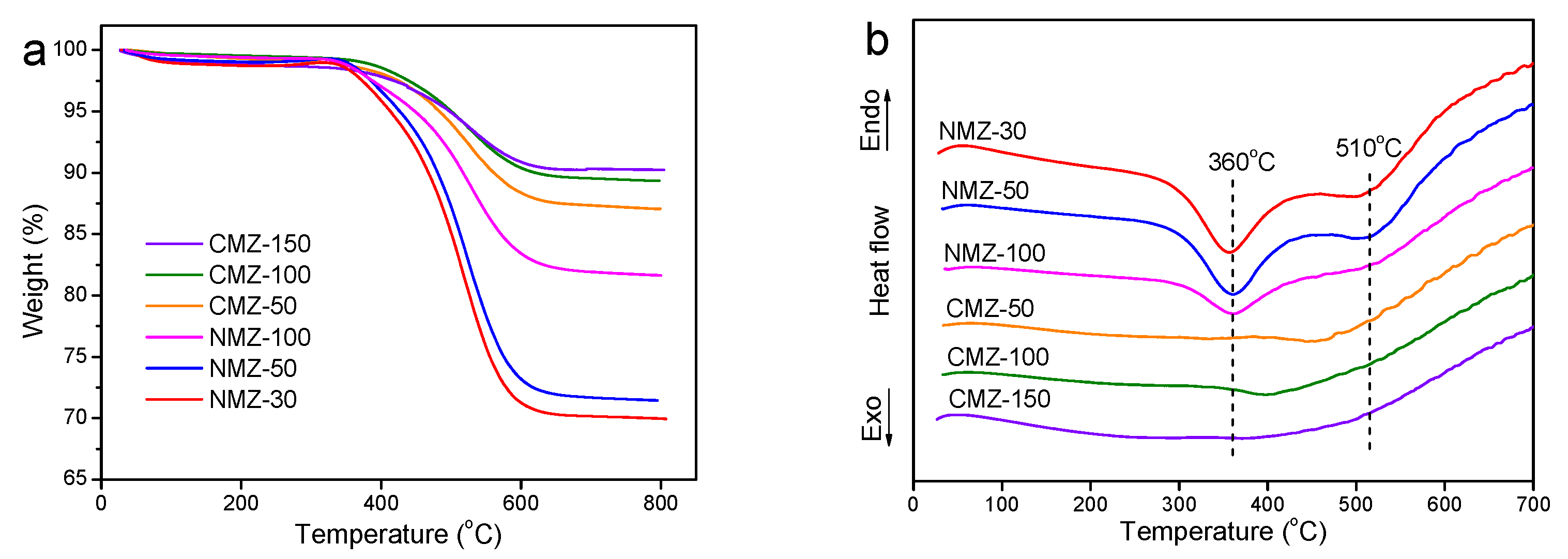
2.1. Characterization Results
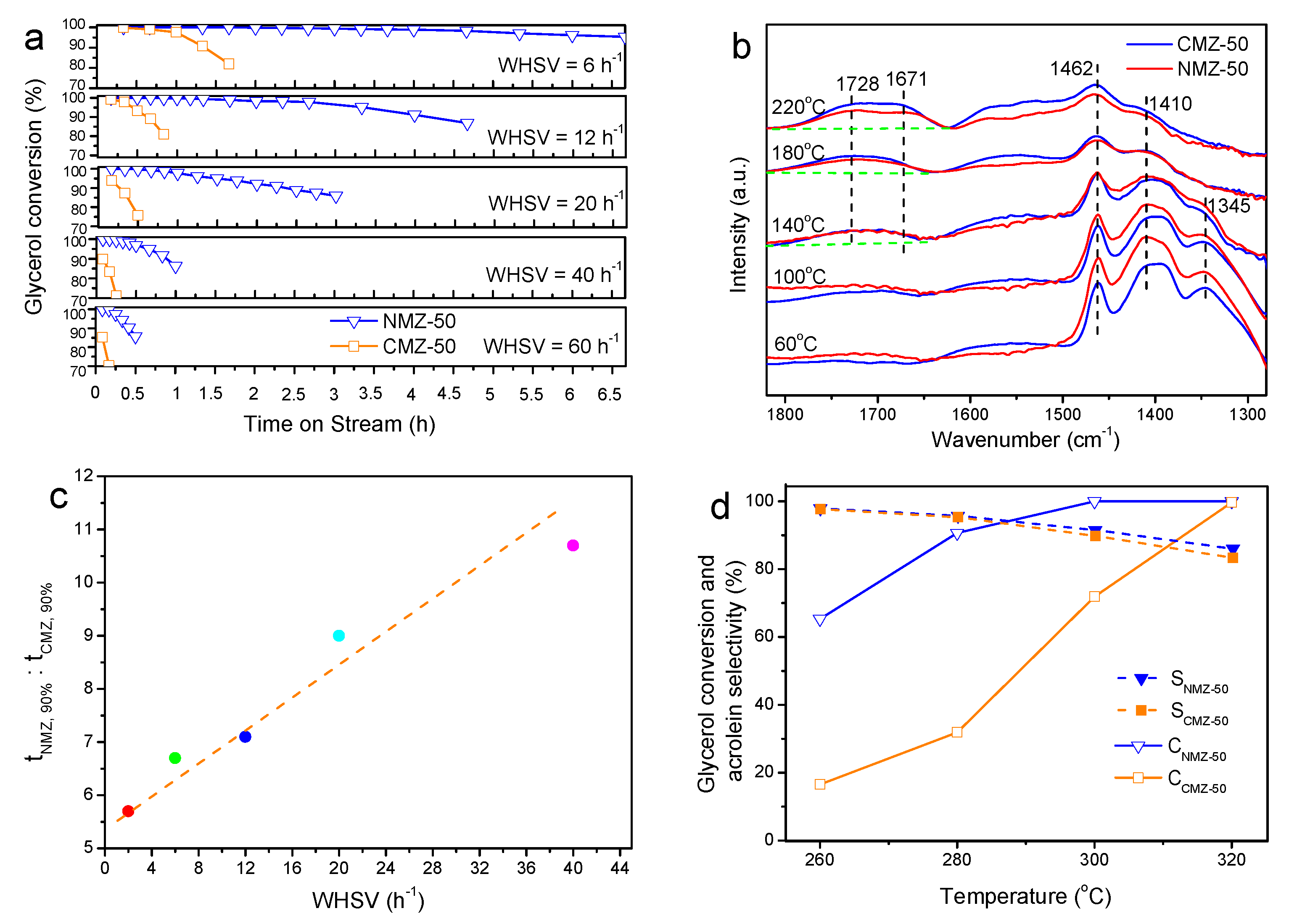
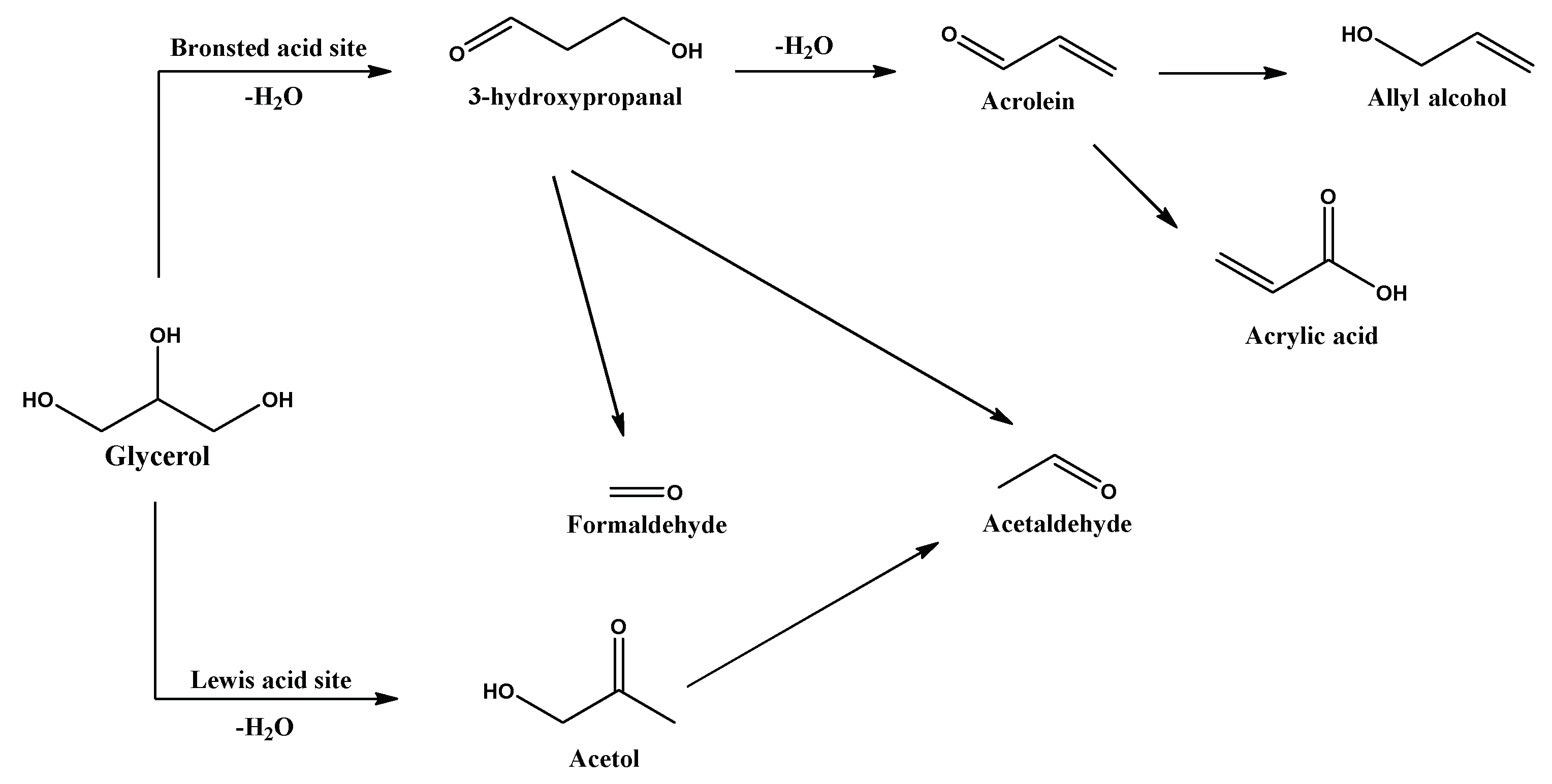
2.2. Effects of Nanosheet Structure on the Catalytic Performance for Glycerol Dehydration
2.3. Effects of Si/Al Ratios
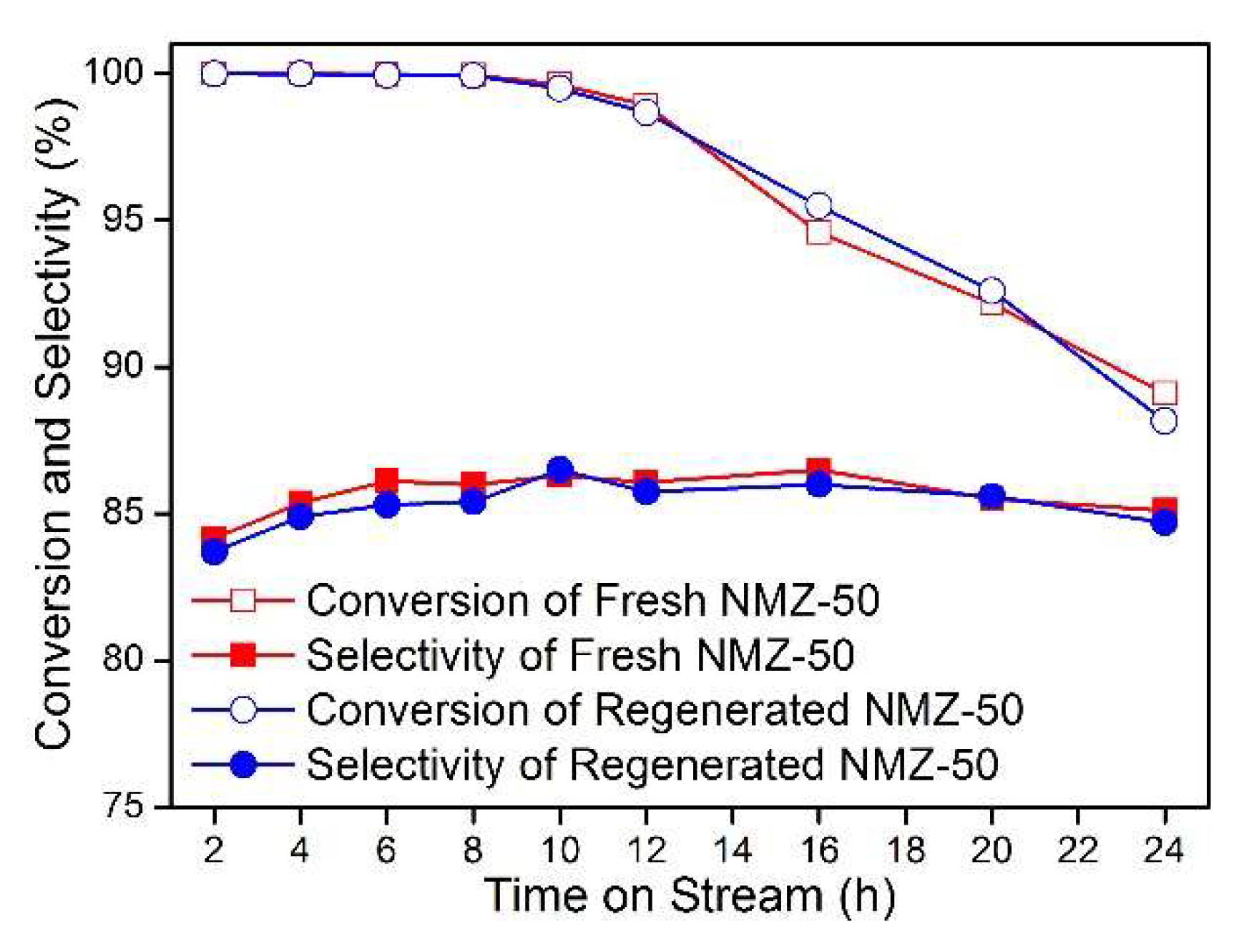
2.4. Catalyst Reutilization
3. Experimental
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B: Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Cespi, D.; Passarini, F.; Mastragostino, G.; Vassura, I.; Larocca, S.; Iaconi, A.; Chieregato, A.; Dubois, J.L.; Cavani, F. Glycerol as feedstock in the synthesis of chemicals: A life cycle analysis for acrolein production. Green Chem. 2015, 17, 343–355. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: gas-phase dehydration of glycerol over 12-tungstophosphoric acid supported on ZrO2 and SiO2. Green Chem. 2008, 10, 1087–1093. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.P.; Contreras, J.L.; Salmones, J.; Tapia, C.; Zeifert, B.; Navarrete, J.; Vazquez, T.; Garcia, D.C. Catalytic Dehydration of Glycerol to Acrolein over a Catalyst of Pd/LaY Zeolite and Comparison with the Chemical Equilibrium. Catalysts 2017, 7, 73. [Google Scholar] [CrossRef]
- Carrico, C.S.; Cruz, F.T.; Santos, M.B.; Oliveira, D.S.; Pastore, H.O.; Andrade, H.M.C.; Mascarenhas, A.J.S. MWW-type catalysts for gas phase glycerol dehydration to acrolein. J. Catal. 2016, 334, 34–41. [Google Scholar] [CrossRef]
- Huang, L.; Qin, F.; Huang, Z.; Zhuang, Y.; Ma, J.; Xu, H.; Shen, W. Hierarchical ZSM-5 Zeolite Synthesized by an Ultrasound-Assisted Method as a Long-Life Catalyst for Dehydration of Glycerol to Acrolein. Ind. Eng. Chem. Res. 2016, 55, 7318–7327. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Dumeignil, F. Recent developments in the field of catalytic dehydration of glycerol to acrolein. ACS Catal. 