Synthesis and Characterization of Highly Stabilized Polymer–Trypsin Conjugates with Autolysis Resistance
Abstract
:1. Introduction
2. Results and Discussion
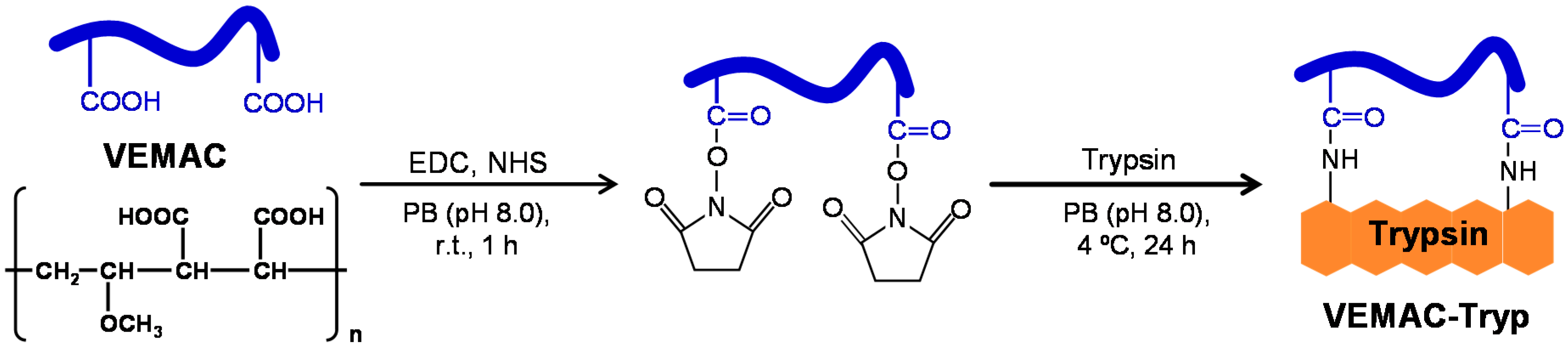
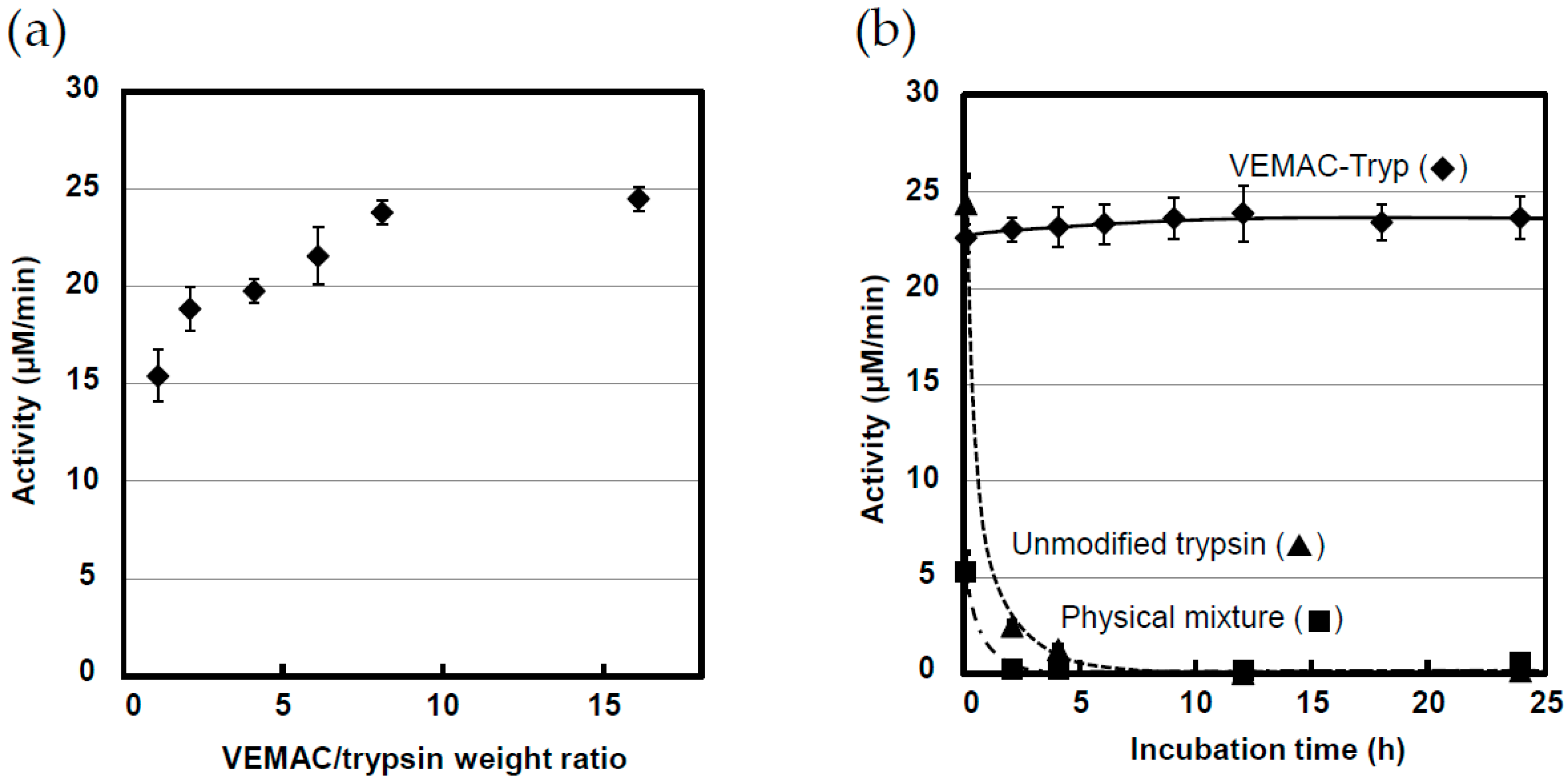
2.1. Synthesis of VEMAC-Tryp and Evaluation of Its Enzymatic Activity
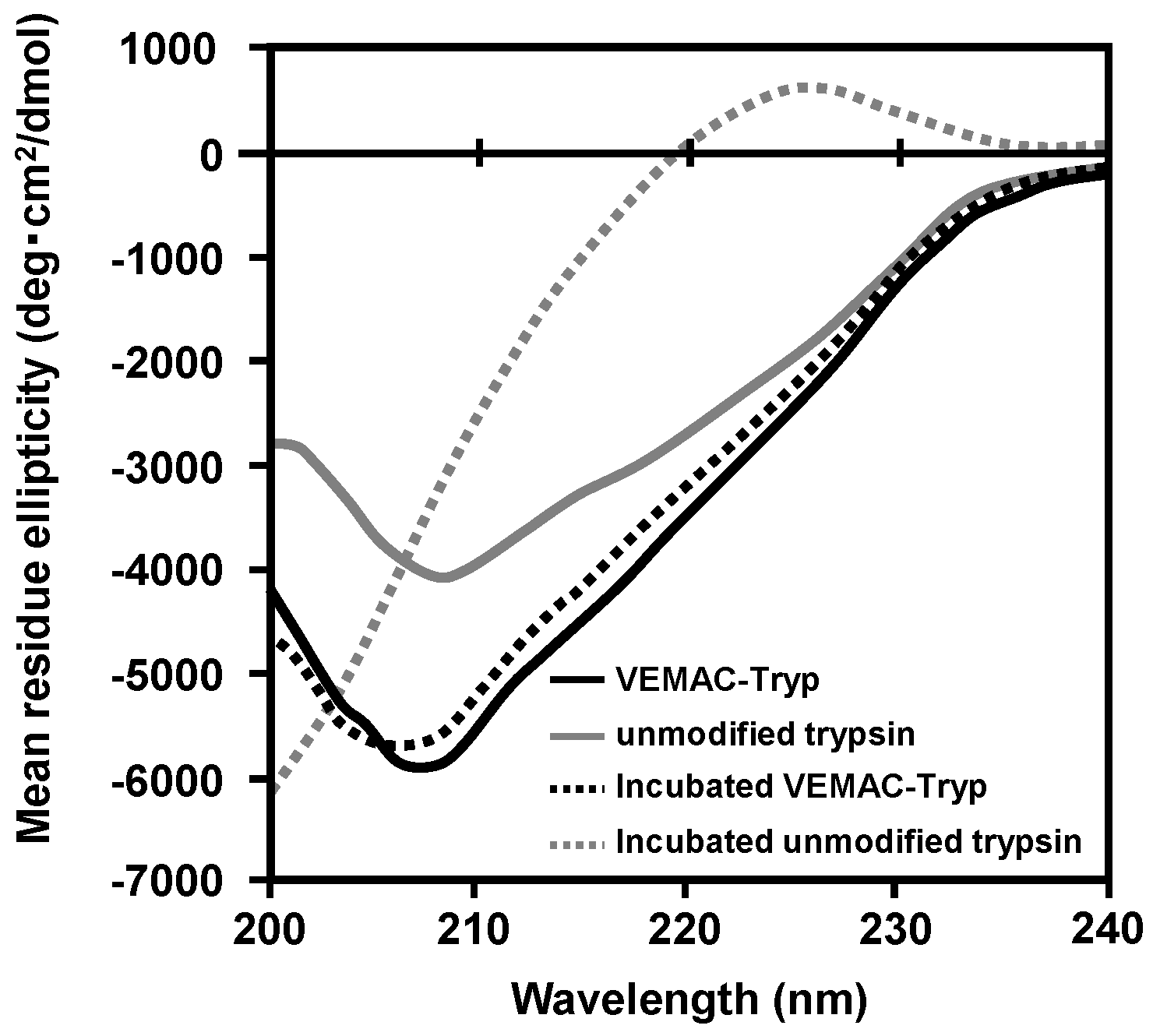
2.2. Physicochemical Characterization of VEMAC-Tryp
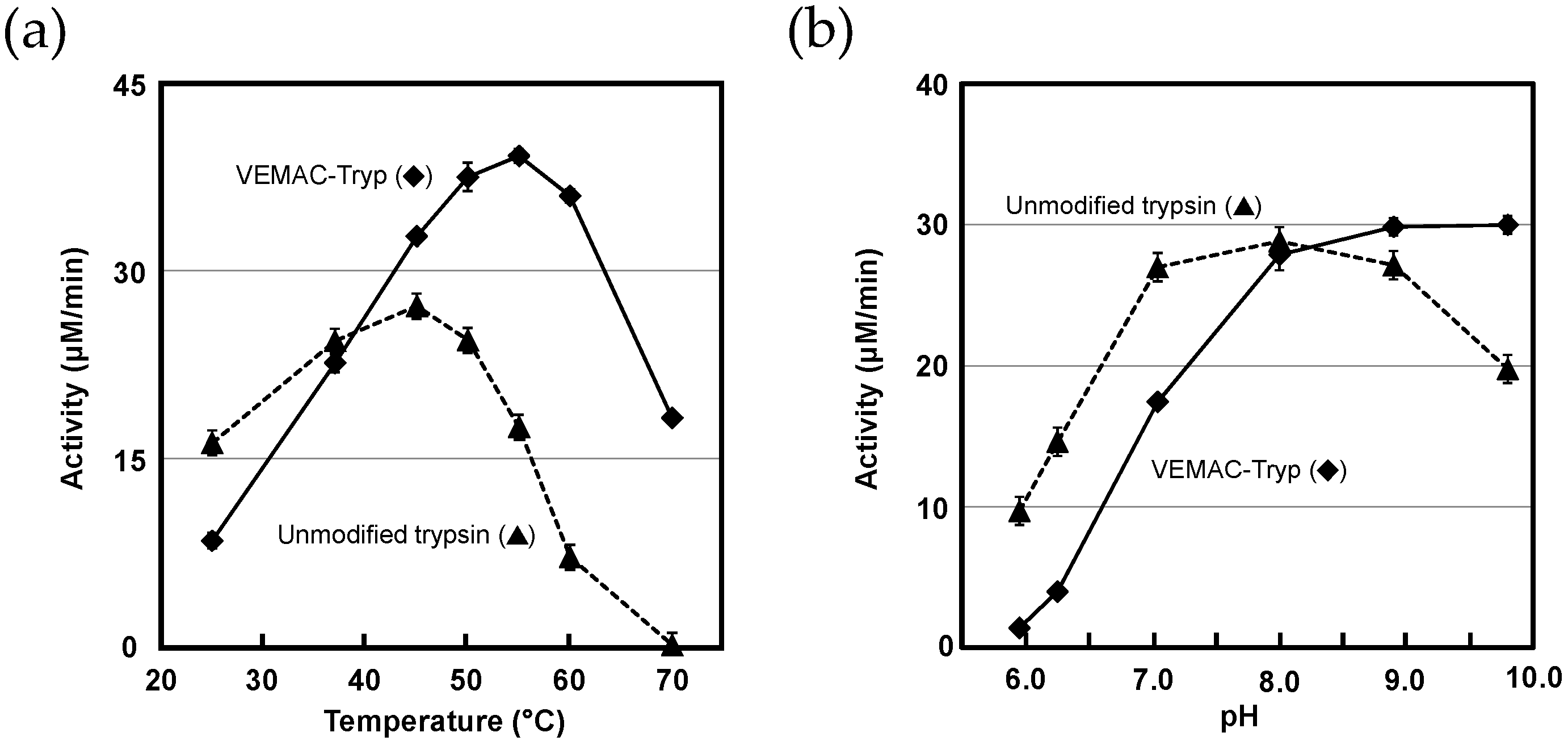
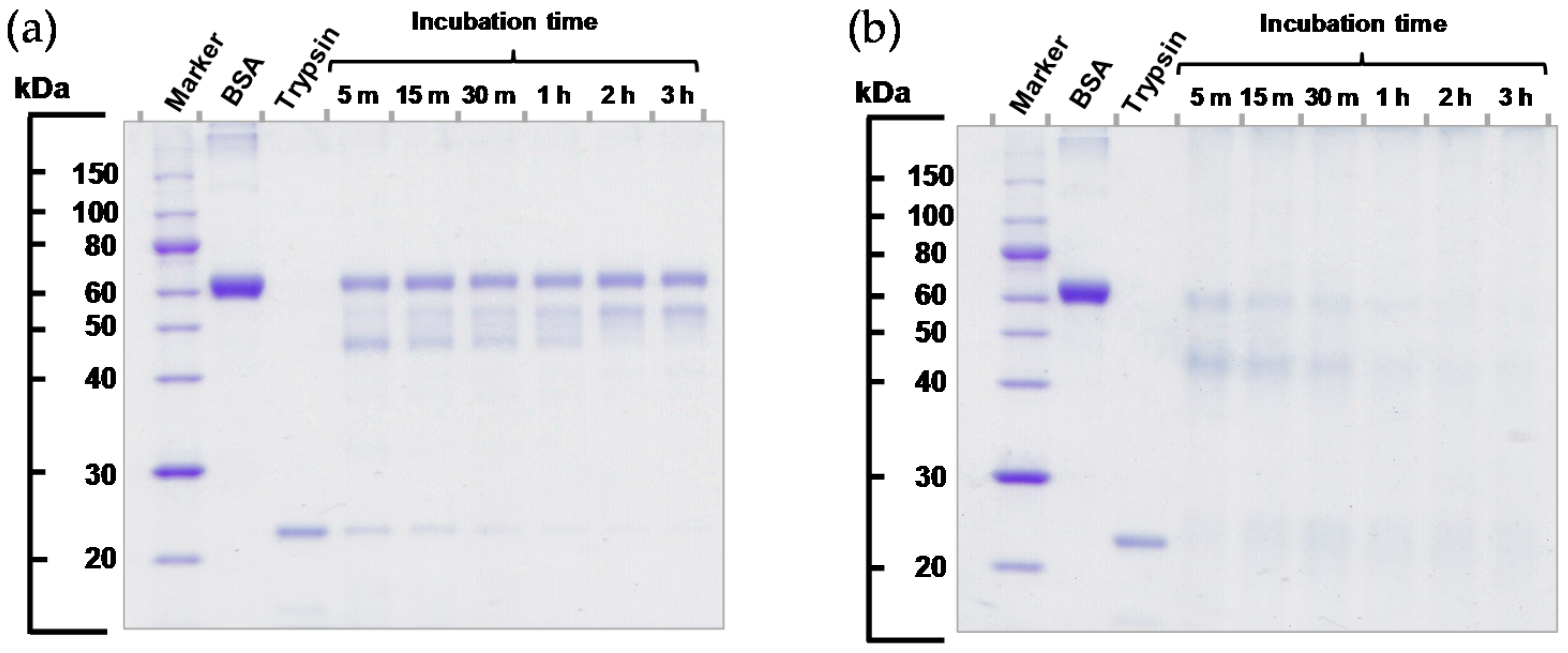
2.3. Protease Activity of VEMAC-Tryp
3. Materials and Methods
3.1. Materials
3.2. Physicochemical Characterization
3.3. Synthesis of VEMAC-Tryp and Preparation of a VEMAC/Trypsin Mixture
3.4. Evaluation of Enzymatic Activity Using a Low Molecular Weight Synthetic Substrate
3.5. Evaluation of Protease Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gauthier, M.A.; Klok, H.-A. Polymer–protein conjugates: An enzymatic activity perspective. Polym. Chem. 2010, 1, 1352–1373. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S. Conjugates of stimuli-responsive polymers and proteins. Prog. Polym. Sci. 2007, 32, 922–932. [Google Scholar] [CrossRef]
- Shakya, A.K.; Sami, H.; Srivastava, A.; Kumar, A. Stability of responsive polymer–protein bioconjugates. Prog. Polym. Sci. 2010, 35, 459–486. [Google Scholar] [CrossRef]
