Zeolite Catalysts for Phenol Benzoylation with Benzoic Acid: Exploring the Synthesis of Hydroxybenzophenones
Abstract
:1. Introduction
| Substrate | Acylating Reagent | Desired Product | Catalyst | Ref. |
|---|---|---|---|---|
| Cyclohexene | Acetic anhydride, acetyl chloride | Acetylcyclohexenes | H-Y, H-beta, H-mordenite | [33] |
| Benzene | Benzoyl chloride | Benzophenone | Ga, In-H-beta | [26] |
| Toluene | Phthalic acid | 2-methylanthraquinone | H-Y, H-beta, H-mordenite | [39] |
| Toluene | Benzoyl chloride | 4-methylbenzophenone | H-beta | [4] |
| Toluene | Acetic anhydride | 4-methylacetophenone | H-beta (nano) | [8] |
| o-Xylene | Benzoyl chloride | 3,4-dimethylbenzophenone | H-beta | [6] |
| m-Xylene | Benzoyl chloride, benzoic anhydride | 2,4-dimethylbenzophenone | H-Y | [40] |
| Naphthalene | Benzoyl chloride | 2-benzoylnaphthalene | H-beta | [5] |
| 2-Methoxynaphthalene | Acetic anhydride | 2-acetyl-6-methoxynaphthalene | H-beta, H-Y, ITQ-7 | [7,13,22,25,41] |
| Biphenyl | Acetic anhydride | 4-acetylbiphenyl | H-Y, H-beta | [12] |
| Biphenyl | Benzoyl chloride | 4-phenylbenzophenone | H-beta | [20] |
| Chlorobenzene | 4-chlorobenozylchloride | 4,4′-dichlorobenzophenone | H-beta | [14] |
| Phenol | Propionyl chloride | 4- and 2-hydroxypropiophenone | H-beta | [10] |
| Phenol (gas-phase) | Acetic acid | Phenyl acetate, o-hydroxyacetophenone | H-Y, H-beta, H-ZSM-5 | [11,30,31] |
| Phenol (gas-phase) | Acetic acid | p- and o-hydroxyacetophenone | Zn-exchanged NaY or ZSM-5 | [42] |