2013, 3, 1819–1834. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Ulgen, A.; Hoelderich, W.F. Conversion of glycerol to acrolein in the presence of WO3/TiO2 catalysts. Appl. Catal. A: Gen. 2011, 400, 34–38. [Google Scholar] [CrossRef]
- Martinuzzi, I.; Azizi, Y.; Zahraa, O.; Leclerc, J.P. Deactivation study of a heteropolyacid catalyst for glycerol dehydration to form acrolein. Chem. Eng. Sci. 2015, 134, 663–670. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol as a potential renewable raw material for acrylic acid production. Green Chem. 2017, 19, 3186–3213. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Capron, M.; Dumeignil, F. Towards the sustainable production of acrolein by glycerol dehydration. ChemSusChem 2009, 2, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, X.P.; Bozell, J.J. A comparative review of petroleum-based and bio-based acrolein production. ChemSusChem 2012, 5, 1162–1180. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Ren, S.; Ye, X.P. Glycerol dehydration to acrolein catalyzed by ZSM-5 zeolite in supercritical carbon dioxide medium. ChemSusChem 2016, 9, 3268–3271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Jiang, Y.; Hunger, M.; Huang, J. Cooperativity of Brønsted and Lewis acid sites on zeolite for glycerol dehydration. ACS Catal. 2014, 4, 1144–1147. [Google Scholar] [CrossRef]
- García-Sancho, C.; Cecilia, J.A.; Moreno-Ruiz, A.; Mérida-Robles, J.M.; Santamaría-onzález, J.; Moreno-Tost, R.; Maireles-Torres, P. Influence of the niobium supported species on the catalytic dehydration of glycerol to acrolein. Appl. Catal. B: Environ. 2015, 179, 139–149. [Google Scholar]
