Preparation of Crosslinked Enzyme Aggregates of a Thermostable Cyclodextrin Glucosyltransferase from Thermoanaerobacter sp. Critical Effect of the Crosslinking Agent
Abstract
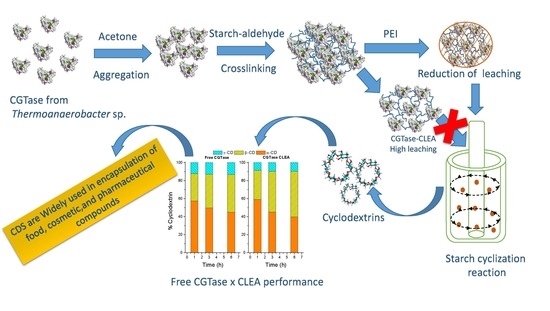
:1. Introduction
2. Results and Discussion
2.1. Selection of Precipitating Agent
2.2. Effect of the Precipitant Concentration on the Precipitated Activity of CGTase
2.3. Effect of Glutaraldehyde Concentration and Reaction Time
2.4. Strategies to Improve the Global Activity Recovery of CGTase CLEAs
2.5. Thermal Inactivation Assay
2.6. Cyclodextrin Production
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Activity Assay
3.3. CLEAs Preparation
3.3.1. Precipitant Selection
3.3.2. Cross-Linking Using Glutaraldehyde
3.4. Evaluation of Macromolecular Cross-Linkers
3.4.1. Preparation of Macromolecular Cross-Linkers
3.4.2. Cross-Linking Procedure Using Macromolecular Cross-Linkers
3.5. Production of Cyclodextrins
3.6. Thermal and Operational Stability
3.7. Chromatographic Method for Analysis of CDs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szejtli, J. The cyclodextrins and their applications in biotechnology. Carbohydr. Polym. 1990, 12, 375–392. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Norman, B.E.; Jorgensen, S.T. Thermoanaerobacter sp. CGTase: Its properties and application. J. Jpn. Soc. Starch Sci. 1992, 39, 101–108. [Google Scholar] [CrossRef]
- Arun, R.; Kumar, C.K.A.; Sravanthi, V.V.N.S.S. Cyclodextrins as drug carrier molecule: A review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Wind, R.D.; Uitdehaag, J.C.M.; Buitelaar, R.M.; Dijkstra, B.W.; Dijkhuizen, L. Engineering of cyclodextrin product specificity and pH optima of the thermostable cyclodextrin glycosyltransferase from Thermoanaerobacterium thermosulfurigenes EM1. J. Biol. Chem. 1998, 273, 5771–5779. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, J.; Li, B.; Yuan, H. High efficiency transformation of stevioside into a single mono-glycosylated product using a cyclodextrin glucanotransferase from Paenibacillus sp. CGMCC 5316. World J. Microbiol. Biotechnol. 2015, 31, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Cui, L.L.; Zhang, S.P.; Zhang, Y.F.; Su, Z.G.; Ma, G.H. Hybrid magnetic cross-linked enzyme aggregates of phenylalanine ammonia lyase from Rhodotorula glutinis. PLoS ONE 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Schoevaart, R.; Van Langen, L.M. Cross-linked enzyme aggregates (CLEAs): A novel and versatile method for enzyme immobilization (a review). Biocatal. Biotransform. 2005, 23, 141–147. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilization in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Vázquez-Duhalt, R.; Mateos-Díaz, J.C.; Guisán, J.M.; Favela-Torres, E. Carrier-free immobilization of lipase from Candida rugosa with polyethyleneimines by carboxyl-activated cross-linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Fonseca, M.; Kopp, W.; Giordano, R.; Fernández-Lafuente, R.; Tardioli, P. Preparation of magnetic cross-linked amyloglucosidase aggregates: Solving some activity problems. Catalysts 2018, 8, 496. [Google Scholar] [CrossRef]