- Falatach, R.; Li, S.; Sloane, S.; McGlone, C.; Berberich, J.A.; Page, R.C.; Averick, S.; Konkolewicz, D. Why synthesize protein–polymer conjugates? The stability and activity of chymotrypsin-polymer bioconjugates synthesized by RAFT. Polymer 2015, 72, 382–386. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Hsieh, Y.-P.; Lin, S.-C. Effect of PEGylation on the activity and stability of horseradish peroxidase and l-N-carbamoylase in aqueous phases. Process Biochem. 2015, 50, 1372–1378. [Google Scholar] [CrossRef]
- Swierczewska, M.; Lee, K.C.; Lee, S. What is the future of PEGylated therapies? Expert Opin. Emerg. Drugs 2015, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Alconcel, S.N.S.; Baas, A.S.; Maynard, H.D. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym. Chem. 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Cummings, C.S.; Koepsel, R.R.; Russell, A.J. Polymer-based protein engineering can rationally tune enzyme activity, pH-dependence, and stability. Biomacromolecules 2013, 14, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Lucius, M.; Falatach, R.; McGlone, C.; Makaroff, K.; Danielson, A.; Williams, C.; Nix, J.C.; Konkolewicz, D.; Page, R.C.; Berberich, J.A. Investigating the impact of polymer functional groups on the stability and activity of lysozyme-polymer conjugates. Biomacromolecules 2016, 17, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Pedroche, J.; del Mar Yust, M.; Mateo, C.; Fernández-Lafuente, R.; Girón-Calle, J.; Alaiz, M.; Vioque, J.; Guisán, J.M.; Millán, F. Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: Correlation between enzyme–support linkages and thermal stability. Enzyme Microb. Technol. 2007, 40, 1160–1166. [Google Scholar] [CrossRef]
