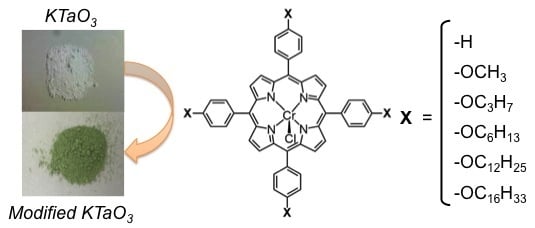
Effect of Porphyrin Molecular Structure on Water Splitting Activity of a KTaO3 Photocatalyst
Abstract
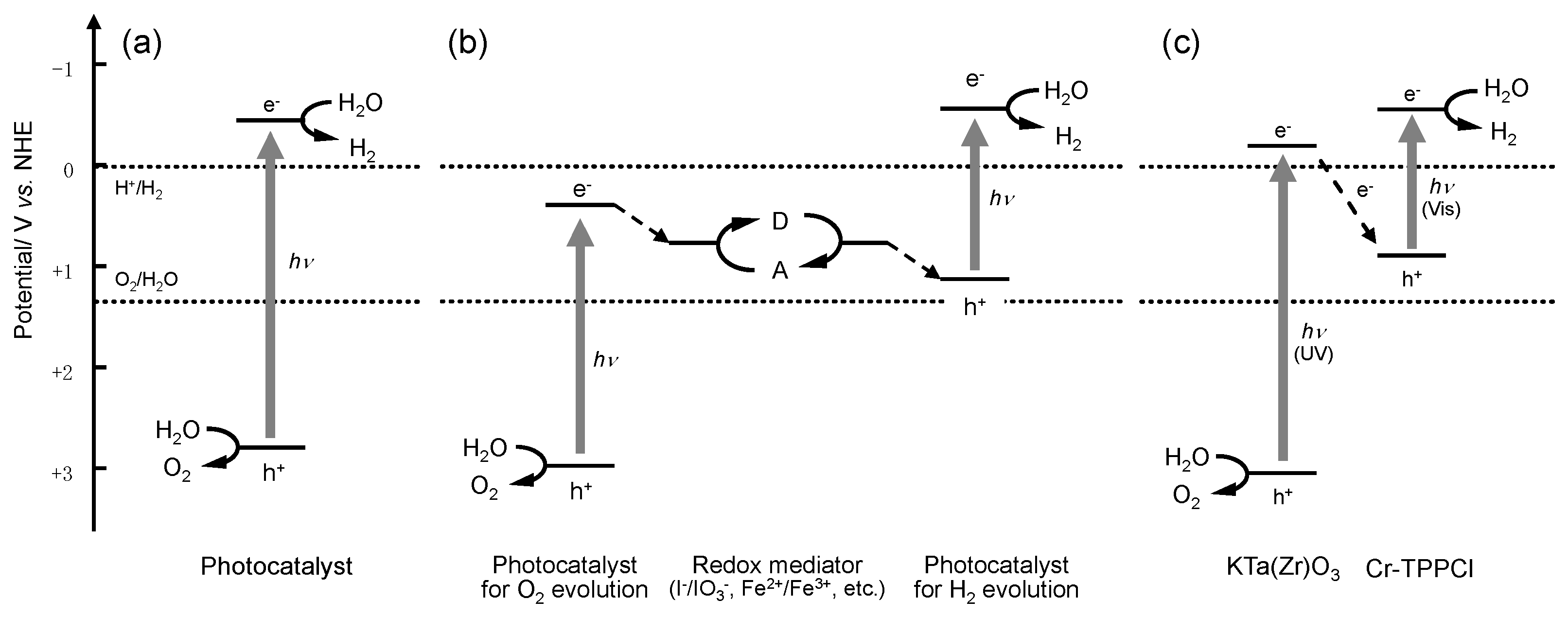
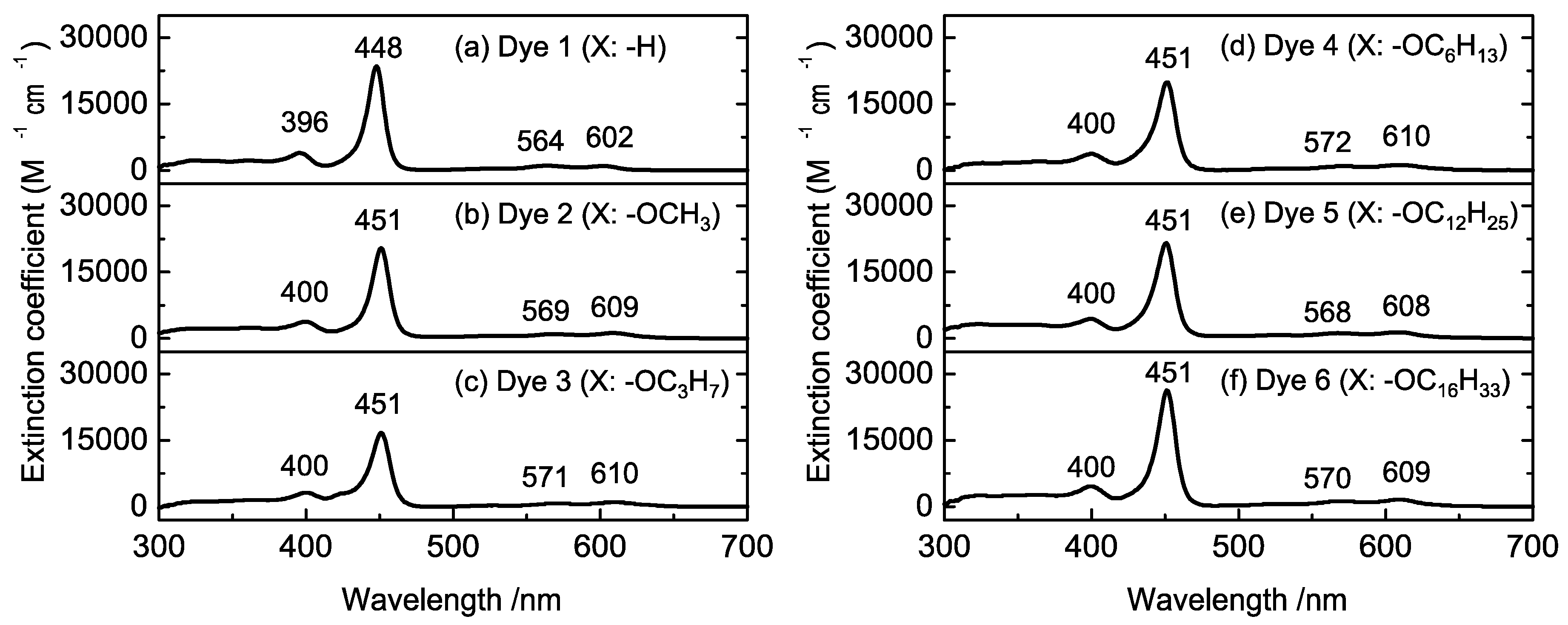
:1. Introduction
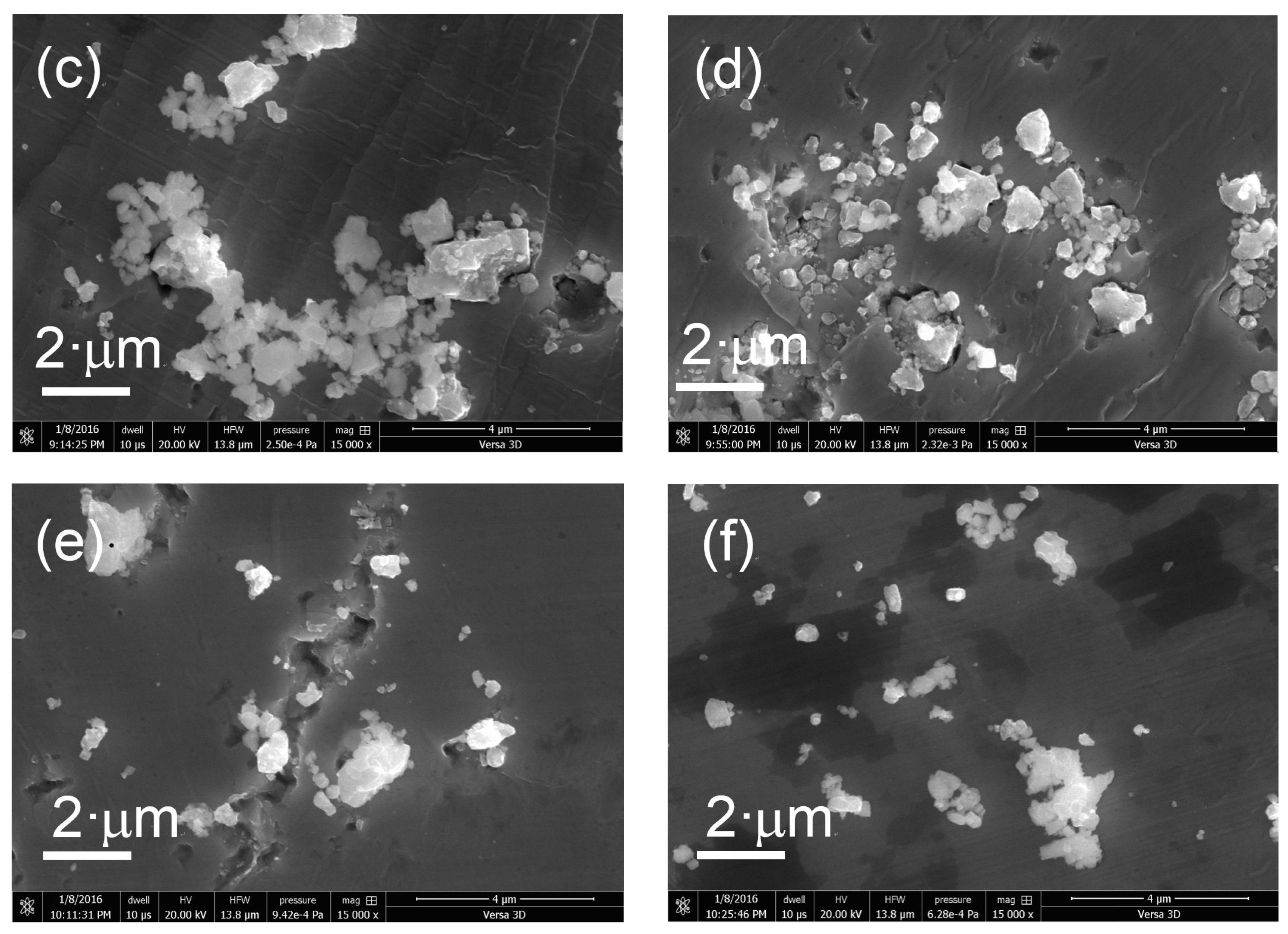
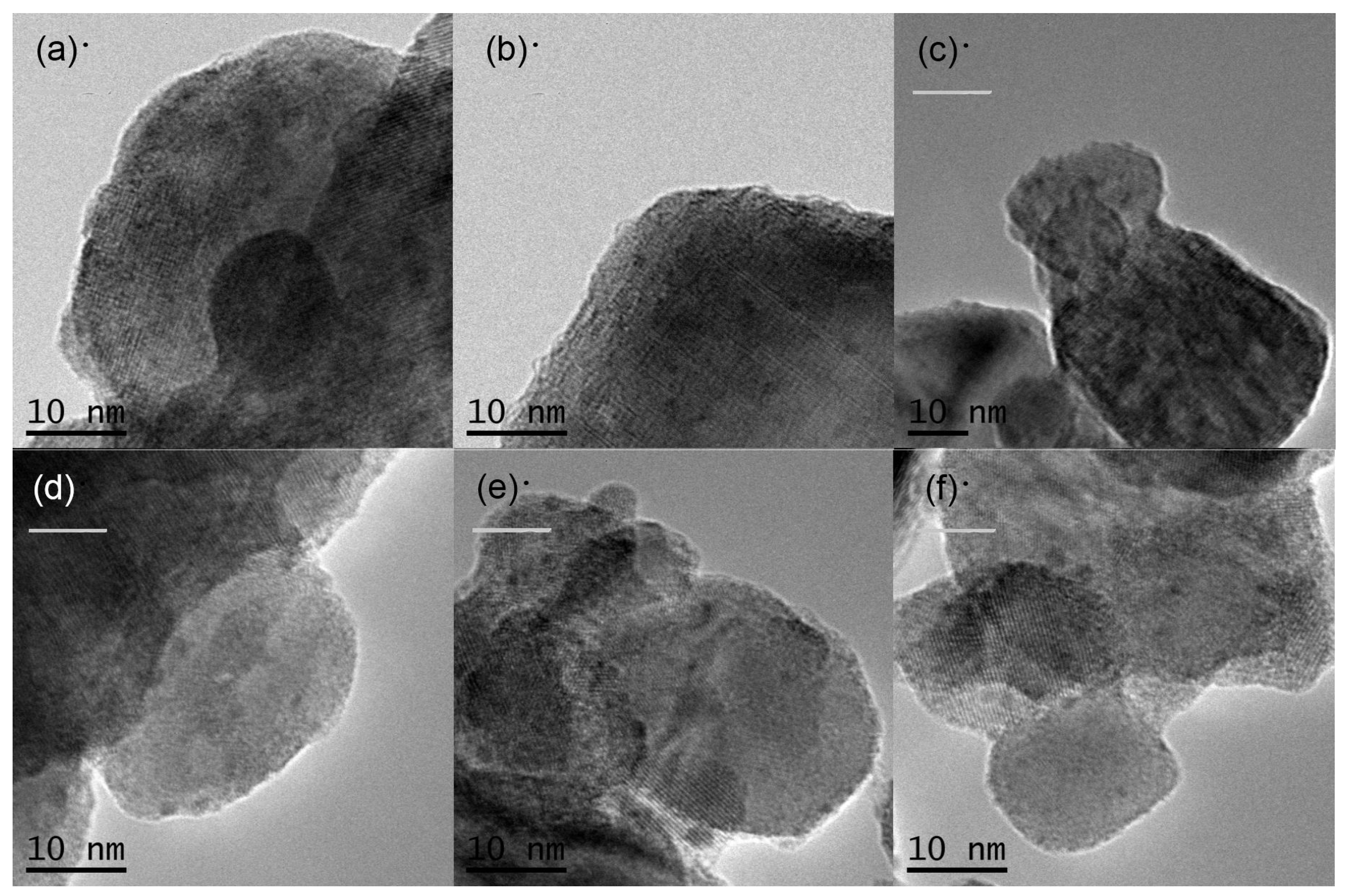
2. Results and Discussion
3. Materials and Methods
3.1. Photocatalyst Preparation
3.1.1. Zr-doped KTaO3 Preparation
3.1.2. Porphyrin Dye Synthesis
3.1.3. Preparation of Porphyrin-Modified KTa(Zr)O3 Photocatalysts
3.2. Photocatalytic Water Splitting Reaction
3.3. Photocatalyst Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NADP+ | Nicotinamide adenine dinucleotide phosphate (oxidized form) |
| TEM | Transmission electron microscopy |
| NMR | Nuclear Magnetic Resonance |
| CSI-MS | Cold-spray ionization mass spectroscopy |
| FIB-SEM | focused ion beam-scanning electron microscopy |
| DMF | N,N-dimethylformamide |
| SEM | Scanning electron microscopy |
References
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- May, M.M.; Lewerenz, H.-J.; Lackner, D.; Dimroth, F.; Hannappel, T. Efficient direct solar-to-hydrogen conversion by in situ interface transformation of a tandem structure. Nat. Commun. 2015, 6, 8286. [Google Scholar] [CrossRef] [PubMed]
