Fabrication and Optimization of a Lipase Immobilized Enzymatic Membrane Bioreactor based on Polysulfone Gradient-Pore Hollow Fiber Membrane
Abstract
:1. Introduction
2. Results and Discussion
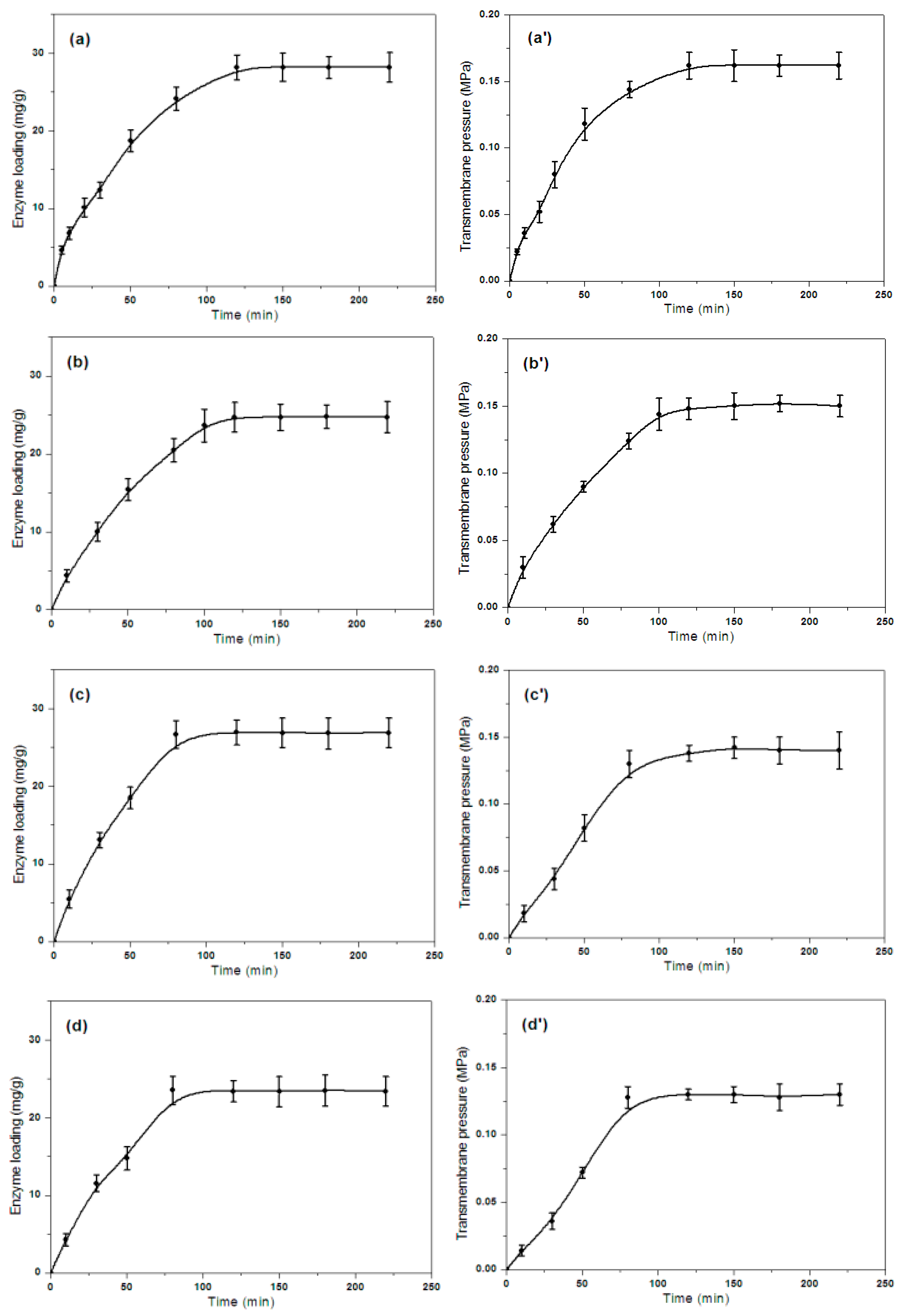
2.1. CRL Immobilization in RGM-PSF Membranes Based EMBR
2.2. Effect of Pore Diameter on the Efficiency of EMBR
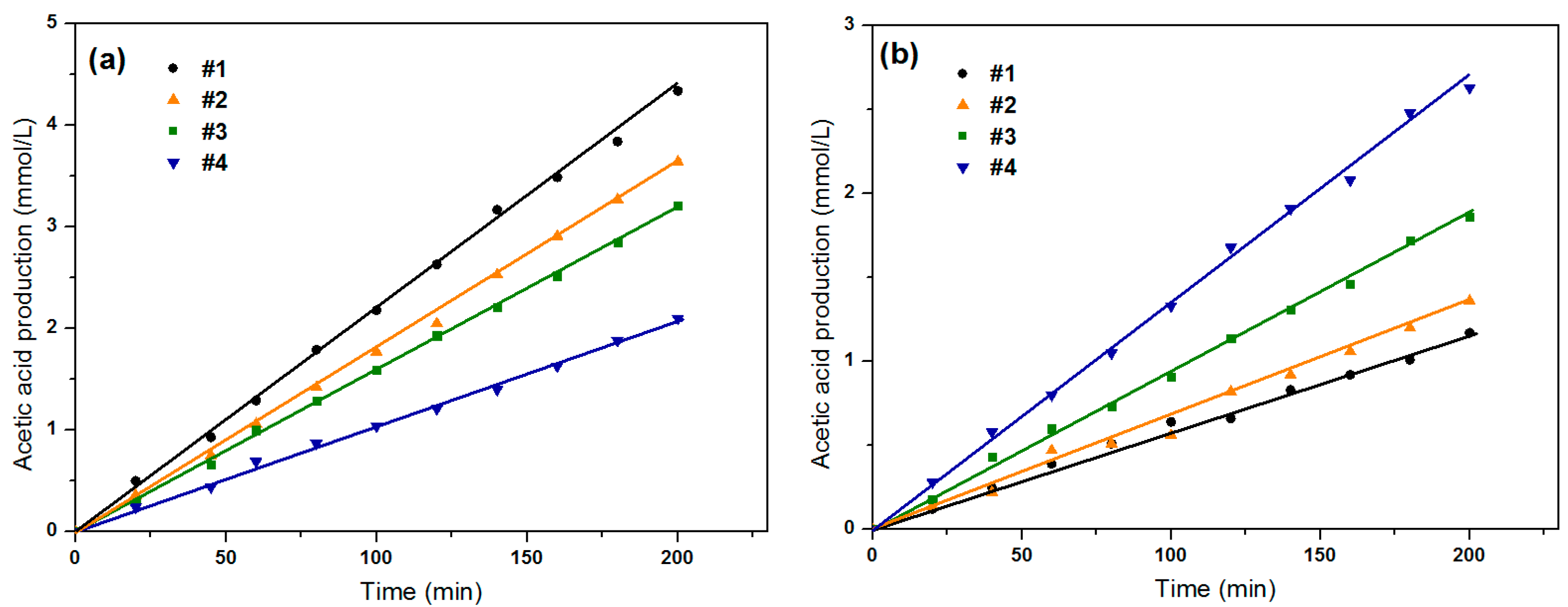
2.3. Effect of Substrate Feed Direction on the Efficiency of EMBR
2.4. Effect of Operational Parameters on the Efficiency of EMBR
2.4.1. Effect of Operation Pressure on the Efficiency of EMBR
2.4.2. Effect of Substrate Concentration on the Efficiency of EMBR
2.4.3. Effect of Temperature on the Efficiency of EMBR
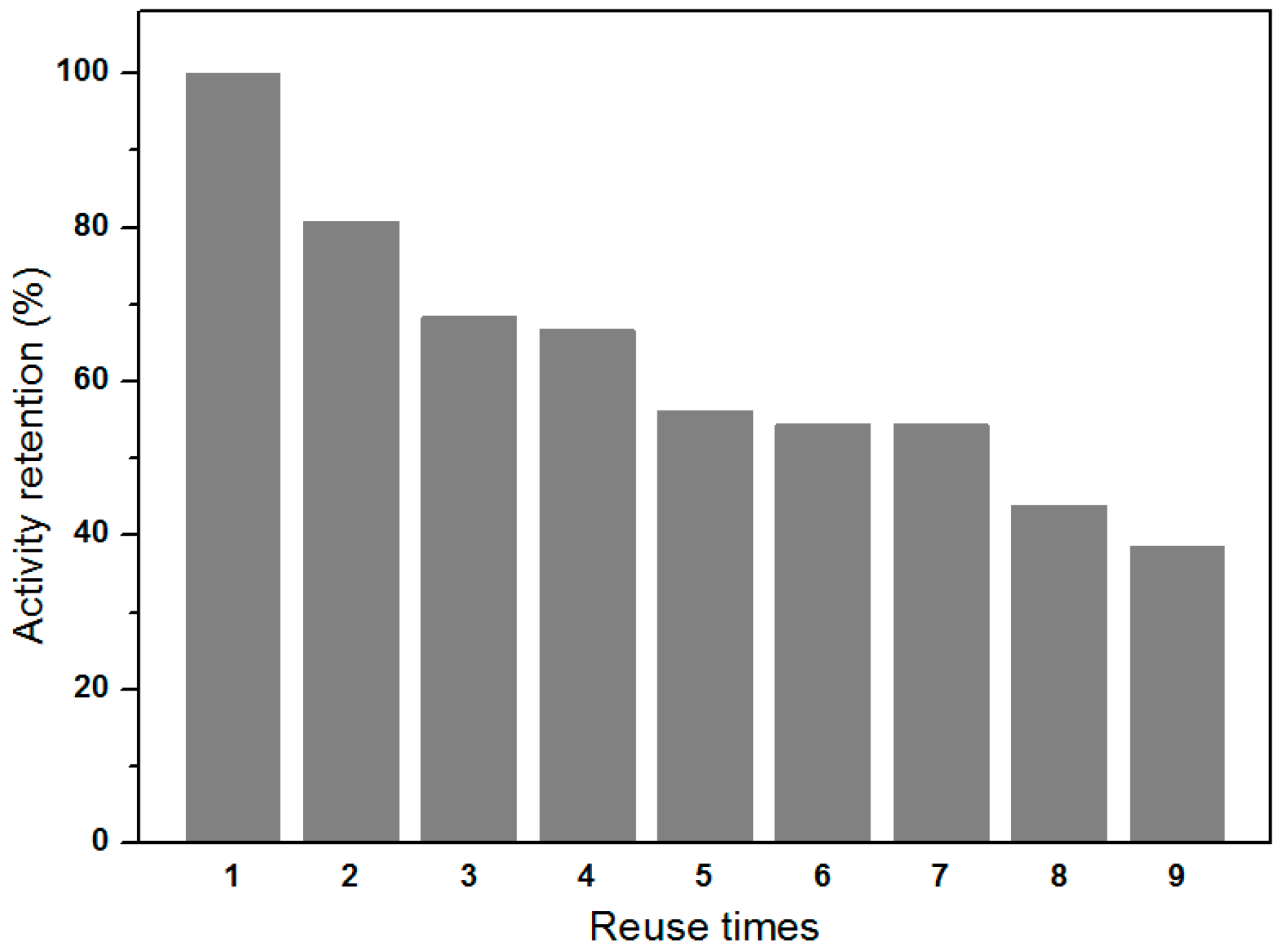
2.5. Reusability of Immobilized Enzymes
3. Materials and Methods
3.1. Materials
3.2. Constrcution of EMBR
3.3. TA Hydrolysis in EMBR
3.4. Reusability of EMBR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Dhake, K.P.; Karoyo, A.H.; Mohamed, M.H.; Wilson, L.D.; Bhanage, B.M. Enzymatic activity studies of pseudomonas cepacia lipase adsorbed onto copolymer supports containing beta-cyclodextrin. J. Mol. Catal. B Enzym. 2013, 87, 105–112. [Google Scholar] [CrossRef]
- Ranieri, G.; Mazzei, R.; Wu, Z.T.; Li, K.; Giorno, L. Use of a ceramic membrane to improve the performance of two-separate-phase biocatalytic membrane reactor. Molecules 2016, 21, 345. [Google Scholar] [CrossRef]
- Cardenas-Fernandez, M.; Hamley-Bennett, C.; Leak, D.J.; Lye, G.J. Continuous enzymatic hydrolysis of sugar beet pectin and L-arabinose recovery within an integrated biorefinery. Bioresour. Technol. 2018, 269, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Morthensen, S.T.; Meyer, A.S.; Jorgensen, H.; Pinelo, M. Significance of membrane bioreactor design on the biocatalytic performance of glucose oxidase and catalase: Free vs. immobilized enzyme systems. Biochem. Eng. J. 2017, 117, 41–47. [Google Scholar] [CrossRef]
