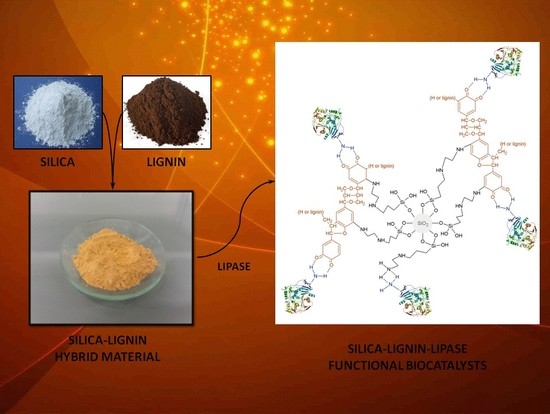
Lipase B from Candida antarctica Immobilized on a Silica-Lignin Matrix as a Stable and Reusable Biocatalytic System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Porous Structure Characterization
2.2. FTIR Spectroscopy
2.3. Elemental Analysis
2.4. Atomic Force Microscopy
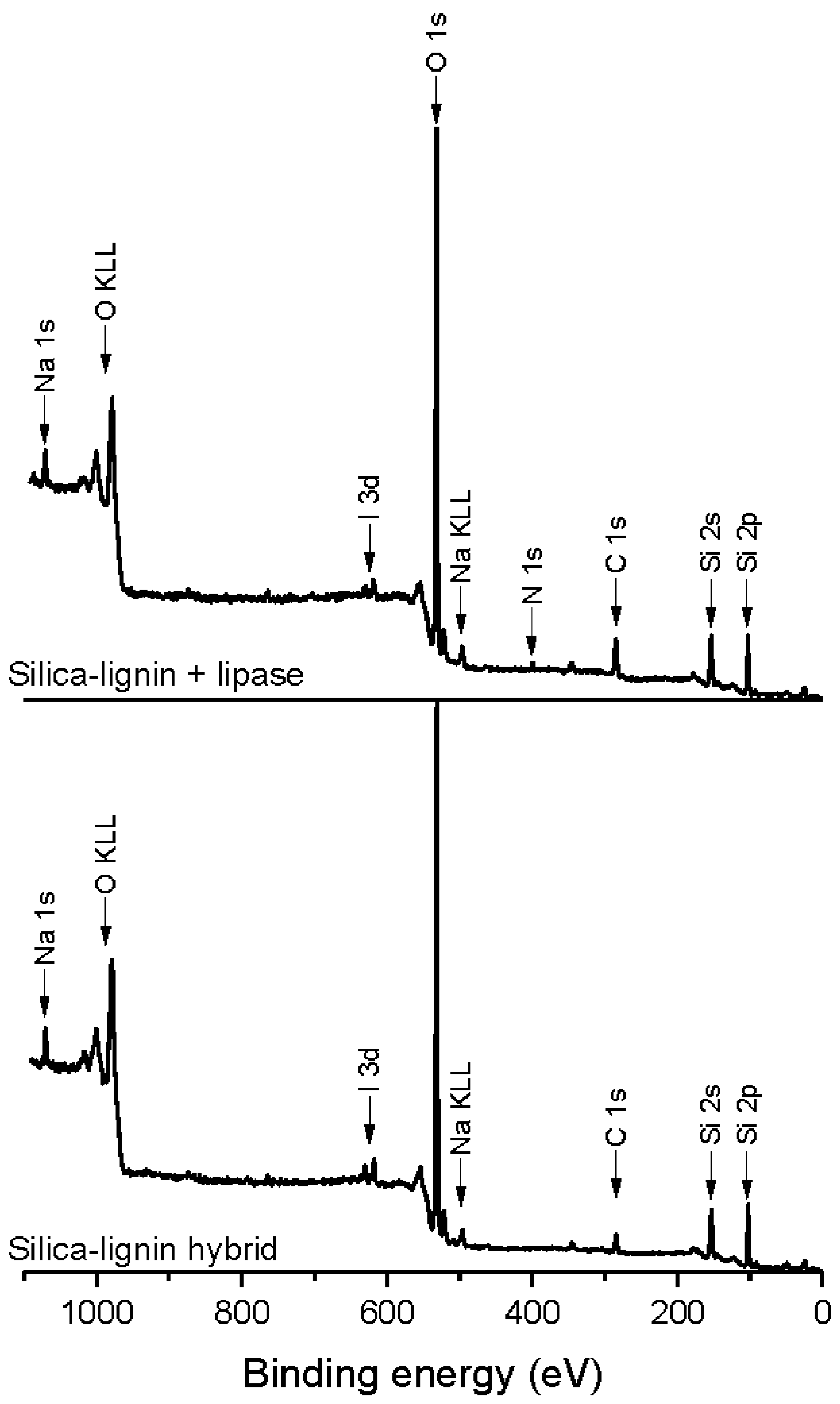
2.5. XPS Analysis
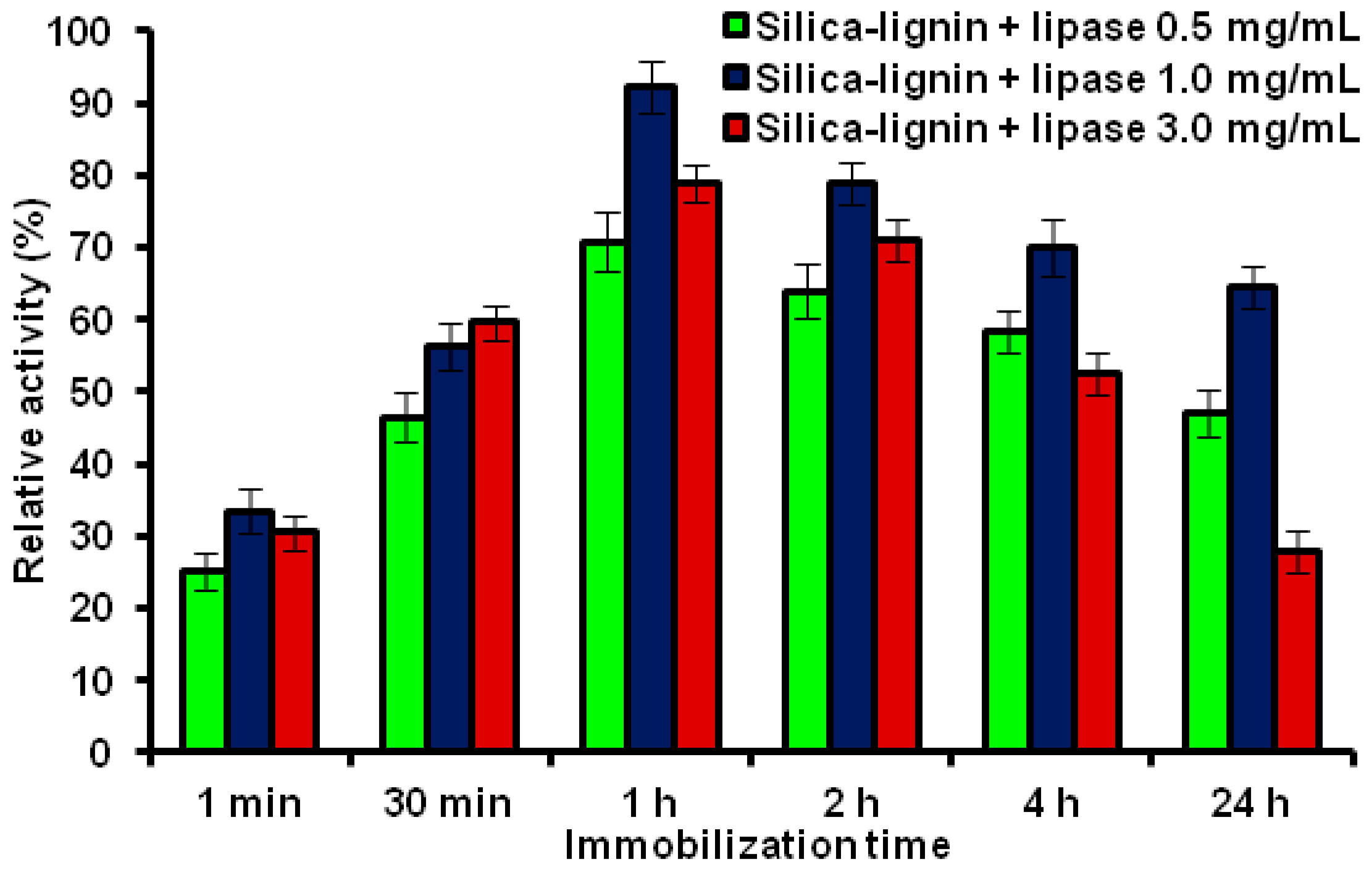
2.6. Desorption Tests of the Immobilized Lipase B from Candida antarctica
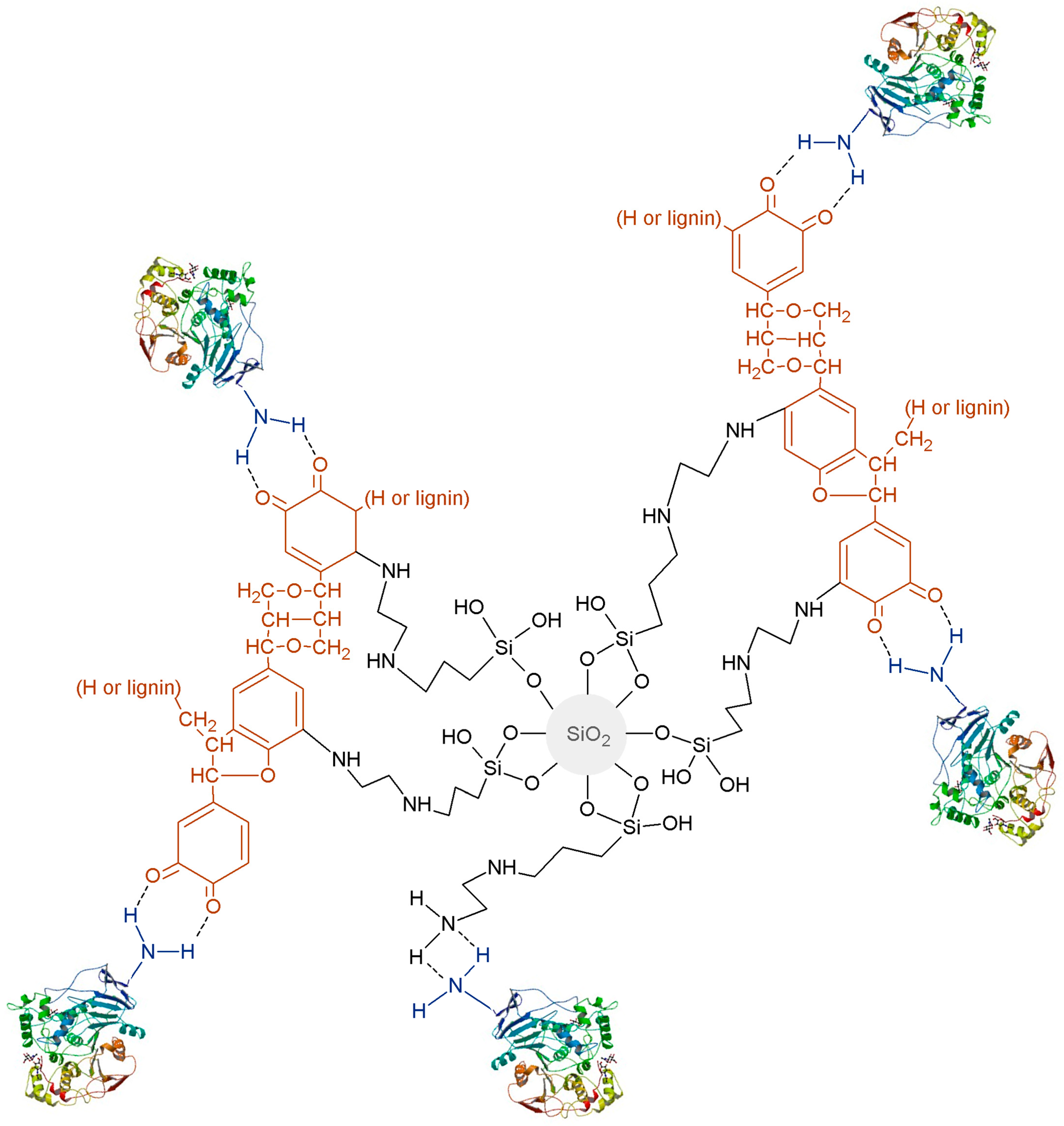
2.7. Suggested Mechanism of Attachment of the Enzyme to the Silica-Lignin Matrix
2.8. Retention of Hydrolytic Activity
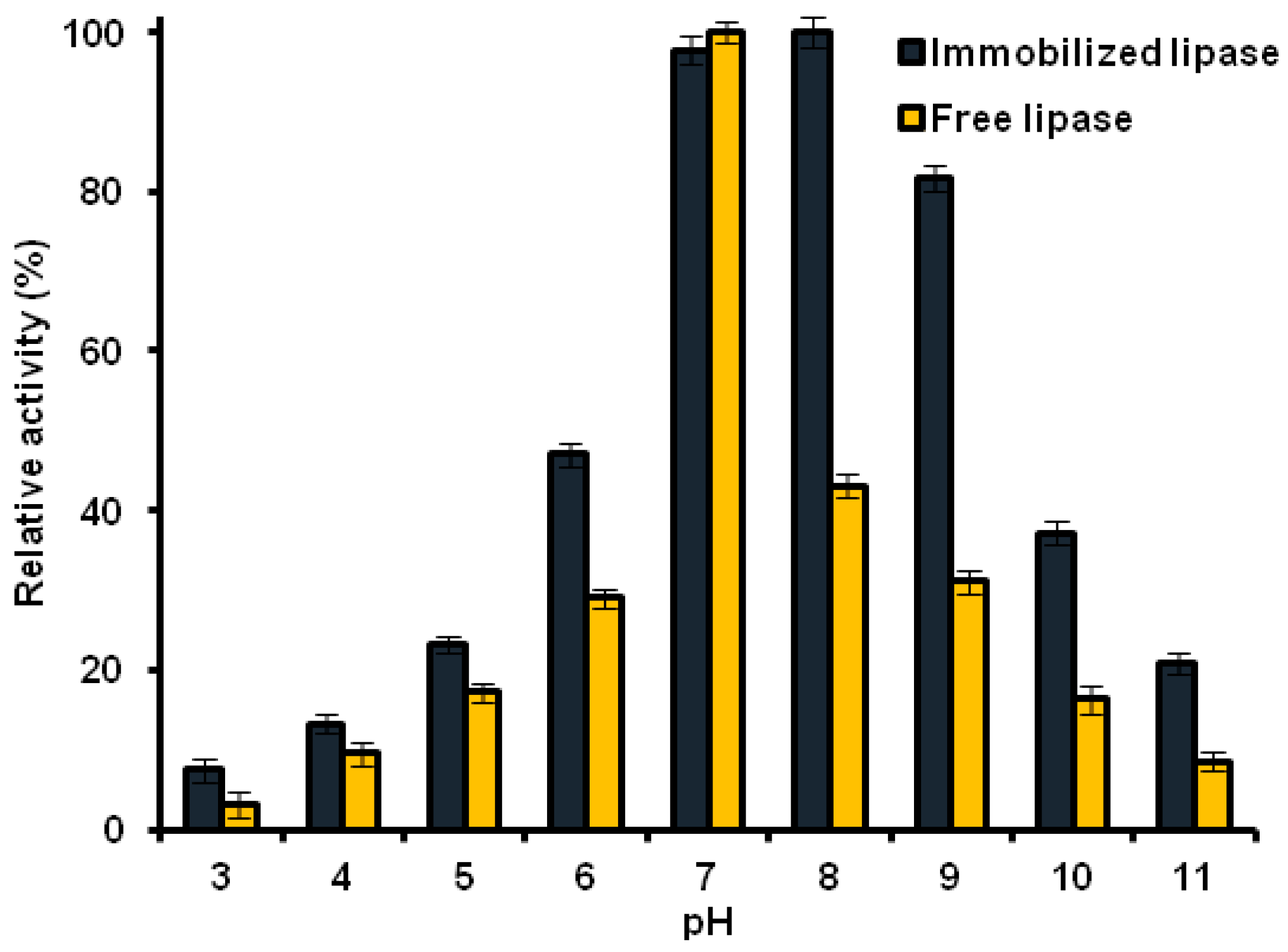
2.8.1. Effect of pH
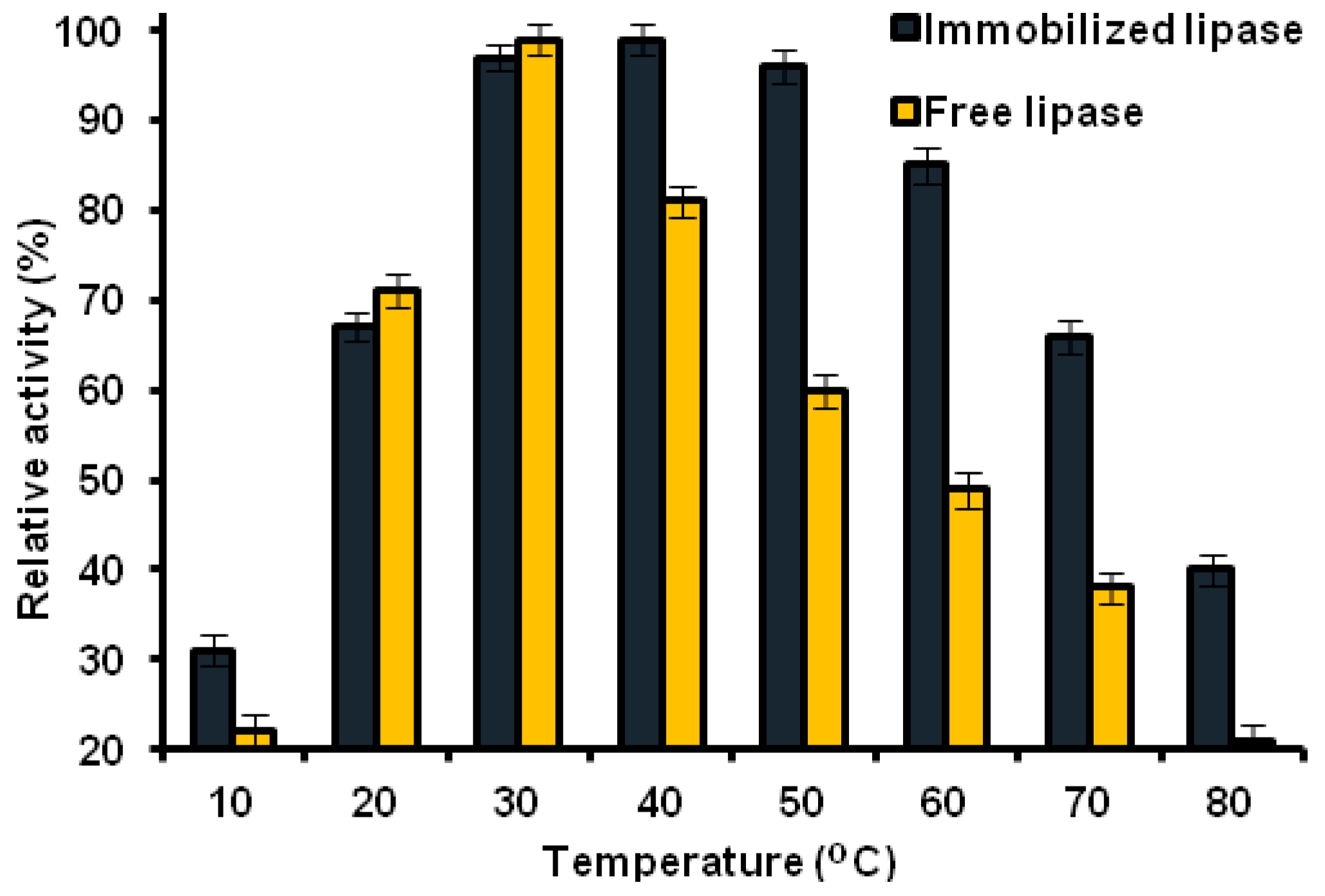
2.8.2. Thermal Stability
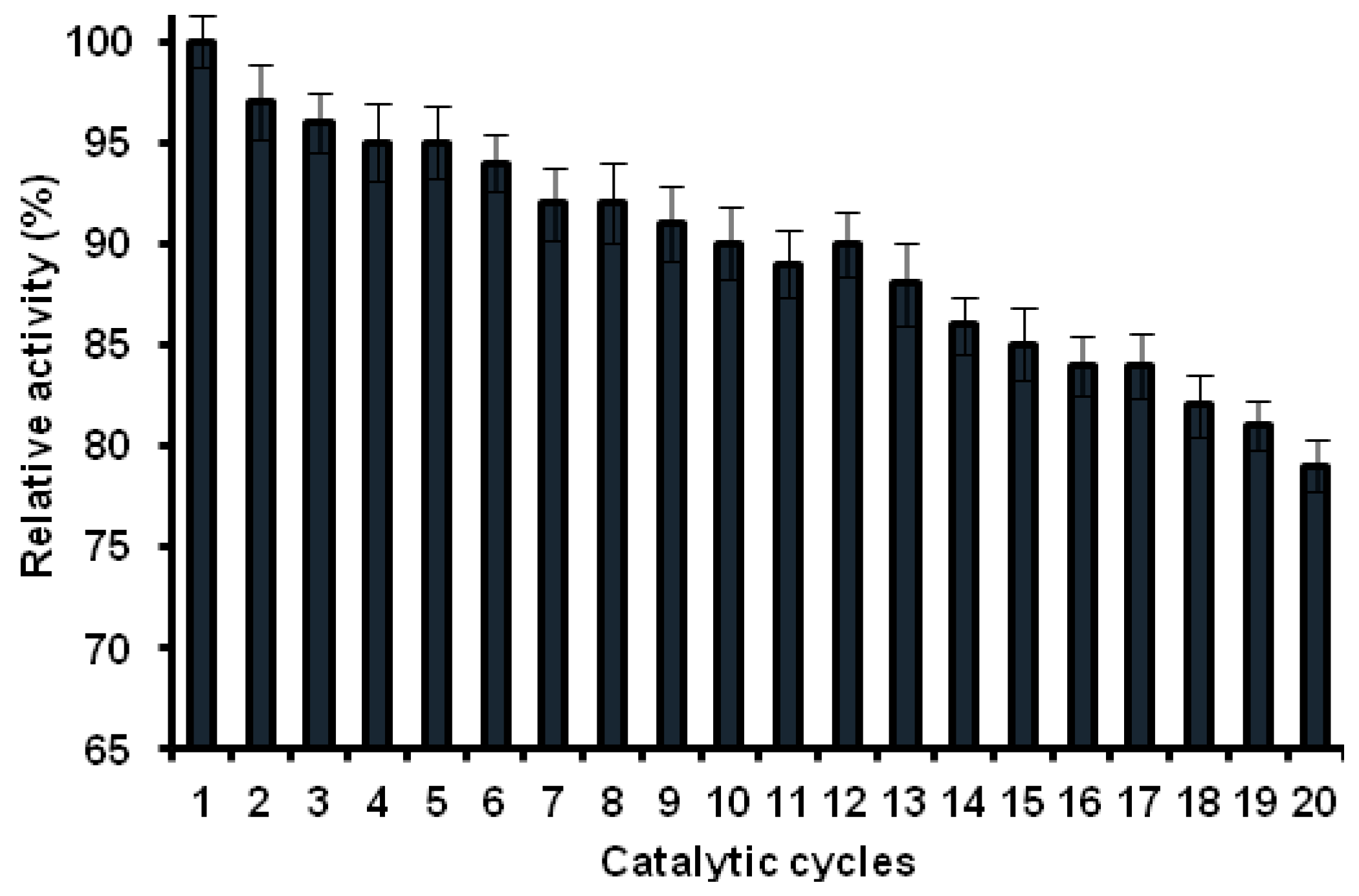
2.8.3. Reusability
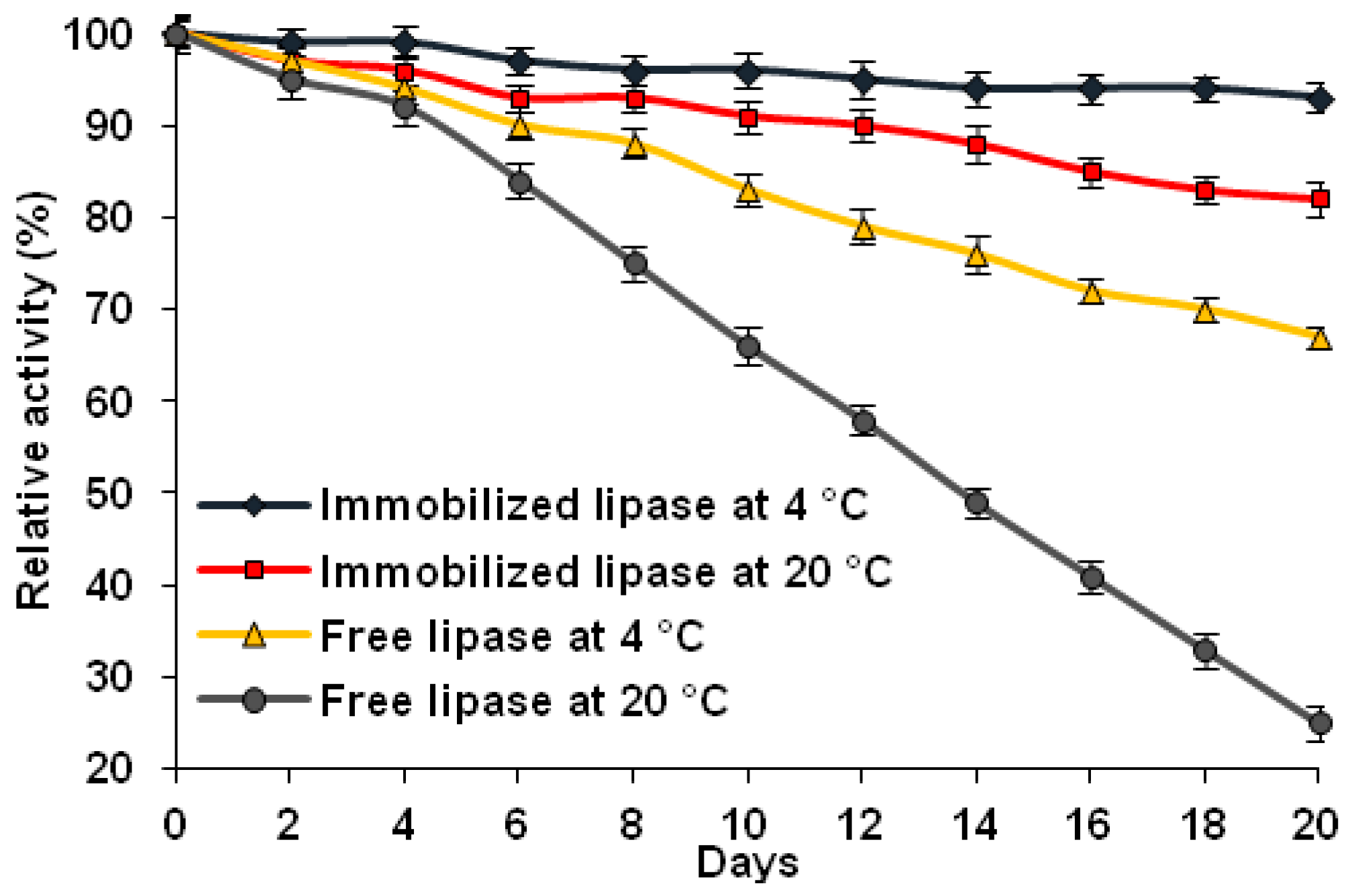
2.8.4. Storage Stability
2.9. Kinetic Parameters
3. Materials and Methods
3.1. Materials
3.2. Matrix Preparation and Lipase Immobilization
3.3. Analysis of the Products Following Immobilization
3.4. Hydrolytic Activity
3.4.1. Effect of pH
3.4.2. Thermal Stability
3.4.3. Reusability
3.4.4. Storage Stability
3.5. Kinetic Parameters
3.6. Desorption of the Immobilized CALB
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Manzo, R.M.; de Sousa, M.; Fenoglio, C.L.; Barro Goncalves, L.R.; Mammarella, E.J. Chemical improvement of chitosan-modified beads for the immobilization of Enterococcus faecium DBFIQ E36 l-arabinose isomerase through multipoint covalent attachment approach. J. Ind. Microbiol. Biotechnol. 2015, 42, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzymes immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties—Application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Khoobi, M.; Motevalizadeh, S.F.; Asadgol, Z.; Forootanfar, H.; Shafiee, A.; Faramarzi, M.A. Synthesis of functionalized polyethylenimine-grafted mesoporous silica spheres and the effect of side arms on lipase immobilization and application. Biochem. Eng. J. 2014, 88, 131–141. [Google Scholar] [CrossRef]