- Peltier, G.; Tolleter, D.; Billon, E.; Cournac, L. Auxiliary electron transport pathways in chloroplasts of microalgae. Photosynth. Res. 2010, 106, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small 2015, 16, 5262–5271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.; Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar]
- Kato, H.; Hori, M.; Konta, R.; Shimodaira, Y.; Kudo, A. Construction of Z-scheme type heterogeneous photocatalysis systems for water splitting into H2 and O2 under visible light irradiation. Chem. Lett. 2004, 33, 1348–1349. [Google Scholar] [CrossRef]
- Sasaki, Y.; Iwase, A.; Kato, H.; Kudo, A. The effect of co-catalyst for Z-scheme photocatalysis systems with an Fe3+/Fe2+ electron mediator on overall water splitting under visible light irradiation. J. Catal. 2008, 259, 133–137. [Google Scholar] [CrossRef]
- Abe, R.; Takata, T.; Sugihara, H.; Domen, K. Photocatalytic overall water splitting under visible light by TaON and WO3 with an IO3−/I−shuttle redox mediator. Chem. Commun. 2005, 3829–3831. [Google Scholar] [CrossRef] [PubMed]
- Higashi, M.; Abe, R.; Ishikawa, A.; Takata, T.; Ohtani, B.; Domen, K. Z-scheme overall water splitting on modified-TaON photocatalysts under visible light (λ < 500 nm). Chem. Lett. 2008, 37, 138–139. [Google Scholar]
- Tabata, M.; Maeda, K.; Higashi, M.; Lu, D.; Takata, T.; Abe, R.; Domen, K. Modified Ta3N5 powder as a photocatalyst for O2 evolution in a two-step water splitting system with an iodate/iodide shuttle redox mediator under visible light. Langmuir 2010, 26, 9161–9165. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Abe, R.; Domen, K. Role and function of ruthenium species as promoters with TaON-based photocatalysts for oxygen evolution in two-step water splitting under visible light. J. Phys. Chem. C 2011, 115, 3057–3564. [Google Scholar] [CrossRef]
- Matoba, T.; Maeda, K.; Domen, K. Activation of BaTaO2N photocatalyst for enhanced non-sacrificial hydrogen evolution from water under visible light by forming a solid solution with BaZrO3. Chem. Eur. J. 2011, 17, 14731–14735. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Lu, D.; Domen, K. Solar-Driven Z-scheme water splitting using modified BaZrO3–BaTaO2N solid solutions as photocatalysts. ACS Catal. 2013, 3, 1026–1033. [Google Scholar] [CrossRef]