- Mazurenko, I.; Etienne, M.; Kohring, G.W.; Lapicque, F.; Walcarius, A. Enzymatic bioreactor for simultaneous electrosynthesis and energy production. Electrochim. Acta 2016, 199, 342–348. [Google Scholar] [CrossRef]
- Straathof, A.J.J.; Panke, S.; Schmid, A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2002, 13, 548–556. [Google Scholar] [CrossRef]
- Liu, C.J.; Saeki, D.; Matsuyama, H. A novel strategy to immobilize enzymes on microporous membranes via dicarboxylic acid halides. RSC Adv. 2017, 7, 48199–48207. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, R.; Piacentini, E.; Gebreyohannes, A.Y.; Giorno, L. Membrane bioreactors in food, pharmaceutical and biofuel applications: State of the art, progresses and perspectives. Curr. Org. Chem. 2017, 21, 1671–1701. [Google Scholar] [CrossRef]
- Guo, Y.Z.; Zhu, X.Y.; Fang, F.; Hong, X.; Wu, H.M.; Chen, D.J.; Huang, X.J. Immobilization of enzymes on a phospholipid bionically modified polysulfone gradient-pore membrane for the enhanced performance of enzymatic membrane bioreactors. Molecules 2018, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, L.F.; Yang, F.L. Highly conductive graphene/PANi-phytic acid modified cathodic filter membrane and its antifouling property in EMBR in neutral conditions. Desalination 2014, 338, 10–16. [Google Scholar] [CrossRef]
- Liao, Y.; Tian, M.; Goh, S.W.; Wang, R.; Fane, A.G. Effects of internal concentration polarization and membrane roughness on phenol removal in extractive membrane bioreactor. J. Membr. Sci. 2018, 563, 309–319. [Google Scholar] [CrossRef]