| Phenol | Benzoic anhydride | p- and o-hydroxybenzophenone | H-beta | [19] |
| Phenol | Acetic anhydride | p- and o-hydroxyacetophenone | H-ZSM5 (Cu-, Co-doped) | [37] |
| Phenol | Acetic acid | p- and o-hydroxyacetophenone | HZSM-5, H-Y | [38,43] |
| Phenol | Phenylacetate | p- and o-hydroxyacetophenone | H-beta | [44] |
| p-Cresol | Acetic acid, propionic acid, butyric acid, etc. | Various o-hydroxy ketones | H-beta | [45] |
| Anisole | Octanoic acid | p-octanoyl anisole | H-beta | [24] |
| Anisole | Hexanoic, octanoic, decanoic acids | 4-methoxyphenylalkylketone | H-beta, H-Y, H-mordenite | [29] |
| Anisole | Acetic anhydride | p- and o-methoxyacetophenone | H- beta, H-Y | [32,36] |
| Guaiacol | Acetic anhydride | 2-methoxyphenyl acetate | H-Ferrierite | [16] |
| Veratrole | Benzoic anhydride, benzoyl chloride | Dimethoxybenzophenone | H-Y, H-beta | [15] |
| Veratrole | Propionyl chloride | 3,4-dimethoxypropiophenone | H-beta | [17] |
| Dimethoxybenzenes | Various acyl chlorides | Various | H-Y | [18] |
| Veratrole | Acetic anhydride | 3-4-dimethoxyacetophenone | H-Y, H-beta | [9,21] |
| Phenylacetate | Fries rearrangement | p- and o-hydroxyacetophenone | H-beta | [23,27,46] |
| Phenylacetate (gas-phase) | Fries rearrangement | p- and o-hydroxyacetophenone | H3PO4/ZSM-5 | [28] |
| Phenylacetate | Fries rearrangement | p- and o-hydroxyacetophenone | H-beta, H-Y, H-ZSM5 | [34] |
| Phenylacetate, phenyl benzoate | Fries rearrangement | p- and o-hydroxyacetophenone | H-ZSM5, H-ZSM12 | [35] |
