1. Introduction
The increase in energy consumption and the effects of global climate change on the environment has driven the effort of the scientific community towards the energetic emancipation from fossil fuels. Indeed, the promotion of new, energy-efficient processes to produce fuels and chemicals from more sustainable sources are under constant investigation. For this reason biomass is seen as a potential source of energy and, more specifically, transportation fuels [
1].
Since biomass is over-functionalized it requires selective oxygen removal reactions (e.g., hydrogenation, hydrogenolysis, dehydration, decarboxylation) to obtain platform chemicals [
2,
3,
4]. Etherification is an important reaction for the production of biofuels, as it reduces the amount of hygroscopic alcohol groups, and increases both the energy content and cetane number [
5,
6]. In contrast, the petrochemical industry employs catalytic processes to introduce oxygen in hydrocarbons [
7]. Advanced tools such as high-throughput screening and conceptual process development techniques, advanced data mining and computational chemistry can play a pivotal role in quickly finding economically attractive catalysts and/or process conditions [
8,
9,
10].
Hoydonxcs
et al. [
11] have given an excellent overview of the current industrial production and application of those products. The most relevant of these is furfuryl alcohol, which has its application in foundry resins. Other specialty chemicals are furoic acid, tetrahydrofurfuryl alcohol and 2-methyl furan. Furfural production is a widely established and long existing industry. In fact, the oldest furfural process uses sulfuric acid to convert oat hulls was started in 1921 in Iowa (United States) by the Quaker Oats Company. Today, corncobs and bagasse are the major feedstock for furfural production [
11,
12]. Furfural is also considered an excellent platform molecule to produce biofuels [
13,
14]. The production of furfuryl ethers is part of Avantium’s YXY process, which deals with the catalytic conversion of plant based materials into bio-based chemicals and bio-plastics [
15].
The current state of the art route for the production of furfuryl ethers is a two-step process. Furfural is first hydrogenated to furfuryl alcohol. This step is followed by a classical Williamson’s reaction, involving an alkali-metal salt of the hydroxyl compound and an alkyl halide [
16]. Especially this last step is hazardous and quite expensive. In this work, the focus is to develop a sustainable route to 2-metoxymethylfuran (furfuryl methylether, FME) by reductive etherification of furfural (
Scheme 1) and identify the preferable class of heterogeneous catalysts selective in the one pot process. The direct reductive etherification of furfural has not been described in literature, but Bethmont
et al. [
17,
18] have reported this route for several aldehydes to ethers in the presence of hydrogen, using catalysts from the platinum group. Various studies have also reported on the catalytic reductive etherification of chemicals using homogeneous catalysts [
19,
20,
21].
Scheme 1.
The direct reductive etherification of furfural to FME.
Scheme 1.
The direct reductive etherification of furfural to FME.
Very few reports refer to the use of heterogeneous catalysts for furanic compounds. Balakrishnan
et al. [
22] used Pt and Pt/Sn supported on alumina catalysts for the etherification and reductive etherification of 5-hydroxymethyl furfural (HMF). Promising yields up to 60% were reported, but a wider catalyst screening was missing. In order to obtain more knowledge of the reductive etherification and preferably improve the yields, an in-depth catalyst screening is required. Modern advanced tools such as high-throughput screening offer significant potential in this direction [
8,
9,
10].
Given the complexity of the final reaction mixture, as side-products are also detected, two reaction pathways are considered (
Scheme 2). The first pathway consists of the formation of furfuryl alcohol (FA) as the intermediate. The FA then undergoes an acid-catalyzed nucleophilic attack by methanol to form the desired ether. This etherification reaction typically requires a strong acid; therefore, the presence of this kind of active site on the support of the catalyst is imperative. The second pathway consists of the formation of the furfural hemiacetal as intermediate, followed by the hydrogenolysis of the formed hemiacetal to obtain the desired ether. This pathway is similar to the pathway suggested by Bethmont
et al. [
18] for the synthesis of aliphatic ethers from aliphatic aldehydes and alcohols. The furfural acetal is also detected in the reaction mixture and is formed as a consequence of two equilibrium reaction steps (see
Scheme 2). It is important to note that the furfural acetal is not considered to be involved in the formation of FME. Bethmont
et al. [
18] demonstrated that the formation of the ether does not occur by direct hydrogenolysis of the ketal or acetal. Indeed, they postulated that the hemiketal or hemiacetal is dehydrated to the enol ether and the latter is subsequently hydrogenated to the desired ether.