- Liu, R.; Wang, T.; Jin, Y. Catalytic dehydration of glycerol to acrolein over HPW supported on Cs+ modified SBA-15. Catal. Today 2014, 233, 127–132. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Investigation of solid acid-base catalysts for gas-phase dehydration of glycerol. Green Chem. 2007, 9, 1130–1136. [Google Scholar] [CrossRef]
- Jia, C.J.; Liu, Y.; Schmidt, W.; Lu, A.H.; Schüth, F. Small-sized HZSM-5 zeolite as highly active catalyst for gas phase dehydration of glycerol to acrolein. J. Catal. 2010, 269, 71–79. [Google Scholar] [CrossRef]
- Possato, L.G.; Diniz, R.N.; Garetto, T.; Pulcinelli, S.H.; Santilli, C.V.; Martins, L. A comparative study of glycerol dehydration catalyzed by micro/mesoporous MFI zeolites. J. Catal. 2013, 300, 102–112. [Google Scholar] [CrossRef]
- Ding, J.F.; Ma, T.L.; Shao, R.; Xu, W.; Wang, P.F.; Song, X.Y.; Guan, R.F.; Yeung, K.L.; Han, W. Gas phase dehydration of glycerol to acrolein on an amino siloxane-functionalized MCM-41 supported Wells-Dawson type H6P2W18O62 catalyst. New J. Chem. 2018, 42, 14271–14280. [Google Scholar] [CrossRef]
- Atia, H.; Armbruster, U.; Martin, A. Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J. Catal. 2008, 258, 71–82. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Gas-phase dehydration of glycerol over Nb2O5 catalyst. J. Catal. 2007, 250, 342–349. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S. Thermo-kinetic and diffusion studies of glycerol dehydration to acrolein using HSiW-gamma-Al2O3 supported ZrO2 solid acid catalyst. Renew. Energy. 2017, 114, 794–804. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Xu, W.; Yun, Z. Dehydration of glycerol to acrolein over Wells-Dawson and Keggin type phosphotungstic acids supported on MCM-41 catalysts. Chem. Eng. J. 2017, 316, 797–806. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jung, K.D.; Park, E.D. A comparative study for gas-phase dehydration of glycerol over H-zeolites. Appl. Catal. A: Gen. 2011, 393, 275–287. [Google Scholar] [CrossRef]