2. Results and Discussion
2.1. Characterization of H-Beta Zeolites
| Sample | Si/Al, Atomic Ratio | Overall Amount of NH3 Desorbed (mmole NH3/g) | Micropore Volume and Area (cm3/g, m2/g) | Mesopore Volume (cm3/g) | Total Pore Volume (cm3/g) | Total Surface Area (m2/g) | Crystallite Size (nm; from XRD) |
|---|---|---|---|---|---|---|---|
| HB-13 | 13 | 0.39 | 0.13, 384 | 0.55 | 0.70 | 575 | 18 |
| HB-38 | 38 | 0.24 | 0.15, 421 | 0.94 | 1.10 | 636 | 16 |
| HB-75 | 75 | 0.11 | 0.15, 429 | 0.99 | 1.16 | 645 | 16 |
| HB-150 | 150 | 0.17 | 0.16, 458 | 0.17 | 0.38 | 641 | 27 |
| HY-3 | 3 | 1.0 * | Nd | Nd | Nd | 584 | Nd |
| HY-7 | 7.5 | 0.1 * | Nd | Nd | Nd | 550 * | Nd |
| HY-100 | 100 | <0.1 * | Nd | Nd | Nd | 814 | Nd |
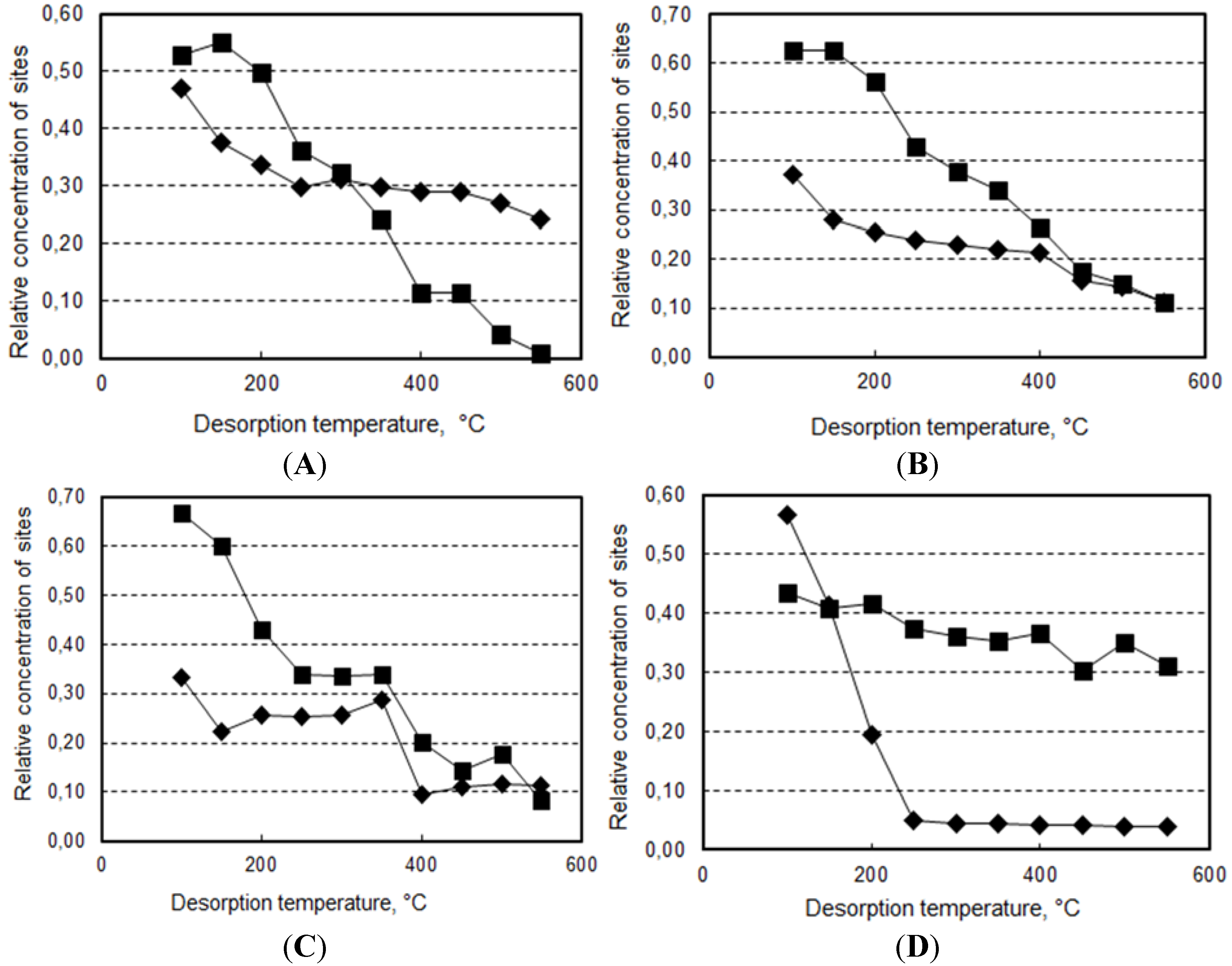

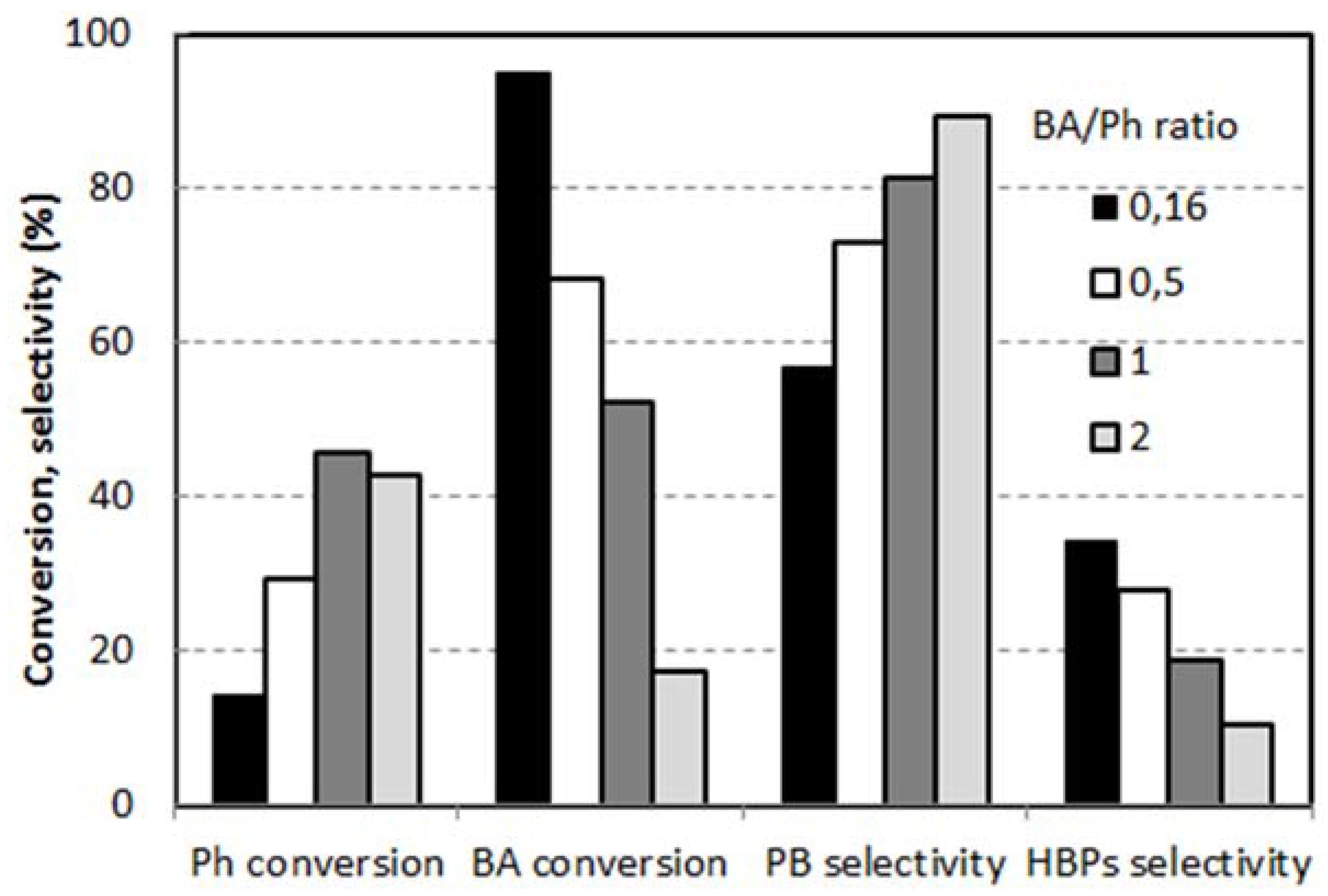
2.2. Reactivity of H-Beta Zeolites
- (a)
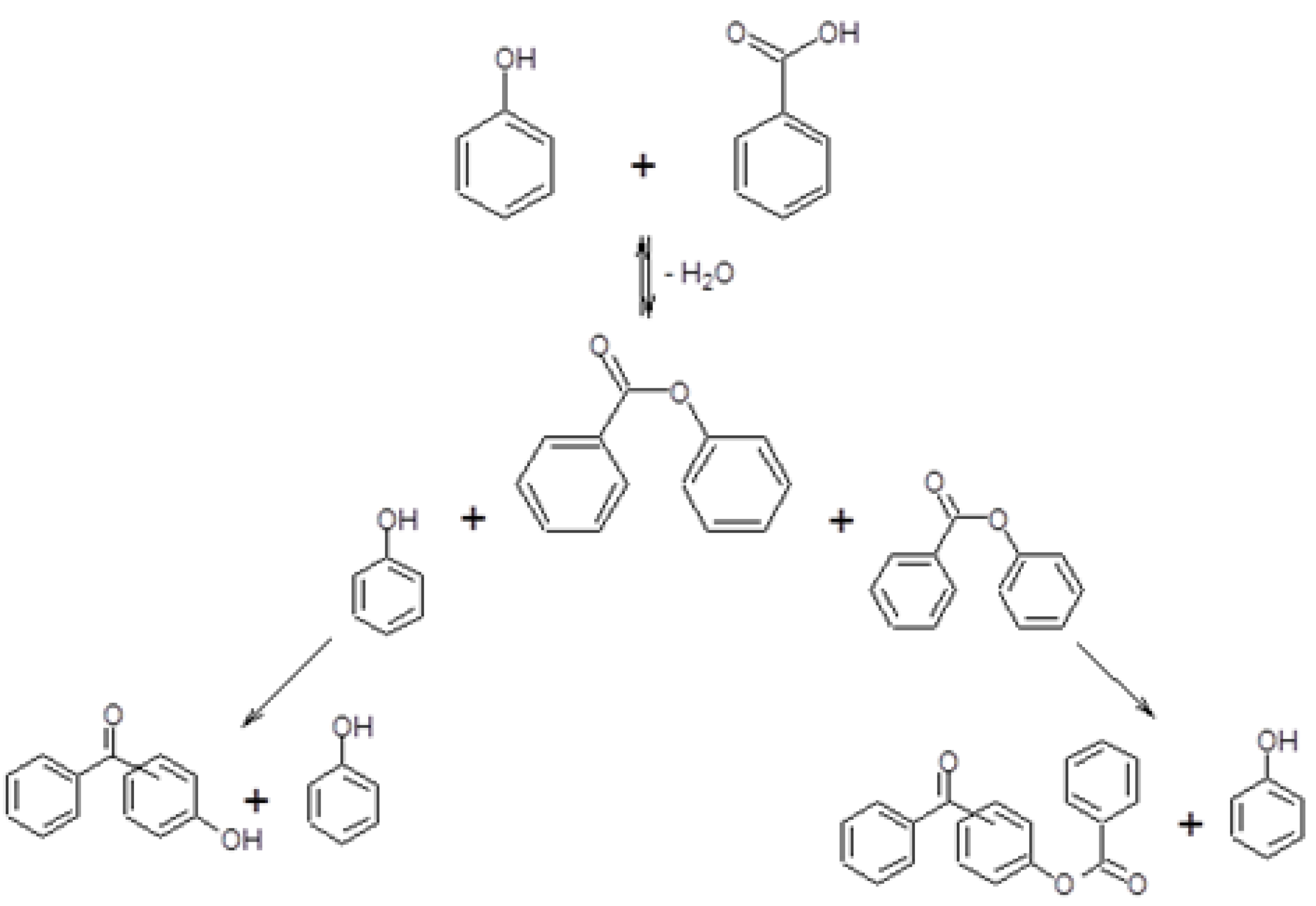
- An intramolecular Fries rearrangement of PB, which does not involve phenol, and leads preferentially to the formation of o-HBP. The greatest contribution of this reaction is registered with samples having the highest Al content, leading to a p-/o-HBP selectivity ratio lower than one with HB-13.
- (b)
- An intermolecular reaction between PB and phenol, which leads to both of the two HBP isomers. The greatest contribution of this reaction is registered with samples having the lowest Al content, leading to a p-/o-HBP selectivity ratio greater than one with HB-150 and HB-75.