- Riccardi, C.M.; Cole, K.S.; Benson, K.R.; Ward, J.R.; Bassett, K.M.; Zhang, Y.; Zore, O.V.; Stromer, B.; Kasi, R.M.; Kumar, C.V. Toward “stable-on-the-table” enzymes: Improving key properties of catalase by covalent conjugation with poly(acrylic acid). Bioconjugate Chem. 2014, 25, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Treetharnmathurot, B.; Dieudonné, L.; Ferguson, E.L.; Schmaljohann, D.; Duncan, R.; Wiwattanapatapee, R. Dextrin–trypsin and ST-HPMA–trypsin conjugates: Enzyme activity, autolysis and thermal stability. Int. J. Pharm. 2009, 373, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hustoft, H.K.; Malerod, H.; Wilson, S.R.; Reubsaet, L.; Lundanes, E.; Greibrokk, T. A critical review of trypsin digestion for LC-MS based proteomics. In Integrative Proteomics; Leung, H.-C., Man, T.-K., Flores, R.J., Eds.; InTech: Rijeka, Croatia, 2012; pp. 73–92. [Google Scholar]
- Havliš, J.; Thomas, H.; Šebela, M.; Shevchenko, A. Fast-response proteomics by accelerated in-gel digestion of proteins. Anal. Chem. 2003, 75, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Sebela, M.; Stosova, T.; Havlis, J.; Wielsch, N.; Thomas, H.; Zdrahal, Z.; Shevchenko, A. Thermostable trypsin conjugates for high-throughput proteomics: Synthesis and performance evaluation. Proteomics 2006, 6, 2959–2963. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Ge, J.; Dong, W.; Liu, Z.; Ouyang, P. Preparation and characterization of a temperature-sensitive sulfobetaine polymer–trypsin conjugate. Biochem. Eng. J. 2006, 30, 48–54. [Google Scholar] [CrossRef]