Scheme 2.
The two proposed pathways for the reductive etherification of furfural.
Scheme 2.
The two proposed pathways for the reductive etherification of furfural.
Since no efficient catalyst for furfural has been described in literature, a wide range of hydrogenation catalysts was evaluated at several reaction conditions using a high-throughput screening approach. The reductive etherification of furfural to FME is carried out at 50 bar hydrogen pressure for 1 h at 80 to 120 °C. In order to ascertain that the best possible metal catalysts were used in the screening campaign, only commercially available cobalt, nickel, copper, platinum, palladium and iridium-based catalysts on various supports were tested. For each metal, the widest available selection of supports was selected between the commercially available catalysts. Carbon, alumina, silica, titania, calcium carbonate and diatomaceous earths were tested (see
Table 1).
2. Results and Discussion
The aim of this research is to identify the most promising family of metal catalysts and reaction conditions for the one-step production of 2-methoxymethylfuran (furfuryl methylether, FME) from furfural, thereby avoiding the synthetic efficiency loss that generally occurs in multistep processes [
23]. A high-throughput program screening 35 commercial catalysts at three temperatures and a fixed reaction time, using GC for analysis was conducted. From the reaction scheme in
Scheme 2 it can be argued that, in the single step reductive etherification of furfural to FME, a multifunctional catalyst is needed with both a hydrogenation and an acidic function. In particular, the acid centers require special attention as they can also catalyze polymerization processes of furfural and furfuryl alcohol [
12,
24], leading to fast deactivation of the catalyst.
It is clear that a fair comparison of the catalysts needs to be based on turn-over-frequencies per active metal (TOF). However, to calculate this TOF one needs to determine the available catalytic surface area or, less preferably, the amount of metal. Due to non-analysis agreements this was not possible for all catalysts. The catalysts were therefore compared using the furfural conversion rate, which was expressed in mmol/g metal/h (see
Table 1). Within the experimental design, nickel, palladium and platinum catalysts showed high conversion rates and variable yields in the desired FME. Copper and nickel catalysts also gave significant conversions, but minimal amounts of FME were observed. The cobalt molybdenum catalysts showed hardly any activity.
Table 1.
Supplier specification and conversion rate of furfural (mmol/g metal/h) for each catalyst tested in the reductive etherification of furfural. “Pre-red” indicates catalysts already present in a “pre-reduced” state.
Table 1.
Supplier specification and conversion rate of furfural (mmol/g metal/h) for each catalyst tested in the reductive etherification of furfural. “Pre-red” indicates catalysts already present in a “pre-reduced” state.