- Guimarães, J.; Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P. Evaluation of strategies to produce highly porous cross-linked aggregates of porcine pancreas lipase with magnetic properties. Molecules 2018, 23, 2993. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Fernández-Lorente, G.; Fernández-Lafuente, R.; Illanes, A.; Guisán, J.M.; Palomo, J.M. CLEAs of lipases and poly-ionic polymers: A simple way of preparing stable biocatalysts with improved properties. Enzym. Microb. Technol. 2006, 39, 750–755. [Google Scholar] [CrossRef]
- Araujo-Silva, R.; Mafra, A.C.O.; Rojas, M.J.; Kopp, W.; Giordano, R.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Maltose production using starch from cassava bagasse catalyzed by cross-linked β-amylase aggregates. Catalysts 2018, 8, 170. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Hidalgo, A.; Alonso, N.; Fernández-Lafuente, R.; Guisán, J.M. Co-aggregation of enzymes and polyethyleneimine: A simple method to prepare stable and immobilized derivatives of glutaryl acylase. Biomacromolecules 2005, 6, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Mafra, A.C.O.; Furlan, F.F.; Badino, A.C.; Tardioli, P.W. Gluconic acid production from sucrose in an airlift reactor using a multi-enzyme system. Bioprocess Biosyst. Eng. 2015, 38, 671–680. [Google Scholar] [CrossRef]
- Galvis, M.; Barbosa, O.; Ruiz, M.; Cruz, J.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Chemical amination of lipase B from Candida antarctica is an efficient solution for the preparation of crosslinked enzyme aggregates. Process Biochem. 2012, 47, 2373–2378. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; van Langen, L.M.; van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef]
- Talekar, S.; Nadar, S.; Joshi, A.; Joshi, G. Pectin cross-linked enzyme aggregates (pectin-CLEAs) of glucoamylase. RSC Adv. 2014, 4, 59444–59453. [Google Scholar] [CrossRef]
- Zhen, Q.; Wang, M.; Qi, W.; Su, R.; He, Z. Preparation of β-mannanase CLEAs using macromolecular cross-linkers. Catal. Sci. Technol. 2013, 3, 1937–1941. [Google Scholar] [CrossRef]
- Talekar, S.; Waingade, S.; Gaikwad, V.; Patil, S.; Nagavekar, N. Preparation and characterization of cross linked enzyme aggregates (CLEAs) of Bacillus amyloliquefaciens alpha amylase. J. Biochem. Technol. 2012, 3, 349–353. [Google Scholar]
- Gupta, K.; Jana, A.K.; Kumar, S.; Maiti, M. Immobilization of amyloglucosidase from SSF of Aspergillus niger by crosslinked enzyme aggregate onto magnetic nanoparticles using minimum amount of carrier and characterizations. J. Mol. Catal. B Enzym. 2013, 98, 30–36. [Google Scholar] [CrossRef]
- Matte, C.R.; Nunes, M.R.; Benvenutti, E.V.; da Natividade Schöffer, J.; Ayub, M.A.Z.; Hertz, P.F. Characterization of cyclodextrin glycosyltransferase immobilized on silica microspheres via aminopropyltrimethoxysilane as a “spacer arm”. J. Mol. Catal. B Enzym. 2012, 78, 51–56. [Google Scholar] [CrossRef]
- Martín, M.T.; Plou, F.J.; Alcalde, M.; Ballesteros, A. Immobilization on Eupergit C of cyclodextrin glucosyltransferase (CGTase) and properties of the immobilized biocatalyst. J. Mol. Catal. B Enzym. 2003, 21, 299–308. [Google Scholar] [CrossRef]
- Tardioli, P.W.; Zanin, G.M.; de Moraes, F.F.; De Moraes, F.F. Production of cyclodextrins in a fluidized-bed reactor using cyclodextrin-glycosyl-transferase. Appl. Biochem. Biotechnol. 2000, 84–86, 1003–1019. [Google Scholar] [CrossRef]