2.3. Reactivity Experiments with PB
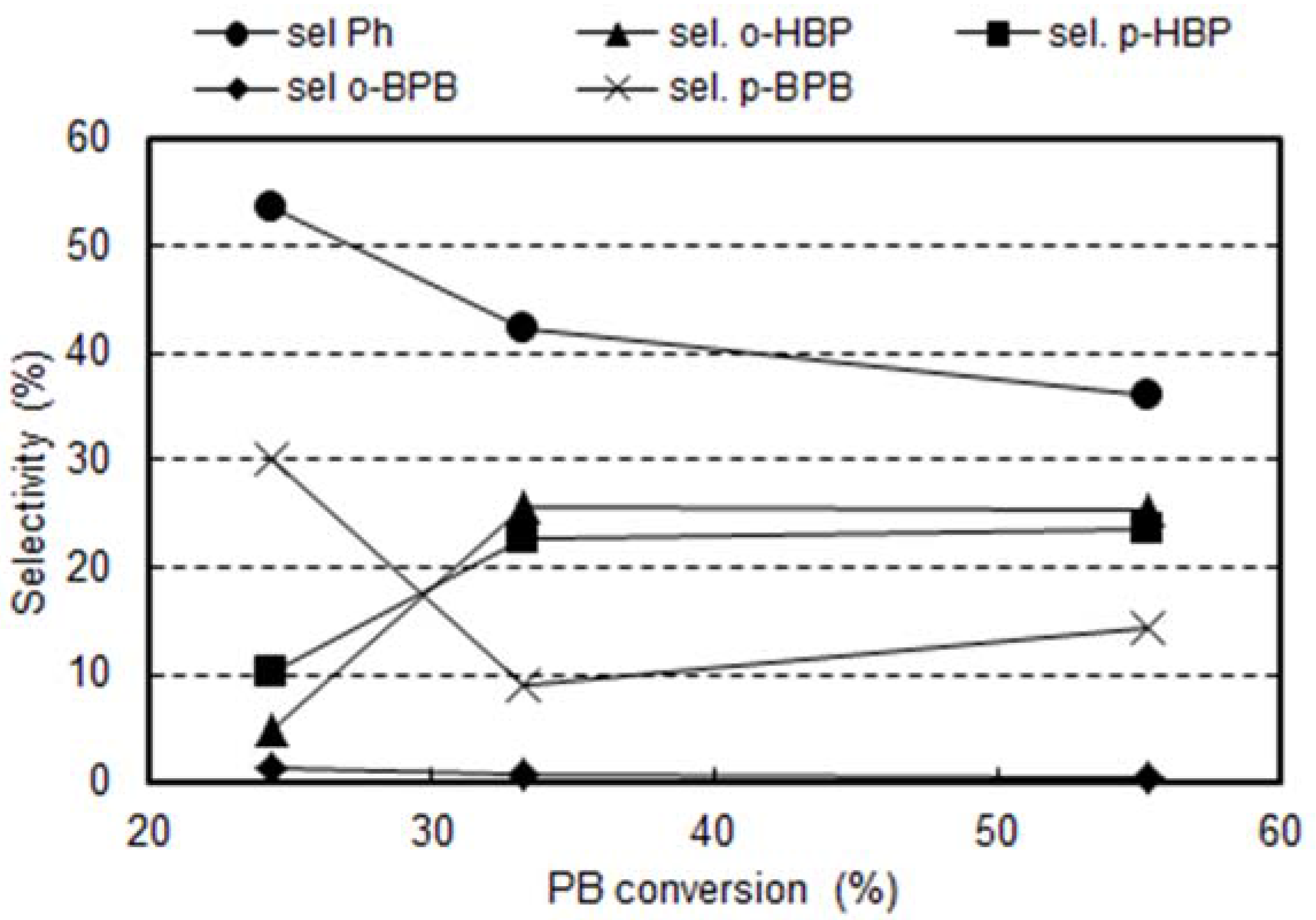
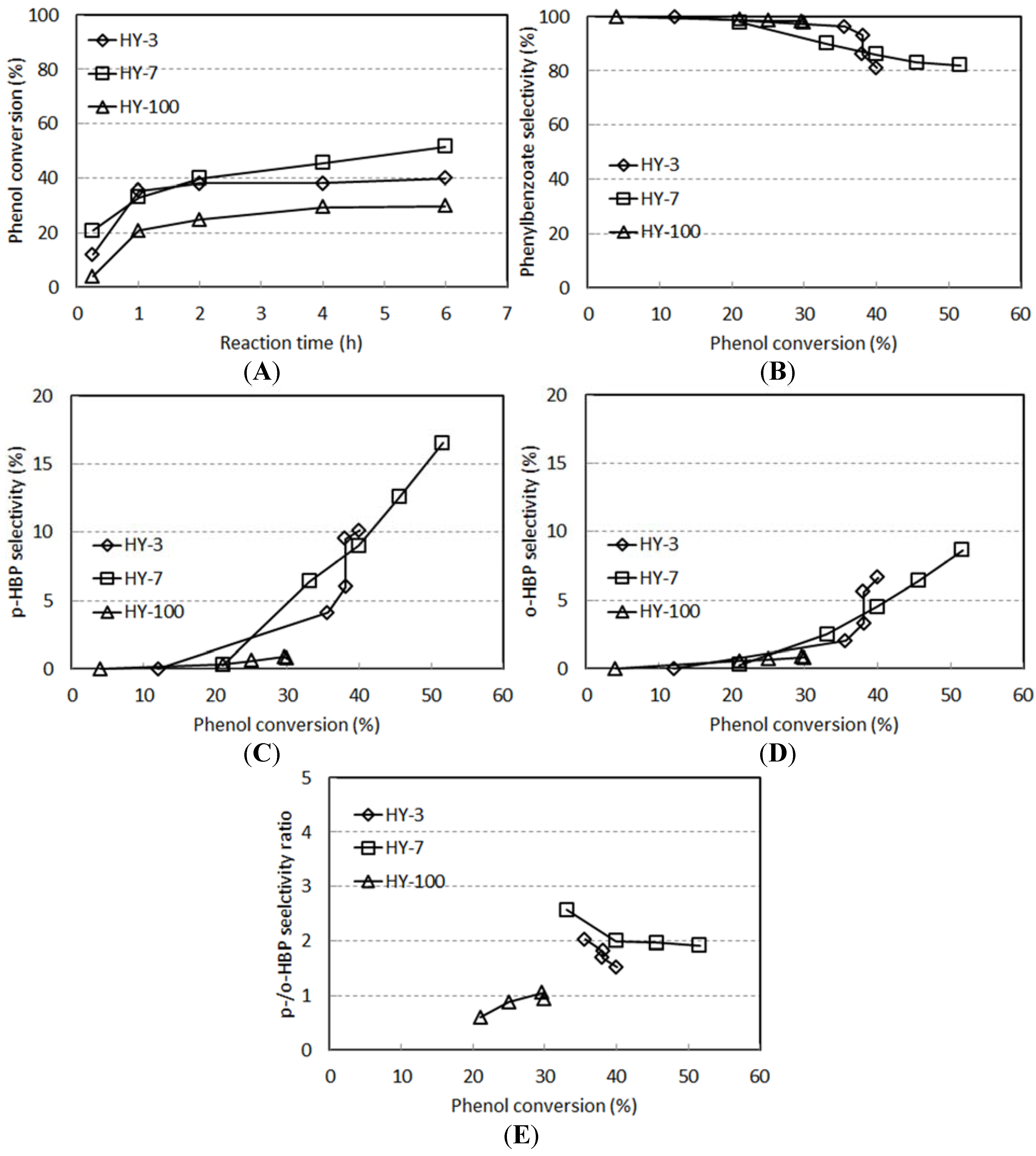
2.4. The Reactivity of H-Y Zeolites

3. Experimental Section
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Sartori, G.; Maggi, R. Use of solid catalysts in Friedel-Crafts acylation reactions. Chem. Rev. 2006, 106, 1077–1104. [Google Scholar] [CrossRef] [PubMed]
- Bejblová, M.; Procházková, D.; Čejka, J. Acylation Reactions over Zeolites and Mesoporous Catalysts. ChemSusChem 2009, 2, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, M.; Cavani, F.; Cimini, M.; Pozzo, L.D.; Maselli, L.; Venerito, D.; Pizzoli, F.; Veronesi, G. An environmentally friendly synthesis of 2,4-dihydroxybenzophenone by the single-step o-mono-benzoylation of 1,3-dihydroxybenzene (resorcinol) and Fries rearrangement of intermediate resorcinol monobenzoate: the activity of acid-treated montmorillonite. Comptes Rendus Chim. 2004, 7, 143–150. [Google Scholar] [CrossRef]
- Singh, A.P.; Bhattacharya, D.; Sharma, S. Benzoylation of toluene with benzoyl chloride over zeolite catalysts. J. Mol. Catal. A 1995, 102, 139–145. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Sharma, S.; Singh, A.P. Selective benzoylation of naphthalene to 2-benzoylnaphthalene using zeolite H-beta catalysts. Appl. Catal. A 1997, 150, 53–62. [Google Scholar] [CrossRef]
- Jacob, B.; Sugunan, S.; Singh, A.P. Selective benzoylation of o-xylene to 3,4-dimethylbenzophenone using various zeolite catalysts. J. Mol. Catal. A 1999, 139, 43–53. [Google Scholar] [CrossRef]
- Andy, P.; Garcia-Martinez, J.; Lee, G.; Gonzalez, H.; Jones, C.W.; Davis, M.E. Acylation of 2-Methoxynaphthalene and Isobutylbenzene over Zeolite Beta. J. Catal. 2000, 192, 215–223. [Google Scholar] [CrossRef]
- Botella, P.; Corma, A.; López-Nieto, J.M.; Valencia, S.; Jacquot, R. Acylation of Toluene with Acetic Anhydride over Beta Zeolites: Influence of Reaction Conditions and Physicochemical Properties of the Catalyst. J. Catal. 2000, 195, 161–168. [Google Scholar] [CrossRef]
- Moreau, P.; Finiels, A.; Meric, P. Acetylation of dimethoxybenzenes with acetic anhydride in the presence of acidic zeolites. J. Mol. Catal. A 2000, 154, 185–192. [Google Scholar] [CrossRef]
- Chaube, V.D.; Moreau, P.; Finiels, A.; Ramaswamy, A.V.; Singh, A.P. Propionylation of phenol to 4-hydroxypropiophenone over zeolite H-beta. J. Mol. Catal. A 2001, 174, 255–264. [Google Scholar] [CrossRef]
- Neves, I.; Jayat, F.; Magnoux, P.; Perot, G.; Ribeiro, F.R.; Gubelmann, M.; Guisnet, M. Acylation of phenol with acetic acid over a HZSM-5 zeolite: the reaction scheme. J. Mol. Catal. 1994, 93, 169–179. [Google Scholar] [CrossRef]
- Escola, J.M.; Davis, M.E. Acylation of biphenyl with acetic anhydride and carboxylic acids over zeolite catalysts. Appl. Catal. A 2001, 214, 111–120. [Google Scholar] [CrossRef]
- Botella, P.; Corma, A.; Sastre, G. Al-ITQ-7, a Shape-Selective Zeolite for Acylation of 2-Methoxynaphthalene. J. Catal. 2001, 197, 81–90. [Google Scholar] [CrossRef]
- Venkatesan, C.; Jaimol, T.; Moreau, P.; Finiels, A.; Ramaswamy, A.V.; Singh, A.P. Liquid phase selective benzoylation of chlorobenzene. Catal. Lett. 2001, 75, 119–123. [Google Scholar] [CrossRef]
- Raja, T.; Singh, A.P.; Ramaswamy, A.V.; Finiels, A.; Moreau, P. Benzoylation of 1,2-dimethoxybenzene with benzoic anhydride and substituted benzoyl chlorides over large pore zeolites. Appl. Catal. A 2001, 211, 31–39. [Google Scholar] [CrossRef]
- Chavan, S.; Anand, R.; Pasupathy, K.; Rao, B. Catalytic acetylation of alcohols, phenols, thiols and amines with zeolite H-FER under solventless conditions. Green Chem. 2001, 320–322. [Google Scholar] [CrossRef]