| No. | Catalyst | Supplier Name | Supplier ID | Furfural Conversion Rate (mmol/g metal/h) |
|---|
| 80 °C | 100 °C | 120 °C |
|---|
| 1 | Co/Mo on alumina | Criterion | DC200 | 470 | 870 | 1030 |
| 2 | CoO/MoO3 on alumina | Unicat | HT-75 | 400 | 570 | 770 |
| 3 | Cu-Zn on alumina | Engelhard | A-002 | 40 | 90 | 90 |
| 4 | Cu-ZnO on alumina | Unicat | MS-900 | 50 | 60 | 90 |
| 5 | CuCrOx | Engelhard | A-003 | 100 | 110 | 110 |
| 6 | Cu | Johnson Matthey | Pricat Cu 60/35T Copper oxide on an inert carrier | 50 | 110 | 100 |
| 7 | Cu | Johnson Matthey | 40/18 P | 100 | 170 | 190 |
| 8 | Cu-Cr-Ba | CRI KataLeuna | KL1970-T3 | 130 | 120 | 210 |
| 9 | Ir on active carbon | Degussa a | L 1082 BB/W 5% | 1380 | 1460 | 1720 |
| 10 | Ir on charcoal | Johnson Matthey | 5% Ir on charcoal | 1360 | 1500 | 940 |
| 11 | Ir on calcium carbonate (Pre-Red) | Alfa Aesar | 41305 | 1220 | 1460 | 1080 |
| 12 | Ir on calcium carbonate | Alfa Aesar | 41305 | 1240 | 1140 | 1420 |
| 13 | Ni on alumina | CRI KataLeuna | KL6562-TL1.2 | 70 | 100 | 230 |
| 14 | Ni on special alumina | CRI KataLeuna | KL6527-CY1.2 | 90 | 100 | 110 |
| 15 | Ni on silica | CRI KataLeuna | KL6503-T | 70 | 80 | 60 |
| 16 | Ni on special silica | CRI KataLeuna | KL6580 | 110 | 120 | 120 |
| 17 | Ni/MoO3 on silica-alumina | CRI KataLeuna | KL9514-CY | 690 | 800 | 860 |
| 18 | Nysofact 120 catalyst on inert support | Engelhard | 120 | 60 | 50 | 50 |
| 19 | Ni on diatomaceous earth | Nikki Chemical Co., Ltd. | NU1101 | 80 | 70 | 90 |
| 20 | Nickel catalyst | Engelhard | A201 | 50 | 70 | 80 |
| 21 | Pricat | Johnson Matthey | Pricat NI 55/5 P | 30 | 30 | 30 |
| 22 | Pricat | Johnson Matthey | Pricat NI 55/5 T (inert carrier) | 30 | 70 | 90 |
| 23 | HTC | Johnson Matthey | HTC NI 200 RPS 2.5 mm | 60 | 80 | 130 |
| 24 | NiMo catalyst | Unicat | HT-86 | 530 | 600 | 730 |
| 25 | Platinum on activated C | Degussa a | F 1002 RE/W 5% Pt | 1440 | 1560 | 1580 |
| 26 | Platinum on titania | Degussa a | n.a. b | 1080 | 980 | 1000 |
| 27 | Pt on alumina | Degussa a | F 1002 XKYA/W 5% | 880 | 580 | 520 |
| 28 | Pt(S) on carbon 5% Pt | Johnson Matthey | Pt/C Sulfided, 5% Pt Type B106032-5 | 460 | 380 | 440 |
| 29 | Pd on activated carbon | Degussa a | E 1002 XU/W 5% Pd | 1360 | 1300 | 1560 |
| 30 | Pd on CaCO3 powder | Degussa a | E 407 R/D 5% | 440 | 840 | 520 |
| 31 | Pd on alumina, JCAT001 (Pre-Red) | Johnson Matthey | JCAT001 | 1490 | 1100 | 1160 |
| 32 | Pd on alumina, JCAT001 | Johnson Matthey | JCAT001 | 160 | 160 | 240 |
| 33 | Palladium on graphite (Pre-Red) | Johnson Matthey | JCAT010 | 1780 | 1700 | 1630 |
| 34 | Palladium on graphite | Johnson Matthey | JCAT010 | 930 | 870 | 1330 |
| 35 | Pd on charcoal powder | Johnson Matthey | 5R87L | 1420 | 1450 | 1520 |
The Space Time Yield (STY) of 2-metoxymethylfuran for all catalysts tested is shown in
Figure 1. From
Figure 1 it is clear that only palladium, platinum and iridium give a significant STY to the desired FME ether. Palladium based catalysts appear to be the most promising in converting furfural to FME. With charcoal as support a STY of 1125 mmol/g supported metal/h at 100 °C was obtained. Among the platinum catalysts the best STY was also obtained with activated carbon as the support (305 mmol/g supported metal/h at 120 °C). The iridium catalysts gave the lowest STYs to FME, with a maximum value of 162 mmol/g supported metal/h, again with charcoal as the support.
Figure 1.
2-metoxymethyl furan (FME) Space Time Yield (mmol/g supported metal/h)
vs. catalyst ID number (see
Table 1). Different color marks were used in the figure for the six active metal catalysts: pink (cobalt), grey (iridium), black (palladium), light blue (copper), green (nickel), violet (platinum).
Figure 1.