- Hemelsoet, K.; Van der Mynsbrugge, J.; De Wispelaere, K.; Waroquier, W.; Van Speybroeck, V. Unraveling the reaction mechanisms governing methanol-to-olefins catalysis by theory and experiment. ChemPhysChem 2013, 14, 1526–1545. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Jung, K.D.; Park, E.D. Gas-phase dehydration of glycerol over ZSM-5 catalysts. Micro. Meso. Mater. 2010, 131, 28–36. [Google Scholar] [CrossRef]
- Kongpatpanich, K.; Nanok, T.; Boekfa, B.; Probst, M.; Limtrakul, J. Structures and reaction mechanisms of glycerol dehydration over H-ZSM-5 zeolite, a density functional theory study. Phys. Chem. Chem. Phys. 2011, 13, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lv, Y.; Qu, Y.; Zhang, G.; Xi, Y.; Phillips, D.L.; Liu, C. A combined experimental and computational study of the catalytic dehydration of glycerol on microporous zeolites: An investigation of the reaction mechanism and acrolein selectivity. Phys. Chem. Chem. Phys. 2013, 15, 20120–20133. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O’Connor, P. Biomass to chemicals: Catalytic conversion of glycerol/water mixtures into acrolein, reaction network. J. Catal. 2008, 257, 163–171. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Z.; Huang, L.; Zhang, H.; Song, K.; Wang, L.; Shi, Z.; Ma, J.; Zhuang, J.; Shen, W.; Zhang, Y.; Xu, H.; Tang, Y. Dehydration of glycerol to acrolein over hierarchical ZSM-5 zeolites: Effects of mesoporosity and acidity. ACS Catal. 2015, 5, 2548–2558. [Google Scholar] [CrossRef]
- Suprun, W.; Lutecki, M.; Haber, T.; Papp, H. Acidic catalysts for the dehydration of glycerol: Activity and deactivation. J. Mol. Catal. A: Chem. 2009, 309, 71–78. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, X.Y.; Rooke, J.C.; Zhang, Y.H.; Yang, X.Y.; Tang, Y.; Xiao, F.S.; Su, B.L. Hierarchically structured zeolites: Synthesis, mass transport properties and applications. J. Mater. Chem. 2012, 22, 17381–17403. [Google Scholar] [CrossRef]