- Ovcharova, A.; Vasilevsky, V.; Borisov, I.; Bazhenov, S.; Volkov, A.; Bildyukevich, A.; Volkov, V. Polysulfone porous hollow fiber membranes for ethylene-ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2017, 183, 162–172. [Google Scholar] [CrossRef]
- Lim, S.; Park, M.J.; Phuntsho, S.; Tijing, L.D.; Nisola, G.M.; Shim, W.G.; Chung, W.J.; Shon, H.K. Dual-layered nanocomposite substrate membrane based on polysulfone/graphene oxide for mitigating internal concentration polarization in forward osmosis. Polymer 2017, 110, 36–48. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Poppe, J.K.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Enzymatic reactors for biodiesel synthesis: Present status and future prospects. Biotechnol. Adv. 2015, 33, 511–525. [Google Scholar] [CrossRef]
- Jochems, P.; Satyawali, Y.; Diels, L.; Dejonghe, W. Enzyme immobilization on/in polymeric membranes: Status, challenges and perspectives in biocatalytic membrane reactors (BMRs). Green Chem. 2011, 13, 1609–1623. [Google Scholar] [CrossRef]
- Bali, N.; Petsi, A.J.; Skouras, E.D.; Burganos, V.N. Three-dimensional reconstruction of bioactive membranes and pore-scale simulation of enzymatic reactions: The case of lactose hydrolysis. J. Membr. Sci. 2017, 524, 225–234. [Google Scholar] [CrossRef]
- Girono, L.; Drioli, E. Biocatalytic membrane reactors: Applications and perspectives. Trends Biotechnol. 2000, 18, 339–349. [Google Scholar] [CrossRef]
- Okobira, T.; Matsuo, A.; Matsumoto, H.; Tanaka, T.; Kai, K.; Minari, C.; Goto, M.; Kawakita, H.; Uezu, K. Enhancement of immobilized lipase activity by design of polymer brushes on a hollow fiber membrane. J. Biosci. Bioeng. 2015, 120, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Nogalska, A.; Ammendola, M.; Portugal, C.A.M.; Tylkowski, B.; Crespo, J.G.; Garcia-Valls, R. Polysulfone biomimetic membrane for CO2 capture. Funct. Mater. Lett. 2018, 11, 1850046. [Google Scholar] [CrossRef]