- Treetharnmathurot, B.; Ovartlarnporn, C.; Wungsintaweekul, J.; Duncan, R.; Wiwattanapatapee, R. Effect of PEG molecular weight and linking chemistry on the biological activity and thermal stability of PEGylated trypsin. Int. J. Pharm. 2008, 357, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, H.F.; Puigserver, A.J. Increased activity and stability of poly(ethylene glycol)-modified trypsin. Enzyme Microb. Technol. 1992, 14, 150–155. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hassanzadeh, F.; Aliabadi, H.S.; Khoraskani, F.R.; Mirian, M.; Behdadfar, B. Targeted delivery of doxorubicin to breast cancer cells by magnetic LHRH chitosan bioconjugated nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Laverty, T.P.; Morris, C.; Andrews, G.P. Statistical modelling of the rheological and mucoadhesive properties of aqueous poly(methylvinylether-co-maleic acid) networks: Redefining biomedical applications and the relationship between viscoelasticity and mucoadhesion. Colloids Surf. B 2016, 144, 125–134. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.C.; Torrisi, B.M.; Al-Zahrani, S.; McCrudden, C.M.; Zaric, M.; Scott, C.J.; Kissenpfennig, A.; McCarthy, H.O.; Donnelly, R.F. Laser-engineered dissolving microneedle arrays for protein delivery: Potential for enhanced intradermal vaccination. J. Pharm. Pharmacol. 