- Chal, R.; Gérardin, C.; Bulut, M.; Donk, S.V. Overview and Industrial Assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.C.; Jo, C.; Kim, T.W.; Kim, C.U.; Jeong, S.Y.; Chae, H.J. Structural and physicochemical effects of MFI zeolite nanosheets for the selective synthesis of propylene from methanol. Micro. Meso. Mater. 2016, 222, 1–8. [Google Scholar] [CrossRef]
- Hu, S.; Shan, J.; Zhang, Q.; Wang, Y.; Liu, Y.; Gong, Y.; Wu, Z.; Dou, T. Selective formation of propylene from methanol over high-silica nanosheets of MFI zeolite. Appl. Catal. A: Gen. 2012, 445, 215–220. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Park, W.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Pillared MFI zeolite nanosheets of a single-unit-cell thickness. J. Am. Chem. Soc. 2010, 132, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Brus, J.; Kobera, L.; Schoefberger, W.; Urbanova, M.; Klein, P.; Sazama, P.; Tabor, E.; Sklenak, S.; Fishchuk, A.V.; Dedecek, J. Structure of framework aluminum Lewis sites and perturbed aluminum atoms in zeolites as determined by Al-27{H-1} REDOR (3Q) MAS NMR Spectroscopy and DFT/Molecular Mechanics. Angew. Chem. Int. Ed. 2015, 54, 541–545. [Google Scholar]
- Bauerl, F.; Karge, H.G. Characterization of coke on zeolites. In Book Molecular Sieves; Springer: Berlin, Germany, 2007; Volume 5, pp. 249–364. [Google Scholar]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Brønsted acid sites in the dehydration of glycerol over niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Oliveira, A.S.D.; Vasconcelos, S.J.S.; Sousa, J.R.; Sousa, F.F.; Filho, J.M.; Oliveira, A.C. Catalytic conversion of glycerol to acrolein over modified molecular sieves: Activity and deactivation studies. Chem. Eng. J. 2011, 168, 765–774. [Google Scholar] [CrossRef]