- Lima, L.N.; Oliveira, G.C.; Rojas, M.J.; de Castro, H.F.; Da Ros, P.C.M.; Mendes, A.A.; Giordano, R.L.C.; Tardioli, P.W. Immobilization of Pseudomonas fluorescens lipase on hydrophobic supports and application in biodiesel synthesis by transesterification of vegetable oils in solvent-free systems. J. Ind. Microbiol. Biotechnol. 2015, 42, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Zniszczol, A.; Herman, A.P.; Szymanska, K.; Mrowiec-Bialon, J.; Walczak, K.Z.; Jarzebski, A.; Boncel, S. Covalently immobilized lipase on aminoalkyl-, carboxy- andhydroxy-multi-wall carbon nanotubes in the enantioselectivesynthesis of Solketal esters. Enzyme Microb. Technol. 2016, 87, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Mendes, A.A.; de Castro, H.F.; Rodrigues, D.S.; Adriano, W.S.; Tardioli, P.W.; Mammarella, E.J.; Giordano, R.C.; Giordano, R.L.C. Multipoint covalent immobilization of lipase on chitosan hybrid hydrogels: Influence of the polyelectrolyte complex type and chemical modification on the catalytic properties of the biocatalysts. J. Ind. Microbiol. Biotechnol. 2011, 38, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; De Oliveira, D. Nanomaterials for biocatalyst immobilization-state of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisen, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Manoela, E.A.; dos Santos, J.C.S.; Freired, D.M.G.; Ruedae, N.; Fernández-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, A.; Teke, M.; Onal, S.; Telefoncu, A. Immobilization of pancreatic lipase on chitin and chitosan. Prep. Biochem. Biotechnol. 2006, 36, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.M.; Pereira, E.B.; de Castro, H.F. Immobilization of lipase on chitin and its use in nonconventional biocatalysis. Biomacromolecules 2004, 5, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rattanaphra, D.; Srinophakun, P. Biodiesel production from crude sunflower oil and crude jatropha oil using immobilized lipase. J. Chem. Eng. Jpn. 2010, 43, 104–108. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, J.Q.; Xu, Z.K. Immobilization of lipase from Candida rugosa on electrospun polysulfone nanofibrous membranes by adsorption. J. Mol. Catal. B: Enzym. 2006, 42, 45–51. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, W.; Jin, L.; Zha, J.; Tao, T.; Lin, Y.; Wang, Z. Synthesis of amine-functionalized Fe3O4@C nanoparticles for lipase immobilization. J. Mater. Chem. A 2014, 2, 18339–18344. [Google Scholar] [CrossRef]