- Mahlicli, F.Y.; Sen, Y.; Mutlu, M.; Altinkaya, S.A. Immobilization of superoxide dismutase/catalase onto polysulfone membranes to suppress hemodialysis-induced oxidative stress: A comparison of two immobilization methods. J. Membr. Sci. 2015, 479, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Ingole, P.; Singh, K.; Bhattacharya, A. Comparative study of the hydrolysis of different oils by lipase-immobilized membranes. J. Appl. Polym. Sci. 2012, 124, E17–E26. [Google Scholar] [CrossRef]
- Saeki, D.; Nagao, S.; Sawada, I.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of antibacterial polyamide reverse osmosis membrane modified with a covalently immobilized enzyme. J. Membr. Sci. 2013, 428, 403–409. [Google Scholar] [CrossRef]
- Liu, J.G.; Cui, Z.F. Optimization of operating conditions for glucose oxidation in an enzymatic membrane bioreactor. J. Membr. Sci. 2007, 302, 180–187. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, J.Q.; Xu, Z.K. Immobilization of lipase from Candida rugosa on electrospun polysulfone nanofibrous membranes by adsorption. J. Mol. Catal. B Enzym. 2006, 42, 45–51. [Google Scholar] [CrossRef]
- Sousa, H.A.; Rodrigues, C.; Klein, E.; Afonso, C.A.M.; Crespo, J.G. Immobilisation of pig liver esterase in hollow fibre membranes. Enzym. Microb. Tech. 2001, 29, 625–634. [Google Scholar] [CrossRef]
- Andre, J.; Borneman, Z.; Wessling, M. Enzymatic conversion in ion-exchange matrix hollow fiber membrnes. Ind. Eng. Chem. Res. 2013, 52, 8635–8644. [Google Scholar] [CrossRef]
- Chen, P.C.; Huang, X.J.; Xu, Z.K. Utilization of a biphasic oil/aqueous cellulose nanofiber membrane bioreactor with immobilized lipase for continuous hydrolysis of olive oil. Cellulose 2014, 21, 407–416. [Google Scholar] [CrossRef]