2-metoxymethyl furan (FME) Space Time Yield (mmol/g supported metal/h)
vs. catalyst ID number (see
Table 1). Different color marks were used in the figure for the six active metal catalysts: pink (cobalt), grey (iridium), black (palladium), light blue (copper), green (nickel), violet (platinum).
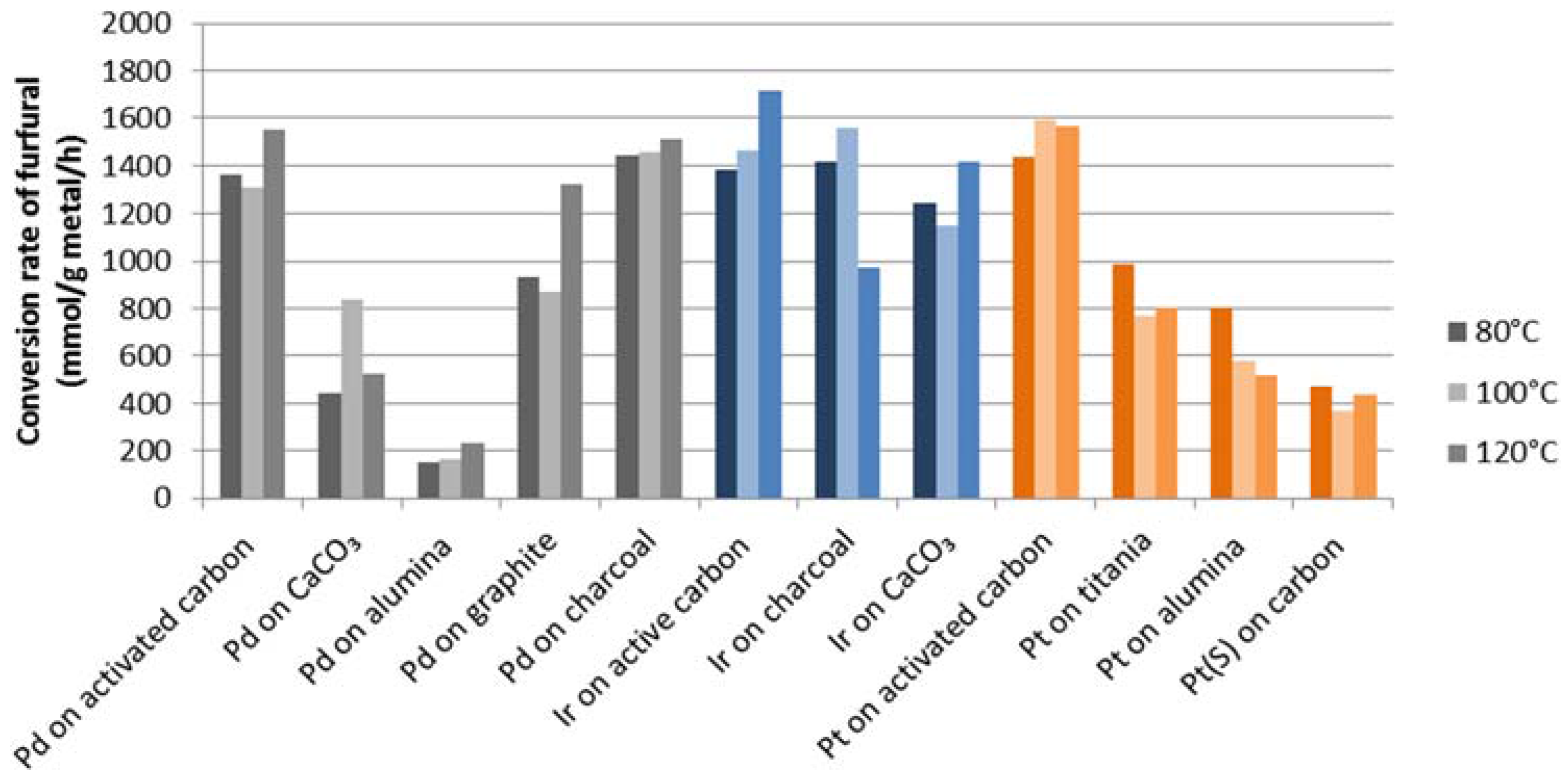
The variation in activity within one group of metal catalysts can be explained by a number of variables, such as metal loading, support, promoters and/or impurities present. This is inherent to the use of commercial materials from different sources, but using these ascertains that a good activity for the particular metal loading on a support is actually available. Despite the different metal loadings, the results reported in
Table 1 strongly indicate that the observed variations are due to specific metal-support interactions. To clarify this, the conversion of furfural for catalysts with 5%
w/
w metal loading is shown in
Figure 2. It can be clearly seen that palladium, platinum and iridium on carbonaceous supports, such as charcoal, graphite and activated carbon, showed the highest activity.