2015, 67, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.-A.; Almeida, P.V.; Mäkilä, E.; Correia, A.; Ferreira, M.P.A.; Kaasalainen, M.; Salonen, J.; Hirvonen, J.; Santos, H.A. Poly(methyl vinyl ether-alt-maleic acid)-functionalized porous silicon nanoparticles for enhanced stability and cellular internalization. Macromol. Rapid Commun. 2014, 35, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Singh, T.R.R.; Woolfson, A.D.; Donnelly, R.F. Electrically enhanced solute permeation across poly(ethylene glycol)–crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels: Effect of hydrogel crosslink density and ionic conductivity. Int. J. Pharm. 2011, 406, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Finne, U.; Kyyrönen, K.; Urtti, A. Drug release from monoisopropyl ester of poly(vinyl methyl ether-maleic anhydride) can be modified by basic salts in the polymer matrix. J. Controlled Release 1989, 10, 189–194. [Google Scholar] [CrossRef]
- Sasai, Y.; Matsuzaki, N.; Kondo, S.; Kuzuya, M. Introduction of carboxyl group onto polystyrene surface using plasma techniques. Surf. Coat. Technol. 2008, 202, 5724–5727. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Fields, R. The measurement of amino groups in proteins and peptides. Biochem. J. 1971, 124, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.; Agoubi, L.L.; Lee, I.; Limpar, M.T.; Lowe, J.W., Jr.; Goh, S.L. Effects of polymer molecular weight on