- Hou, C.; Zhou, L.; Zhu, H.; Wang, X.; Hu, N.; Zeng, F.; Wang, L.; Yin, H. Mussel-inspired surface modification of magnetic@graphite nanosheets composite for efficient Candida rugosa lipase immobilization. J. Ind. Microbiol. Biotechnol. 2015, 42, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Le, D.M.; Sorensen, H.R.; Knudsen, N.O.; Meyer, A.S. Implications of silica on biorefineries—Interactions with organic material and mineral elements in grasses. Biofuels Bioprod. Biorefin. 2015, 9, 109–121. [Google Scholar] [CrossRef]
- Szymanska, K.; Odrozek, K.; Zniszczol, A.; Torrelo, G.; Resch, V.; Hanefeld, U.; Jarzebski, A.B. MsAcT in siliceous monolithic microreactors enables quantitative ester synthesis in water. Catal. Sci. Technol. 2016, 6, 4882–4888. [Google Scholar] [CrossRef]
- Szymanska, K.; Pietrowska, M.; Kocurek, J.; Maresz, K.; Koreniuk, A.; Mrowiec-Białon, J.; Widlak, P.; Magner, E.; Jarzebski, A. Low back-pressure hierarchically structured multichannel microfluidic bioreactors for rapid protein digestion—Proof of concept. Chem. Eng. J. 2016, 287, 148–154. [Google Scholar] [CrossRef]
- Yu, W.H.; Tong, D.S.; Fang, M.; Shao, P.; Zhou, C.H. Cloning, expression and enzymatic characterization of an aldo-keto reductase from Candida albicans XP1463. J. Mol. Catal. B: Enzym. 2015, 111, 43–50. [Google Scholar] [CrossRef]
- Zou, B.; Hu, Y.; Yu, D.; Jiang, L.; Liu, W.; Song, P. Functionalized ionic liquid modified mesoporous silica SBA-15: A novel, designable and efficient carrier for porcine pancreas lipase. Colloids Surf. B: Biointerfaces 2011, 88, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, Y.; Diao, X.; Luo, G.; Dai, Y. Effect of pore diameter and cross-linking method on the immobilization efficiency of Candida rugosa lipase in SBA-15. Bioresour. Technol. 2010, 101, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2015, 2, 1072–1092. [Google Scholar] [CrossRef]