Figure 2.
Conversion rate of furfural (mmol/g metal/h) for noble metals at 5% w/w metal loading.
Figure 2.
Conversion rate of furfural (mmol/g metal/h) for noble metals at 5% w/w metal loading.
Figure 3 shows the Space Time Yield (STY) for 2-methoxymethylfuran for 5%
w/
w palladium, platinum and iridium on various supports. It can be seen that among all carbon supported catalysts, palladium on charcoal had the best performance, both in terms yield and activity. With this catalyst up to 1125 mmol/g metal/h of FME was produced at 100 °C while the furfural conversion rate reached 1450 mmol/g metal/h. Palladium on graphite also showed good results, producing 800 mmol/g metal/h of FME at 120 °C with a furfural conversion rate of 1330 mmol/g metal/h. Instead, iridium and platinum catalysts, although showing high conversions, did not demonstrate significant selectivity to the desired FME.
Figure 3.
Space Time Yields (mmol/g metal/h) for 2-methoxymethylfuran production for noble metals with 5% w/w metal loading.
Figure 3.
Space Time Yields (mmol/g metal/h) for 2-methoxymethylfuran production for noble metals with 5% w/w metal loading.
Figure 4 shows the yield percentage to FME
versus the conversion of furfural. The line represents 100% selectivity. With palladium on charcoal catalysts a maximum selectivity of 77% was achieved.
Figure 4.
Yield percentage to 2-methoxymethylfuran vs. conversion of furfural for noble metals with 5% w/w metal loading. Dashed line represents 100% selectivity to FME.
Figure 4.
Yield percentage to 2-methoxymethylfuran vs. conversion of furfural for noble metals with 5% w/w metal loading. Dashed line represents 100% selectivity to FME.
The reduced palladium on graphite catalysts show a higher selectivity to FME than the equivalent pre-reduced ones, although the conversion rate of furfural is lower (see
Table 1). This shows that the reduction procedure with hydrogen promotes the formation of the aimed FME with respect to the other products detected in the case of palladium on graphite catalysts. Iridium and platinum catalysts, although showing significant conversion of furfural, do not have sufficiently high productivity/selectivity to FME.
Our results show poor performance of the Pt/Al
2O
3 catalyst, which was reported to have good performance in HMF etherification by Balakrishnan
et al. [
22], albeit in combination with Amberlyst-15, a strongly acidic ion exchange resin. The use of a single catalyst, instead of a combination of two catalysts (especially a combination of an organic resin and an alumina-based catalyst) is preferable from an industrial perspective. The deposition of acid-catalyzed furfural polymerization products on the catalyst surface is expected in long-term operation and the catalyst will require periodic regeneration.
The findings in this screening study are in agreement with those of Bethmont
et al. [
17] who describe palladium on charcoal catalysts in the direct synthesis of ethers from aliphatic aldehydes and ketones, and primary and secondary alcohols. In their work the good catalytic properties of palladium on charcoal were ascribed to its low efficiency of palladium for the competitive carbonyl reduction into further hydrogenation products. However, they also stated that this synthesis method is limited by the impossibility of using an aromatic aldehyde due to the fast reduction of the carbonyl group to alcohols [
18]. This suggests that the reactivity of the carbonyl group plays a crucial role in the one-step reductive etherification of aldehydes. In this respect furfural appears to be an interesting molecule in the reductive etherification to FME. The formation of furfuryl alcohol confirms the aromatic nature of the six electrons on the furan ring. However, furfural is also able to react as an aliphatic aldehyde under our reaction conditions, giving the desired ether in good yields.