the size, activity, and stability of PEG-functionalized trypsin. Biomacromolecules 2010, 11, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J.; Prvan, T. Current statistical methods for estimating the Km and Vmax of Michaelis-Menten kinetics. Biochem. Educ. 1996, 24, 196–206. [Google Scholar] [CrossRef]
- Plochocka, K.; Liu, X.; Tallon, M.A.; Musa, O.M. The quintessential alternating copolymer family: Alkyl vinyl ether co-maleic anhydride copolymers. In Handbook of Maleic Anhydride Based Materials: Syntheses, Properties and Applications; Musa, O.M., Ed.; Springer: Cham, Switzerland, 2016; pp. 211–250. [Google Scholar]
- Oupický, D.; Ulbrich, K.; Říhová, B. Conjugates of semitelechelic poly[N-(2-hydroxypropyl) methacrylamide] with enzymes for protein delivery. J. Bioact. Compat. Polym. 1999, 14, 213–231. [Google Scholar]
- Duncan, R.; Gilbert, H.R.P.; Carbajo, R.J.; Vicent, M.J. Polymer masked−unmasked protein therapy. 1. Bioresponsive dextrin−trypsin and −melanocyte stimulating hormone conjugates designed for α-amylase activation. Biomacromolecules 2008, 9, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Altikatoglu, M.; Celebi, M. Enhanced stability and decolorization of Coomassie Brilliant Blue R-250 by dextran aldehyde-modified horseradish peroxidase. Artif. Cells Blood Substit. Immobil. Biotechnol. 2011, 39, 185–190. [Google Scholar] [CrossRef] [PubMed]
| Sample | Vmax (mM/min) 1 | Km (mM) 1 | Kcat (s−1) 1 | Kcat/Km |
|---|---|---|---|---|