- Zdarta, J.; Klapiszewski, L.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Ehrlich, H.; Maciejewski, H.; Stelling, A.L.; Jesionowski, T. Chitin-lignin material as a novel matrix for enzyme immobilization. Mar. Drugs 2015, 13, 2424–2446. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Nowacka, M.; Milczarek, G.; Jesionowski, T. Physicochemical and electrokinetic properties of silica/lignin biocomposites. Carbohydr. Polym. 2013, 94, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Klapiszewski, Ł.; Milczarek, G. Kraft lignin and silica as precursors of advanced composite materials and electroactive blends. J. Mater. Sci. 2014, 49, 1376–1385. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 sol–gel coated PVDF membrane. J. Membr. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Zdarta, J.; Sałek, K.; Kołodziejczak-Radzimska, A.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Norman, M.; Klapiszewski, Ł.; Bartczak, P.; Kaczorek, E.; Jesionowski, T. Immobilization of Amano Lipase A onto Stöber silica surface: Process characterization and kinetic studies. Open Chem. 2015, 13, 138–148. [Google Scholar] [CrossRef]
- Souza, R.L.; Resende, W.C.; Barao, C.E.; Zanin, G.M.; de Castro, H.F.; Santos, O.A.A.; Fricks, A.T.; Figueiredo, R.T.; Lima, Á.S.; Soares, C.M.F. Influence of the use of Aliquat 336 in the immobilization procedure in sol-gel of lipase from Bacillus sp. ITP-001. J. Mol. Catal. B: Enzym. 2012, 84, 152–159. [Google Scholar] [CrossRef]
- Gao, Z.; Zhan, W.; Guo, Y.; Wang, Y.; Guo, Y.; Lu, G. Aldehyde propyl-functionalized mesostructured cellular foams: Efficient supports for immobilization of penicillin G acylase. J. Mol. Catal. B: Enzym. 2014, 105, 111–117. [Google Scholar] [CrossRef]
- Wong, P.T.T.; Nong, R.K.; Caputo, T.A.; Godwin, T.A.; Rigas, B. Infrared spectroscopy of exfoliated human cervical cells: Evidence of extensive structural changes during carcinogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 10988–10992. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, M.; Della Ventura, B.; Mita, D.G.; Manolova, N.; Stoilova, O.; Rashkov, I.; Lepore, M. FT-IR microscopy characterization of sol–gel layers prior and after glucose oxidase immobilization for biosensing applications. J. Sol-Gel Sci. Technol. 2011, 57, 204–211. [Google Scholar] [CrossRef]