- Copeland, J.R.; Foo, G.S.; Harrison, L.A.; Sievers, C. In situ ATR-IR study on aqueous phase reforming reactions of glycerol over a Pt/gamma-Al2O3 catalyst. Catal. Today. 2013, 205, 49–59. [Google Scholar] [CrossRef]
- Yi, X.D.; Zhang, X.B.; Weng, W.Z.; Wan, H.L. Studies on the reaction pathways for the selective oxidation of propane to acrolein over MoPO/SiO2 catalyst by IR spectroscopy. J. Mol. Catal. A: Chem. 2007, 277, 202–209. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Copeland, J.R.; Pelt, A.H.V.; Crittenden, J.C.; Sievers, C. Stability of Pt/gamma-Al2O3 Catalysts in Model Biomass Solutions. Top Catal. 2012, 55, 162–174. [Google Scholar] [CrossRef]
- Schmidt, F.; Hoffmann, C.; Giordanino, F.; Bordiga, S.; Simon, P.; Carrillo-Cabrera, W.; Kaskel, S. Coke location in microporous and hierarchical ZSM-5 and the impact on the MTH reaction. J. Catal. 2013, 307, 238–245. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, C.; Xue, Y.; Wu, J.; Wang, J.; Fan, W. Graphene oxide: An efficient acid catalyst for alcoholysis and esterification reactions. ChemCatChem 2014, 6, 3080–3083. [Google Scholar] [CrossRef]
- Madeira, F.F.; Ben Tayeb, K.; Pinard, L.; Vezin, H.; Maury, S.; Cadran, N. Ethanol transformation into hydrocarbons on ZSM-5 zeolites: Influence of Si/Al ratio on catalytic performances and deactivation rate. Study of the radical species role. Appl. Catal A: Gen. 2012, 443, 171–180. [Google Scholar] [CrossRef]



| Sample | Si/Ala | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | ||||
|---|---|---|---|---|---|---|---|
| Total | Microb | Externalc | Total | Mesod | |||
| NMZ-30 | 28 | 543 | 271 | 272 | 0.67 | 0.63 | |