- Jaimol, T.; Moreau, P.; Finiels, A.; Ramaswamy, A.V.; Singh, A.P. Selective propionylation of veratrole to 3,4-dimethoxypropiophenone using zeolite H-beta catalysts. Appl. Catal. A 2001, 214, 1–10. [Google Scholar] [CrossRef]
- Bigi, F.; Carloni, S.; Flego, C.; Maggi, R.; Mazzacani, A.; Rastelli, M.; Sartori, G. HY zeolite-promoted electrophilic acylation of methoxyarenes with linear acid chlorides. J. Mol. Catal. A 2002, 178, 139–146. [Google Scholar] [CrossRef]
- Chaube, V.D.; Moreau, P.; Finiels, A.; Ramaswamy, A.V.; Singh, A.P. A novel single step selective synthesis of 4-hydroxybenzophenone (4-HBP ) using zeolite H-beta. Catal. Lett. 2002, 79, 89–94. [Google Scholar] [CrossRef]
- Chidambaram, M.; Venkatesan, C.; Moreau, P.; Finiels, A.; Ramaswamy, A.V.; Singh, A.P. Selective benzoylation of biphenyl to 4-phenylbenzophenone over zeolite H-beta. Appl. Catal. A 2002, 224, 129–140. [Google Scholar] [CrossRef]
- Guignard, C.; Pédron, V.; Richard, F.; Jacquot, R.; Spagnol, M.; Coustard, J.M.; Pérot, G. Acylation of veratrole by acetic anhydride over Hβ and HY zeolites: Possible role of di- and triketone by-products in the deactivation process. Appl. Catal. A 2002, 234, 79–90. [Google Scholar] [CrossRef]
- Meric, P.; Finiels, A.; Moreau, P. Kinetics of 2-methoxynaphthalene acetylation with acetic anhydride over dealuminated HY zeolites. J. Mol. Catal. A 2002, 189, 251–262. [Google Scholar] [CrossRef]
- Wang, H.; Zou, Y. Modified beta zeolite as catalyst for Fries rearrangement reaction. Catal. Lett. 2003, 86, 163–167. [Google Scholar] [CrossRef]
- Beers, A.E.W.; Van Bokhoven, J.A.; De Lathouder, K.M.; Kapteijn, F.; Moulijn, J.A. Optimization of zeolite Beta by steaming and acid leaching for the acylation of anisole with octanoic acid: A structure-activity relation. J. Catal. 2003, 218, 239–248. [Google Scholar] [CrossRef]
- Botella, P.; Corma, A.; Navarro, M.T.; Rey, F.; Sastre, G. On the shape selective acylation of 2-methoxynaphthalene over polymorph C of Beta (ITQ-17). J. Catal. 2003, 217, 406–416. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Jana, S.K.; Patil, N.S.; Bhargava, S.K. Friedel-Crafts type benzylation and benzoylation of aromatic compounds over HY zeolite modified by oxides or chlorides of gallium and indium. Microporous Mesoporous Mater. 2003, 57, 21–35. [Google Scholar] [CrossRef]
- Heitling, E.; Roessner, F.; Van Steen, E. Origin of catalyst deactivation in Fries rearrangement of phenyl acetate over zeolite H-Beta. J. Mol. Catal. A 2004, 216, 61–65. [Google Scholar] [CrossRef]
- Ghiaci, M.; Abbaspur, A.; Kalbasi, R.J. Internal versus external surface active sites in ZSM-5 zeolite: Part 1. Fries rearrangement catalyzed by modified and unmodified H3PO4/ZSM-5. Appl. Catal. A 2006, 298, 32–39. [Google Scholar] [CrossRef]
- Wagholikar, S.G.; Niphadkar, P.S.; Mayadevi, S.; Sivasanker, S. Acylation of anisole with long-chain carboxylic acids over wide pore zeolites. Appl. Catal. A 2007, 317, 250–257. [Google Scholar] [CrossRef]
- Padró, C.L.; Sad, M.E.; Apesteguía, C.R. Acid site requirements for the synthesis of o-hydroxyacetophenone by acylation of phenol with acetic acid. Catal. Today 2006, 116, 184–190. [Google Scholar] [CrossRef]
- Padró, C.L.; Apesteguía, C.R. Acylation of phenol on solid acids: Study of the deactivation mechanism. Catal. Today 2005, 107–108, 258–265. [Google Scholar] [CrossRef]
- Freese, U.; Heinrich, F.; Roessner, F. Acylation of aromatic compounds on H-Beta zeolites. Catal. Today 1999, 49, 237–244. [Google Scholar] [CrossRef]