- Jesionowski, T.; Krysztafkiewicz, A. Comparison of the techniques used to modify amorphous hydrated silicas. J. Non-Cryst. Solids 2000, 277, 45–57. [Google Scholar] [CrossRef]
- Tomizuka, N.; Ota, Y.; Yamada, K. Lipase from Candida cylindracea II. Amino acid composition, carbohydrate component, and some physical properties. Agric. Biol. Chem. 1966, 30, 1090–1096. [Google Scholar]
- Klapiszewski, Ł.; Bartczak, P.; Wysokowski, M.; Jankowska, M.; Kabat, K.; Jesionowski, T. Silica conjugated with kraft lignin and its use as a novel ‘green’ sorbent for hazardous metal ions removal. Chem. Eng. J. 2015, 260, 684–693. [Google Scholar] [CrossRef]
- Ghosh, T.; Sarkar, P.; Turner, A.P.F. A novel third generation uric acid biosensor using uricase electro-activated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry 2015, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeyapragasam, T.; Saraswathi, R. Electrochemical biosensing of carbofuran based on acetylcholinesterase immobilized onto iron oxide–chitosan nanocomposite. Sens. Actuators B: Chem. 2014, 191, 681–687. [Google Scholar] [CrossRef]
- Wysokowski, M.; Klapiszewski, Ł.; Moszyński, D.; Bartczak, P.; Szatkowski, T.; Majchrzak, I.; Siwińska-Stefańska, K.; Bazhenov, V.V.; Jesionowski, T. Modification of chitin with kraft lignin and development of new biosorbents for removal of cadmium(II) and nickel(II) ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.S.; Luca, A.C.; Pelendritis, M.; Terenghi, G.; Downes, S.; Schroeder, S.L.M. Qantitative analysis of complex amino acids and RGD peptides by X-ray photoelectron spectroscopy (XPS). Surf. Interface Anal. 2013, 45, 1238–1246. [Google Scholar] [CrossRef]
- Peirce, S.; Tacias-Pascacio, V.G.; Russo, M.E.; Marzocchella, A.; Virgen-Ortiz, J.J.; Fernandez-Lafuente, R. Stabilization of Candida antarctica lipase B (CALB) immobilized on octyl agarose by treatment with polyethyleneimine (PEI). Molecules 2016, 21, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, A. Lipase protein engineering. Biochim. Biophys. Acta 2000, 1543, 223–238. [Google Scholar] [CrossRef]