- Amud, A.E.; Da Silva, G.R.P.; Tardioli, P.W.; Soares, C.M.F.; Moraes, F.F.; Zanin, G.M. Methods and supports for immobilization and stabilization of cyclomaltodextrin glucanotransferase from Thermoanaerobacter. Appl. Biochem. Biotechnol. 2008, 146, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Tardioli, P.W.P.W.; Zanin, G.M.G.M.; de Moraes, F.F.F. Characterization of Thermoanaerobacter cyclomaltodextrin glucanotransferase immobilized on glyoxyl-agarose. Enzym. Microb. Technol. 2006, 39, 1270–1278. [Google Scholar] [CrossRef]
- da Natividade Schöffer, J.; Klein, M.P.; Rodrigues, R.C.; Hertz, P.F. Continuous production of β-cyclodextrin from starch by highly stable cyclodextrin glycosyltransferase immobilized on chitosan. Carbohydr. Polym. 2013, 98, 1311–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, M.T.; Angeles Cruces, M.; Alcalde, M.; Plou, F.J.; Bernabé, M.; Ballesteros, A. Synthesis of maltooligosyl fructofuranosides catalyzed by immobilized cyclodextrin glucosyltransferase using starch as donor. Tetrahedron 2004, 60, 529–534. [Google Scholar] [CrossRef]
- Kim, P.S.; Shin, H.D.; Park, J.K.; Lee, Y.H. Immobilization of cyclodextrin glucanotransferase on amberlite IRA-900 for biosynthesis of transglycosylated xylitol. Biotechnol. Bioprocess Eng. 2000, 5, 174–180. [Google Scholar] [CrossRef]
- Martín, M.T.T.; Alcalde, M.; Plou, F.J.F.J.; Ballesteros, A. Covalent immobilization of cyclodextrin glucosyltransferase (CGTase) in activated silica and Sepharose. Indian J. Biochem. Biophys. 2002, 39, 229–234. [Google Scholar] [PubMed]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA®s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Guisan, J.M. Strategies for enzyme stabilization by intramolecular crosslinking with bifunctional reagents. Enzym. Microb. Technol. 1995, 17, 517–523. [Google Scholar] [CrossRef]
- Mafra, A.C.O.; Kopp, W.; Beltrame, M.B.; Giordano, R.L.C.; Ribeiro, M.P.A.; Tardioli, P.W.; de Lima Camargo Giordano, R.; de Arruda Ribeiro, M.P.; Tardioli, P.W. Diffusion effects of bovine serum albumin on cross-linked aggregates of catalase. J. Mol. Catal. B Enzym. 2016, 133, 107–116. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Fernández-Lafuente, R.; Rodríguez, V.; Mateo, C.; Penzol, G.; Hernández-Justiz, O.; Irazoqui, G.; Villarino, A.; Ovsejevi, K.; Batista, F.; Guisán, J.M. Stabilization of multimeric enzymes via immobilization and post-immobilization techniques. J. Mol. Catal. B Enzym. 1999, 7, 181–189. [Google Scholar] [CrossRef]
- Tardioli, P.W.; Fernández-Lafuente, R.; Guisán, J.M.; Giordano, R.L.C. Design of new immobilized-stabilized carboxypeptidase a derivative for production of aromatic free hydrolysates of proteins. Biotechnol. Prog. 2003, 19, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.M.; Guisán, J.M. Protecting effect of competitive inhibitors during very intense insolubilized enzyme-activated support multipoint attachments: Trypsin (amine)-agarose (aldehyde) system. Enzym. Microb. Technol. 1988, 10, 227–232. [Google Scholar] [CrossRef]
- Borges, D.G.; Baraldo Junior, A.; Farinas, C.S.; Giordano, R.L.C.; Tardioli, P.W. Enhanced saccharification of sugarcane bagasse using soluble cellulase supplemented with immobilized β-glucosidase. Bioresour. Technol. 2014, 167, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, S.T.; Tangney, M.; Starnes, R.L.; Amemiya, K. Cloning and nucleotide sequence of a thermostable cyclodextrin glycosyltransferase gene from Thermoanaerobacter sp. ATCC 53627 and its expression in Escherichia coli. Biotechnol. Let. 1997, 19, 1027–1031. [Google Scholar] [CrossRef]