- Armengol, E.; Corma, A.; Fernández, L.; García, H.; Primo, J. Acid zeolites as catalysts in organic reactions. Acetylation of cyclohexene and 1-methylcyclohexene. Appl. Catal. A 1997, 158, 323–335. [Google Scholar] [CrossRef]
- Heidekum, A.; Harmer, M.A.; Hoelderich, W.F. Highly Selective Fries Rearrangement over Zeolites and Nafion in Silica Composite Catalysts: A Comparison. J. Catal. 1998, 176, 260–263. [Google Scholar] [CrossRef]
- Vogt, A.; Kouwenhoven, H.W.; Prins, R. Fries rearrangement over zeolitic catalysts. Appl. Catal. A 1995, 123, 37–49. [Google Scholar] [CrossRef]
- Wei, H.; Liu, K.; Xie, S.; Xin, W.; Li, X.; Liu, S.; Xu, L. Determination of different acid sites in Beta zeolite for anisole acylation with acetic anhydride. J. Catal. 2013, 307, 103–110. [Google Scholar] [CrossRef]
- Subba Rao, Y.V.; Kulkarni, S.J.; Subrahmanyam, M.; Rama Rao, A.V. An improved acylation of phenol over modified ZSM-5 catalysts. Appl. Catal. A 1995, 133, L1–L6. [Google Scholar] [CrossRef]
- Padró, C.L.; Apesteguía, C.R. Gas-phase synthesis of hydroxyacetophenones by acylation of phenol with acetic acid. J. Catal. 2004, 226, 308–320. [Google Scholar] [CrossRef]
- Hou, Q.; Zheng, B.; Bi, C.; Luan, J.; Zhao, Z.; Guo, H.; Wang, G.; Li, Z. Liquid-phase cascade acylation/dehydration over various zeolite catalysts to synthesize 2-methylanthraquinone through an efficient one-pot strategy. J. Catal. 2009, 268, 376–383. [Google Scholar] [CrossRef]
- Fang, R.; Harvey, G.; Kouwenhoven, H.W.; Prins, R. Effects of non-framework alumina in the acylation of xylene over USY. Appl. Catal. A 1995, 130, 67–77. [Google Scholar] [CrossRef]
- Patil, S.P.; Yadav, G.D. Selective acylation of 2 methoxynaphthalene by large pore zeolites: Catalyst selection through molecular modeling. Comput. Biol. Chem. 2003, 27, 393–404. [Google Scholar] [CrossRef]
- Padró, C.L.; Rey, E.A.; González Peña, L.F.; Apesteguía, C.R. Activity, selectivity and stability of Zn-exchanged NaY and ZSM5 zeolites for the synthesis of o-hydroxyacetophenone by phenol acylation. Microporous Mesoporous Mater. 2011, 143, 236–242. [Google Scholar] [CrossRef]
- Guisnet, M.; Lukyanov, D.B.; Jayat, F.; Magnoux, P.; Hap, H. Kinetic modeling of phenol acylation with acetic acid on HZSM5. Ind. Eng. Chem. Res. 1995, 34, 1624–1629. [Google Scholar] [CrossRef]
- Rohan, D.; Canaff, C.; Magnoux, P.; Guisnet, M. Origin of the deactivation of HBEA zeolites during the acylation of phenol with phenylacetate. J. Mol. Catal. A 1998, 129, 69–78. [Google Scholar] [CrossRef]
- Chandra Shekara, B.M.; Jai Prakash, B.S.; Bhat, Y.S. Microwave-induced deactivation-free catalytic activity of BEA zeolite in acylation reactions. J. Catal. 2012, 290, 101–107. [Google Scholar] [CrossRef]
- Jayat, F.; Picot, M.J.S.; Guisnet, M. Solvent effects in liquid phase Fries rearrangement of phenyl acetate over a HBEA zeolite. Catal. Lett. 1996, 41, 181–187. [Google Scholar] [CrossRef]
- Li, A.-X.; Li, T.-S.; Ding, T.-H. Montmorillonite K-10 and KSF as remarkable acetylation catalysts. Chem. Commun. 1997, 1389–1390. [Google Scholar] [CrossRef]
- Li, T.; Li, A. Acylation of alcohols, phenols, thiols and amines. J. Chem. Soc. Perkin Trans. 1 1998, 1913–1917. [Google Scholar] [CrossRef]
- Choudary, B.M.; Bhaskar, V.; Lakshmi Kantam, M.; Koteswara Rao, K.; Raghavan, K.V. Acylation of alcohols with carboxylic acids; the evolution of compatible acidic sites in montmorillonites. Green Chem. 2000, 2, 67–70. [Google Scholar] [CrossRef]
- Békássy, S.; Farkas, J.; Agai, B.; Figueras, F. Selectivity of C-versus O-acylation of diphenols by clay catalysts. I. Acylation of resorcinol with phenylacetyl chloride. Top. Catal. 2000, 13, 287–290. [Google Scholar] [CrossRef]