- Milczarek, G.; Ignatas, O. Renewable cathode materials from biopolymer/conjugated polymer interpenetrating networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Klapiszewski, L.; Milczarek, G. Structural and electrochemical properties of multifunctional silica/lignin materials. Mater. Chem. Phys. 2014, 147, 1049–1057. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Yang, J.; Hu, Y. Preparation of lignin-silica hybrids and its application in intumescent flame-retardant poly(lactic acid) system. High Perform. Polym. 2012, 24, 738–745. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernandez-Lorente, G.; Pedroche, J.; Fernandez-Lafuente, R.; Guisan, J.M. Epoxy sepabeads: A novel epoxy support for stabilization of industrial enzymes via very intense multipoint covalent attachment. Biotechnol. Prog. 2002, 18, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Torres, R.; Fernandez-Lorente, G.; Ortiz, C.; Fuentes, M.; Hidalgo, A.; Lopez-Gallego, F.; Abian, O.; Palomo, J.M.; Betancor, L.; et al. Epoxy-amino groups: A new tool for improved immobilization of proteins by the epoxy method. Biomacromolecules 2003, 4, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how? Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.; Patwardhan, S.V. Controlling performance of lipase immobilised on bioinspired silica. J. Mater. Chem. B 2013, 1, 1164–1174. [Google Scholar] [CrossRef]
- Emregul, E.; Sungur, S.; Akbulut, U. Polyacrylamide-gelatine carrier system used for invertase immobilization. Food Chem. 2006, 97, 591–597. [Google Scholar] [CrossRef]
- Dong, L.; Ge, C.; Qin, P.; Chen, Y.; Xu, Q. Immobilization and catalytic properties of Candida lipolytic lipase on surface of organic intercalated and modified MgAl-LDHs. Solid State Sci. 2014, 31, 8–15. [Google Scholar] [CrossRef]
- Xie, W.; Wang, J. Enzymatic production of biodiesel from soybean oil by using immobilized lipase on Fe3O4/poly(styrene-methacrylic acid) magnetic microsphere as a biocatalyst. Energy Fuels 2014, 28, 2624–2631. [Google Scholar] [CrossRef]
- Tutarb, H.; Yilmaza, E.; Pehlivan, E.; Yilmaz, M. Immobilization of Candida rugosa lipase on sporopollenin from Lycopodium clavatum. Int. J. Biol. Macromol. 2009, 4, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, M.A. Immobilization of Aspergillus niger NRC 107 xylanase and beta-xylosidase, and properties of the immobilized enzymes. Appl. Biochem. Biotechnol. 