| NMZ-50 | 46 | 563 | 267 | 296 | 0.71 | 0.66 | |
| NMZ-100 | 94 | 432 | 241 | 191 | 0.53 | 0.45 | |
| CMZ-50 | 48 | 355 | 262 | 93 | 0.21 | 0.10 | |
| CMZ-100 | 96 | 363 | 275 | 88 | 0.22 | 0.12 | |
| CMZ-150 | 143 | 370 | 279 | 91 | 0.21 | 0.11 | |
| Sample | TOS (h) | Conversion (%) | Product Selectivity (%) | ||
|---|---|---|---|---|---|
| Acrolein | Acetol | Othersb | |||
| NMZ-30 | 4 | 99.7 | 81.8 | 7.9 | 10.3 |
| 8 | 98.3 | 82.8 | 8.4 | 8.8 | |
| NMZ-50 | 4 | 99.8 | 85.4 | 4.9 | 9.7 |
| 8 | 99.7 | 85.9 | 5.3 | 8.8 | |
| NMZ-100 | 4 | 99.2 | 86.8 | 4.7 | 8.5 |
| 8 | 97.1 | 86.3 | 5.2 | 8.5 | |
| CMZ-50 | 4 | 91.3 | 84.3 | 5.1 | 10.6 |
| 8 | <80.0 | - | - | - | |
| CMZ-100 | 4 | 95.4 | 84.7 | 4.8 | 10.5 |
| 8 | 89.9 | 85.1 | 5.0 | 9.9 | |
| CMZ-150 | 4 | 93.5 | 86.5 | 4.7 | 8.8 |
| 8 | 87.3 | 85.8 | 4.4 | 9.8 | |
| Sample | TOSa (h) | Coke Contentb (%) | Coke Deposition Ratec (gcoke gcat−1 h−1) | Surface Aread (m2 g−1)/Percentage Remained (%) | ||
|---|---|---|---|---|---|---|
| Total | Micro | External | ||||
| NMZ-50 | 36 | 27.5 | 1.05 × 10−2 | 296/52.6 | 99/37.1 | 197/66.6 |
| CMZ-50 | 7 | 11.7 | 1.89 × 10−2 | 66/18.5 | 42/16.0 | 24/25.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, J.; Li, Z.; Zhu, S.; Liu, H.; Li, J.; Wang, J.; Fan, W. Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein. Catalysts 2019, 9, 121. https://doi.org/10.3390/catal9020121
Shan J, Li Z, Zhu S, Liu H, Li J, Wang J, Fan W. Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein. Catalysts. 2019; 9(2):121. https://doi.org/10.3390/catal9020121
Chicago/Turabian StyleShan, Jianfeng, Zhikai Li, Shanhui Zhu, Huan Liu, Junfen Li, Jianguo Wang, and Weibin Fan. 2019. "Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein" Catalysts 9, no. 2: 121. https://doi.org/10.3390/catal9020121