Selectivity to Different Product Groups
Regarding the selectivity to different product groups for each type of metal, several trends are observed. The only metal that gave significant amounts of FME was palladium. This metal, however, also showed signs of consecutive hydrogenolysis of the aldehyde group (
Figure S1, Supplementary Information). In particular 5%
w/
w Pd/charcoal, recognized as the most promising catalyst in the reductive etherification of furfural, showed significant selectivity to furfuryl alcohol (FA) as well (around 25%). Moreover, at high temperature (120 °C) the selectivity to 2-methylfuran (2-MF), obtained by further hydrogenolysis of FA, becomes important (always < 10%). 2-MF represents a highly attractive product due to its unique fuel properties and it has recently been proposed as a promising biofuel component, mixed with gasoline [
25,
26].
The other metal catalysts (iridium, nickel, copper and to a lesser extent platinum) give FA as the main reaction product. These results are in agreement with the industrial use of both copper chromite and Raney nickel catalysts in the synthesis of FA in the gas-phase reaction [
27]. In particular, Cu-based catalysts (copper chromite, Raney copper, Cu/Al
2O
3) have been widely employed, as they do not cleave the C–O bond and only show minor activity in C–C cleavage and ring hydrogenation. However, the use of the toxic chromium based catalyst has serious environmental concerns in its preparation, handling and disposal [
26].
Ultimately our data are in accordance with the previous study of Bethmont
et al. [
17,
18] on the synthesis of ethers from aliphatic aldehydes and alcohols, stating the difficulty of obtaining ethers from an aromatic aldehyde. Palladium catalysts on carbonaceous supports were demonstrated to convert the aromatic aldehyde furfural to the desired ether in good yield. Palladium on charcoal showed the highest activity and selectivity to 2-methoxymethylfuran (77%) of all catalysts under the conditions tested.
4. Conclusions
The results of this work clearly indicate that palladium is the metal of choice for the direct formation of 2-metoxymethylfuran (FME) from furfural. Carbon-based supports, i.e., activated carbon, charcoal and graphite are preferred. This was shown by the high activity and selectivity of the commercial catalysts with both these functionalities in the one-pot reductive etherification. Carbon supports generally possess a high specific surface area, a developed pore space and especially controllable chemical surface properties. The functionalities present in the form of surface oxides (e.g., carboxylic, phenolic, lactonic, ether groups) are responsible for both the acid/base and redox properties of the activated carbon, and for the anchoring of the metal particles, resulting in good metal dispersion.
Further optimization of this catalytic system is needed, because 5%
w/
w Pd on charcoal did not demonstrate sufficient selectivity to 2-metoxymethylfuran (FME), with significant amounts of furfuryl alcohol (FA) and 2-methylfuran (2-MF) as the major by-products. Carbon supports are widely used for noble metal hydrogenation catalysts, including in the hydrogenation furfural [
28], due to their wide range of specific surface areas, tailored pore size distributions and controllable chemical surface properties [
29].
Since the acidity of the support in the reductive etherification of furfural, as assumed from our work and in the work of Bethmont [
17,
18], plays a crucial role in the mechanism of FME formation, a further study of the support surface as well as of the size and dispersion of the metal particles is imperative. The acidity of the carbon support is related to the functionalization treatment of the carbon itself, and can be improved by doping and other methods [
30].
In conclusion, this study has identified the Pd on charcoal as the preferable catalyst for the direct reductive etherification of furfural, and provides indications on aspects for further improvement. Moreover, it is possible to affirm that furfural, although it is an aromatic molecule, undergoes the direct etherification to FME with 5%
w/
w Pd on charcoal catalyst in good yields. This aromaticity could be a limitation in the direct synthesis of ethers from aldehydes and alcohols as stated by Bethmont
et al. [
17,
18], because of the competitive reduction of the carbonyl group to furfuryl alcohol. Nevertheless, the actual performances are already largely superior to those for the catalysts earlier reported in literature. This study is an important starting point for the further optimization of this reaction and the required catalysts.