| Unmodified trypsin | 0.059 ± 0.004 | 1.34 ± 0.12 | 1.18 ± 0.08 | 0.88 |
| VEMAC-Tryp | 0.041 ± 0.001 | 0.73 ± 0.01 | 0.80 ± 0.02 | 1.10 |
| Sample | Diameter (nm) 1 | Zeta-Potential (mV) 1 |
|---|---|---|
| Unmodified trypsin | 3.3 ± 0.7 | −15.3 ± 9.4 |
| VEMAC-Tryp | 21.6 ± 4.2 | −32.6 ± 11.3 |
| Sample | α-Helix (%) | β-Sheet (%) | Turns (%) | Random (%) |
|---|---|---|---|---|
| Unmodified trypsin | 7.2 | 40.4 | 18.3 | 34.1 |
| After incubation | 0 | 26.5 | 31.5 | 42.1 |
| VEMAC-Tryp | 5.6 | 41.7 | 18.0 | 34.7 |
| After incubation | 4.5 | 42.3 | 17.7 | 35.5 |
| pH Value | Diameter (nm) 1 |
|---|---|
| 5.9 | 4.0 ± 1.1 |
| 8.0 | 21.6 ± 4.2 |
| 9.8 | 20.9 ± 6.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasai, Y.; Kanno, H.; Doi, N.; Yamauchi, Y.; Kuzuya, M.; Kondo, S.-i. Synthesis and Characterization of Highly Stabilized Polymer–Trypsin Conjugates with Autolysis Resistance. Catalysts 2017, 7, 4. https://doi.org/10.3390/catal7010004
Sasai Y, Kanno H, Doi N, Yamauchi Y, Kuzuya M, Kondo S-i. Synthesis and Characterization of Highly Stabilized Polymer–Trypsin Conjugates with Autolysis Resistance. Catalysts. 2017; 7(1):4. https://doi.org/10.3390/catal7010004
Chicago/Turabian StyleSasai, Yasushi, Hiroshi Kanno, Naoki Doi, Yukinori Yamauchi, Masayuki Kuzuya, and Shin-ichi Kondo. 2017. "Synthesis and Characterization of Highly Stabilized Polymer–Trypsin Conjugates with Autolysis Resistance" Catalysts 7, no. 1: 4. https://doi.org/10.3390/catal7010004