- Van Der Veen, B.A.; Van Alebeek, G.J.W.M.; Uitdehaag, J.C.M.; Dijkstra, B.W.; Dijkhuizen, L. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur. J. Biochem. 2000, 267, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Cruz, J.; Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Villalonga, R.; Fernandez-Lafuente, R. Inactivation of immobilized trypsin under dissimilar conditions produces trypsin molecules with different structures. RSC Adv. 2016, 6, 27329–27334. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vikmon, M. Rapid and simple spectrophotometric method for determination of micro-amounts of cyclodextrins. In Proceedings of the First International Symposium on Cyclodextrins. Advance in Inclusion Science; Szejtli, J., Ed.; Springer: Dordrecht, The Netherlands, 1982; Volume 1, pp. 69–74. [Google Scholar]
- Giordano, R.C.; Giordano, R.L.C.; Prazeres, D.M.F.; Cooney, C.L. Analysis of a Taylor–Poiseuille vortex flow reactor—I: Flow patterns and mass transfer characteristics. Chem. Eng. Sci. 1998, 53, 3635–3652. [Google Scholar] [CrossRef]
- Giordano, R.L.C.; Giordano, R.C.; Cooney, C.L. Performance of a continuous Taylor–Couette–Poiseuille vortex flow enzymic reactor with suspended particles. Process Biochem. 2000, 35, 1093–1101. [Google Scholar] [CrossRef]
- Resende, M.M.; Sousa, R.; Tardioli, P.W.; Giordano, R.L.C.; Giordano, R.C. Enzymatic tailor-made proteolysis of whey in a vortex flow reactor. AIChE J. 2005, 51, 314–322. [Google Scholar] [CrossRef]
- Rojas, M.J. Recovery of Starch from Cassava Bagasse for Cyclodextrin Production Catalyzed by Cross-Linked CGTase Aggregates. Ph.D. Thesis, Federal University of São Carlos, São Carlos, Brazil, 2017. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Rojas, M.J.; Castral, T.C.; Giordano, R.L.C.; Tardioli, P.W. Development and validation of a simple high performance liquid chromatography—Evaporative light scattering detector method for direct quantification of native cyclodextrins in a cyclization medium. J. Chromatogr. A 2015, 1410, 140–146. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, M.J.; Amaral-Fonseca, M.; Zanin, G.M.; Fernandez-Lafuente, R.; Giordano, R.d.L.C.; Tardioli, P.W. Preparation of Crosslinked Enzyme Aggregates of a Thermostable Cyclodextrin Glucosyltransferase from Thermoanaerobacter sp. Critical Effect of the Crosslinking Agent. Catalysts 2019, 9, 120. https://doi.org/10.3390/catal9020120
Rojas MJ, Amaral-Fonseca M, Zanin GM, Fernandez-Lafuente R, Giordano RdLC, Tardioli PW. Preparation of Crosslinked Enzyme Aggregates of a Thermostable Cyclodextrin Glucosyltransferase from Thermoanaerobacter sp. Critical Effect of the Crosslinking Agent. Catalysts. 2019; 9(2):120. https://doi.org/10.3390/catal9020120
Chicago/Turabian StyleRojas, Mayerlenis Jimenez, Murilo Amaral-Fonseca, Gisella Maria Zanin, Roberto Fernandez-Lafuente, Raquel de Lima Camargo Giordano, and Paulo Waldir Tardioli. 2019. "Preparation of Crosslinked Enzyme Aggregates of a Thermostable Cyclodextrin Glucosyltransferase from Thermoanaerobacter sp. Critical Effect of the Crosslinking Agent" Catalysts 9, no. 2: 120. https://doi.org/10.3390/catal9020120