- Ma, S.S.K.; Maeda, K.; Hisatomi, T.; Tabata, M.; Kudo, A.; Domen, K. A redox-mediator-free solar-driven Z-Scheme water-splitting system consisting of modified Ta3N5 as an oxygen-evolution photocatalyst. Chem. Eur. J. 2013, 19, 7480–7486. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Inoue, T.; Ida, S.; Ishihara, T. Long-time charge separation in porphyrin/KTa(Zr)O3 as water splitting photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 18031–18037. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Inoue, T.; Kaneko, K. Ishihara, Charge-transfer mechanism in Pt/KTa(Zr)O3 photocatalysts modified with porphyrinoids for water splitting. Chem. Eur. J. 2009, 15, 12862–12870. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Ono, N.; Inoue, T.; Matsumoto, H.; Ishihara, T. Dye-sensitizer effects on a Pt/KTa(Zr)O3 catalyst for the photocatalytic splitting of water. Angew. Chem. Int. Ed. 2006, 45, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Nagatomo, M.; Seto, C.; Ida, S.; Ishihara, T. Dye-modification effects on water splitting activity of GaN:ZnO photocatalyst. J. Photochem. Photobio. A 2013, 272, 41–48. [Google Scholar] [CrossRef]
- Hagiwara, H.; Nagatomo, M.; Seto, C.; Ida, S.; Ishihara, T. Dye modification effects on TaON for photocatalytic hydrogen production from water. Catalysts 2013, 3, 614–624. [Google Scholar] [CrossRef]
- Rutherford, A.W.; Faller, P. Photosystem II: Evolutionary perspectives. Phil. Trans. R. Soc. Lond. B 2003, 358, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-aggregates: From serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chem. Int. Ed. 2011, 50, 3376. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Fukada, K.; Wakami, H.; Nishiyabu, R.; Pasternack, R.F. Factors influencing self-aggregation tendencies of cationic porphyrins in aqueous solution. J. Am. Chem. Soc. 2000, 122, 7494–7502. [Google Scholar] [CrossRef]
- Král, V.; Schmidtchem, F.P.; Lang, K.; Berger, M. Anion-controlled assembly of porphyrin-bicyclic guanidine conjugates. Org. Lett. 2002, 1, 51–54. [Google Scholar] [CrossRef]
- Miyajima, N.; Furuta, N.; Wakunami, S.; Mizutani, T. Functionalization of silica surface by tetrahydroxyporphyrin via Si-O linkages. Bull. Chem. Soc. Jpn. 2011, 84, 794–801. [Google Scholar]
- Chen, A.P.; Chisholm, M.H.; Gallucci, J.C.; Zhang, X.; Zhou, Z. Binding of propylene oxide to porphyrin- and Salen-M(III) cations, where M = Al, Ga, Cr, and Co. Inorg. Chem. 2005, 44, 2588–2595. [Google Scholar] [CrossRef] [PubMed]