- Jankovič, L.; Komadel, P. Metal cation-exchanged montmorillonite catalyzed protection of aromatic aldehydes with Ac2O. J. Catal. 2003, 218, 227–233. [Google Scholar] [CrossRef]
- De Castro, C.; Primo, J.; Corma, A. Heteropolyacids and large-pore zeolites as catalysts in acylation reactions using α, β-unsaturated organic acids as acylating agents. J. Mol. Catal. A 1998, 134, 215–222. [Google Scholar] [CrossRef]
- Castro, C.; Corma, A.; Primo, J. On the acylation reactions of anisole using α,β-unsaturated organic acids as acylating agents and solid acids as catalysts: A mechanistic overview. J. Mol. Catal. A 2002, 177, 273–280. [Google Scholar] [CrossRef]
- Kozhevnikova, E.F.; Rafiee, E.; Kozhevnikov, I.V. Fries rearrangement of aryl esters catalysed by heteropoly acid: Catalyst regeneration and reuse. Appl. Catal. A 2004, 260, 25–34. [Google Scholar] [CrossRef]
- Sarsani, V.S.R.; Lyon, C.J.; Hutchenson, K.W.; Harmer, M.A.; Subramaniam, B. Continuous acylation of anisole by acetic anhydride in mesoporous solid acid catalysts: Reaction media effects on catalyst deactivation. J. Catal. 2007, 245, 184–190. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Anderson, J.A. FTIR and reaction studies of the acylation of anisole with acetic anhydride over supported HPA catalysts. J. Catal. 2004, 228, 225–233. [Google Scholar] [CrossRef]
- Yadav, G.D.; Asthana, N.S.; Kamble, V.S. Cesium-substituted dodecatungstophosphoric acid on K-10 clay for benzoylation of anisole with benzoyl chloride. J. Catal. 2003, 217, 88–99. [Google Scholar] [CrossRef]
- Yadav, G.D.; Asthana, N.S.; Kamble, V.S. Friedel-crafts benzoylation of p-xylene over clay supported catalysts: Novelty of cesium substituted dodecatungstophosphoric acid on K-10 clay. Appl. Catal. A 2003, 240, 53–69. [Google Scholar] [CrossRef]
- Yadav, G.D.; Asthana, N.S.; Salgaonkar, S.S. Regio-selective benzoylation of xylenes over caesium modified heteropolyacid supported on K-10 clay. Clean Technol. Environ. Policy 2004, 6, 105–113. [Google Scholar] [CrossRef]
- Yadav, G.D.; George, G. Single step synthesis of 4-hydroxybenzophenone via esterification and Fries rearrangement: Novelty of cesium substituted heteropoly acid supported on clay. J. Mol. Catal. A 2008, 292, 54–61. [Google Scholar] [CrossRef]
- Heravi, M.M.; Behbahani, F.K.; Bamoharram, F.F. H14[NaP5W30O110]: A heteropoly acid catalyzed acetylation of alcohols and phenols in acetic anhydride. J. Mol. Catal. A 2006, 253, 16–19. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Friedel-Crafts acylation and related reactions catalysed by heteropoly acids. Appl. Catal. A 2003, 256, 3–18. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Jana, S.K.; Mandale, A.B. Highly active, reusable and moisture insensitive catalyst obtained from basic Ga-Mg-hydrotalcite anionic clay for Friedel-Crafts type benzylation and acylation reactions. Catal. Lett. 2001, 74, 95–98. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Jana, S.K.; Narkhede, V.S. Benzylation and benzoylation of substituted benzenes over solid catalysts containing Ga- and Mg-oxides and/or chlorides derived from Ga-Mg-hydrotalcite by its HCl pre-treatment or calcination. Appl. Catal. A 2002, 235, 207–215. [Google Scholar] [CrossRef]
- Veverkova, E.; Meciarova, M.; Gotov, B.; Toma, S. Microwave assisted acylation of methoxyarenes catalyzed by EPZG catalyst. Green Chem. 2002, 4, 361–365. [Google Scholar] [CrossRef]
- Trunschke, A.; Deutsch, J.; Müller, D.; Lieske, H.; Quaschning, V.; Kemnitz, E. Nature of surface deposits on sulfated zirconia used as catalyst in the benzoylation of anisole. Catal. Lett. 2002, 83, 271–279. [Google Scholar] [CrossRef]