1993, 38, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Narwal, S.K.; Saun, N.K.; Gupta, R. Characterization and catalytic properties of free and silica-bound lipase: A comparative study. J. Oleo Sci. 2014, 63, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Rouf, A.; Shahb, B.A.; Mahajana, N.; Chaubey, A.; Tanejaba, S.C. Arthrobacter sp. lipase catalyzed kinetic resolution of BINOL: The effect of substrate immobilization. J. Mol. Catal. B: Enzym. 2014, 101, 35–39. [Google Scholar] [CrossRef]
- Zivkovic, L.T.I.; Zivkovic, L.S.; Babic, B.M.; Kokunesoski, M.J.; Jokic, B.M.; Karadzic, I.M. Immobilization of Candida rugosa lipase by adsorption onto biosafe meso/macroporous silica and zirconia. Biochem. Eng. J. 2015, 93, 73–83. [Google Scholar] [CrossRef]
- Ho, L.J.; Lee, D.H.; Lim, J.S.; Um, B.H.; Park, C.; Kang, S.W.; Kim, S.W. Optimization of the process for biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. J. Microbiol. Biotechnol. 2008, 18, 1927–1931. [Google Scholar]
- Chen, Y.; Liu, J.; Xia, C.; Zhao, C.; Ren, Z.; Zhang, W. Immobilization of lipase on porous monodisperse chitosan microspheres. Biotechnol. Appl. Biochem. 2014, 62, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Ren, X.Y.; Liu, Y.M.; Wei, Y.; Qing, L.S.; Liao, X. Covalent immobilization of porcine pancreatic lipase on carboxyl-activated magnetic nanoparticles: Characterization and application for enzymatic inhibition assays. Mater. Sci. Eng.: C 2014, 38, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Jiang, X.P.; Li, Y.; Zeng, S.; Zhang, Y.W. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Krysztafkiewicz, A.; Rager, B.; Jesionowski, T. The effect of surface modification on physicochemical properties of precipitated silica. J. Mater. Sci. 1997, 32, 1333–1339. [Google Scholar] [CrossRef]
- Jesionowski, T.; Ciesielczyk, F.; Krysztafkiewicz, A. Influence of selected alkoxysilanes on dispersive properties and surface chemistry of spherical silica precipitated in emulsion media. Mater. Chem. Phys. 2010, 119, 65–74. [Google Scholar] [CrossRef]