| Dye | X | Amount of dye/μmol | Gas Formation Rate/μmol h−1 | |
|---|---|---|---|---|
| H2 | O2 | |||
| 1 | −H | 0.57 | 40.0 | 22.7 |
| 2 | −OCH3 | 0.49 | 53.7 | 29.4 |
| 3 | −OC3H7 | 0.43 | 26.8 | 12.8 |
| 4 | −OC6H13 | 0.36 | 30.3 | 14.9 |
| 5 | −OC12H25 | 0.28 | 34.7 | 19.1 |
| 6 | −OC16H33 | 0.24 | 43.1 | 23.9 |
| None | - | 0.0 | 7.8 | 4.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagiwara, H.; Higashi, K.; Watanabe, M.; Kakigi, R.; Ida, S.; Ishihara, T. Effect of Porphyrin Molecular Structure on Water Splitting Activity of a KTaO3 Photocatalyst. Catalysts 2016, 6, 42. https://doi.org/10.3390/catal6030042
Hagiwara H, Higashi K, Watanabe M, Kakigi R, Ida S, Ishihara T. Effect of Porphyrin Molecular Structure on Water Splitting Activity of a KTaO3 Photocatalyst. Catalysts. 2016; 6(3):42. https://doi.org/10.3390/catal6030042
Chicago/Turabian StyleHagiwara, Hidehisa, Kohei Higashi, Motonori Watanabe, Ryota Kakigi, Shintaro Ida, and Tatsumi Ishihara. 2016. "Effect of Porphyrin Molecular Structure on Water Splitting Activity of a KTaO3 Photocatalyst" Catalysts 6, no. 3: 42. https://doi.org/10.3390/catal6030042