- Ulbricht, M.; Riedel, M.; Marx, U. Novel photochemical surface functionalization of polysulfone ultrafiltration membranes for covalent immobilization of biomolecules. J. Membr. Sci. 1996, 120, 239–259. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Chen, C.; Chen, P.C.; Gao, Q.L.; Fang, F.; Li, J.; Huang, X.J. High-performance enzymatic membrane bioreactor based on a radial gradient of pores in a PSF membrane via facile enzyme immobilization. RSC Adv. 2016, 6, 30804–30812. [Google Scholar] [CrossRef]
- Huang, X.J.; Wang, L.W.; Zhang, L.L.; Gao, Q.L. Superhydrophilic and Gradient Hole Structured Hollow Fiber Membrane. CN201410081610.9, 28 May 2014. [Google Scholar]
- Gao, Q.L.; Fang, F.; Chen, C.; Zhu, X.Y.; Li, J.; Tang, H.Y.; Zhang, Z.B.; Huang, X.J. Facile approach to silica-modified polysulfone microfiltration membranes for oil-in-water emulsion separation. RSC Adv. 2016, 6, 41323–41330. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-C.; Ma, Z.; Zhu, X.-Y.; Chen, D.-J.; Huang, X.-J. Fabrication and Optimization of a Lipase Immobilized Enzymatic Membrane Bioreactor based on Polysulfone Gradient-Pore Hollow Fiber Membrane. Catalysts 2019, 9, 495. https://doi.org/10.3390/catal9060495
Chen P-C, Ma Z, Zhu X-Y, Chen D-J, Huang X-J. Fabrication and Optimization of a Lipase Immobilized Enzymatic Membrane Bioreactor based on Polysulfone Gradient-Pore Hollow Fiber Membrane. Catalysts. 2019; 9(6):495. https://doi.org/10.3390/catal9060495
Chicago/Turabian StyleChen, Peng-Cheng, Zhen Ma, Xue-Yan Zhu, Da-Jing Chen, and Xiao-Jun Huang. 2019. "Fabrication and Optimization of a Lipase Immobilized Enzymatic Membrane Bioreactor based on Polysulfone Gradient-Pore Hollow Fiber Membrane" Catalysts 9, no. 6: 495. https://doi.org/10.3390/catal9060495