- Klapiszewski, L.; Zdarta, J.; Szatkowski, T.; Wysokowski, M.; Nowacka, M.; Szwarc-Rzepka, K.; Bartczak, P.; Siwińska-Stefańska, K.; Ehrlich, H.; Jesionowski, T. Silica/lignosulfonate hybrid materials: Preparation and characterization. Cent. Eur. J. Chem. 2014, 12, 719–735. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]




| Immobilization Time | Enzyme Solution Concentration (mg/cm3) | Porous Structure Characterization | ||
|---|---|---|---|---|
| BET Surface Area (m2/g) | Total Volume of Pores (cm3/g) | Mean Size of Pores (nm) | ||
| Silica | 262 | 0.12 | 3.84 | |
| Silica-lignin matrix | 194 | 0.09 | 2.72 | |
| 1 min | 0.5 | 161 | 0.08 | 2.46 |
| 1 h | 157 | 0.07 | 2.41 | |
| 24 h | 155 | 0.07 | 2.21 | |
| 1 min | 1.0 | 159 | 0.07 | 2.42 |
| 1 h | 152 | 0.06 | 2.23 | |
| 24 h | 147 | 0.05 | 2.14 | |
| 1 min | 3.0 | 147 | 0.05 | 2.39 |
| 1 h | 145 | 0.05 | 2.26 | |
| 24 h | 140 | 0.05 | 2.09 | |
| Immobilization Time | Enzyme Solution Concentration (mg/cm3) | Elemental Content (%) | |||
|---|---|---|---|---|---|
| C | H | N | S | ||
| Silica | 0.18 | 0.78 | - | - | |
| Silica-lignin matrix | 3.91 | 0.91 | 0.14 | 0.12 | |
| 1 min | 0.5 | 4.28 | 1.05 | 0.18 | 0.11 |
| 1 h | 4.47 | 1.07 | 0.20 | 0.13 | |
| 24 h | 5.12 | 1.14 | 0.22 | 0.11 | |
| 1 min | 1.0 | 4.57 | 1.10 | 0.18 | 0.10 |
| 1 h | 4.87 | 1.20 | 0.19 | 0.11 | |
| 24 h | 5.34 | 1.34 | 0.23 | 0.13 | |
| 1 min | 3.0 | 5.35 | 1.23 | 0.39 | 0.12 |
| 1 h | 5.89 | 1.34 | 0.40 | 0.12 | |
| 24 h | 6.23 | 1.46 | 0.41 | 0.11 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdarta, J.; Klapiszewski, L.; Jedrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida antarctica Immobilized on a Silica-Lignin Matrix as a Stable and Reusable Biocatalytic System. Catalysts 2017, 7, 14. https://doi.org/10.3390/catal7010014
Zdarta J, Klapiszewski L, Jedrzak A, Nowicki M, Moszynski D, Jesionowski T. Lipase B from Candida antarctica Immobilized on a Silica-Lignin Matrix as a Stable and Reusable Biocatalytic System. Catalysts. 2017; 7(1):14. https://doi.org/10.3390/catal7010014
Chicago/Turabian StyleZdarta, Jakub, Lukasz Klapiszewski, Artur Jedrzak, Marek Nowicki, Dariusz Moszynski, and Teofil Jesionowski. 2017. "Lipase B from Candida antarctica Immobilized on a Silica-Lignin Matrix as a Stable and Reusable Biocatalytic System" Catalysts 7, no. 1: 14. https://doi.org/10.3390/catal7010014