- Zane, F.; Melada, S.; Signoretto, M.; Pinna, F. Active and recyclable sulphated zirconia catalysts for the acylation of aromatic compounds. Appl. Catal. A 2006, 299, 137–144. [Google Scholar] [CrossRef]
- Signoretto, M.; Breda, A.; Somma, F.; Pinna, F.; Cruciani, G. Mesoporous sulphated zirconia by liquid-crystal templating method. Microporous Mesoporous Mater. 2006, 91, 23–32. [Google Scholar] [CrossRef]
- Ghedini, E.; Signoretto, M.; Pinna, F.; Cerrato, G.; Morterra, C. Gas and liquid phase reactions on MCM-41/SZ catalysts. Appl. Catal. B 2006, 67, 24–33. [Google Scholar] [CrossRef]
- Ghedini, E.; Signoretto, M.; Pinna, F.; Cruciani, G. Mesoporous silica-zirconia systems for catalytic applications. Catal. Lett. 2008, 125, 359–370. [Google Scholar] [CrossRef]
- Melada, S.; Signoretto, M.; Somma, F.; Pinna, F.; Cerrato, G.; Meligrana, G.; Morterra, C. Gas-and liquid-phase reactions on sulphated zirconia prepared by precipitation. Catal. Lett. 2004, 94, 193–198. [Google Scholar] [CrossRef]
- Deutsch, J.; Trunschke, A.; Mu, D. Different acylating agents in the synthesis of aromatic ketones on sulfated zirconia. Catal. Lett. 2003, 88, 9–15. [Google Scholar] [CrossRef]
- Signoretto, M.; Torchiaro, A.; Breda, A.; Pinna, F.; Cerrato, G.; Morterra, C. Study on reuse of metal oxide-promoted sulphated zirconia in acylation reactions. Appl. Catal. B 2008, 84, 363–371. [Google Scholar] [CrossRef]
- Ratnam, K.J.; Reddy, R.S.; Sekhar, N.S.; Kantam, M.L.; Figueras, F. Sulphated zirconia catalyzed acylation of phenols, alcohols and amines under solvent free conditions. J. Mol. Catal. A 2007, 276, 230–234. [Google Scholar] [CrossRef]
- Breda, A.; Signoretto, M.; Ghedini, E.; Pinna, F.; Cruciani, G. Acylation of veratrole over promoted SZ/MCM-41 catalysts: Influence of metal promotion. Appl. Catal. A 2006, 308, 216–222. [Google Scholar] [CrossRef]
- Clark, J.H.; Dekamin, M.G.; Moghaddam, F.M. Genuinely catalytic Fries rearrangement using sulfated zirconia. Green Chem. 2002, 4, 366–368. [Google Scholar] [CrossRef]
- Patil, P.T.; Malshe, K.M.; Kumar, P.; Dongare, M.K.; Kemnitz, E. Benzoylation of anisole over borate zirconia solid acid catalyst. Catal. Commun. 2002, 3, 411–416. [Google Scholar] [CrossRef]
- Chidambaram, M.; Venkatesan, C.; Rajamohanan, P.R.; Singh, A.P. Synthesis of acid functionalized mesoporous Zr-O-SO2-CF3 catalysts; heterogenization of CF3SO3H over mesoporous Zr(OH)4. Appl. Catal. A 2003, 244, 27–37. [Google Scholar] [CrossRef]
- Sakthivel, R.; Prescott, H.A.; Deutsch, J.; Lieske, H.; Kemnitz, E. Synthesis, characterization, and catalytic activity of SO4/Zr1−xSnxO2. Appl. Catal. A 2003, 253, 237–247. [Google Scholar] [CrossRef]
- Chidambaram, M.; Curulla-Ferre, D.; Singh, A.P.; Anderson, B.G. Synthesis and characterization of triflic acid-functionalized mesoporous Zr-TMS catalysts: Heterogenization of CF3SO3H over Zr-TMS and its catalytic activity. J. Catal. 2003, 220, 442–456. [Google Scholar] [CrossRef]
- Parambadath, S.; Chidambaram, M.; Singh, A.P. Synthesis, characterization and catalytic properties of benzyl sulphonic acid functionalized Zr-TMS catalysts. Catal. Today 2004, 97, 233–240. [Google Scholar] [CrossRef]
- Landge, S.M.; Chidambaram, M.; Singh, A.P. Benzoylation of toluene with p-toluoyl chloride over triflic acid functionalized mesoporous Zr-TMS catalyst. J. Mol. Catal. A 2004, 213, 257–266. [Google Scholar] [CrossRef]
- Gawande, M.B.; Deshpande, S.S.; Sonavane, S.U.; Jayaram, R.V. A novel sol-gel synthesized catalyst for Friedel-Crafts benzoylation reaction under solvent-free conditions. J. Mol. Catal. A 2005, 241, 151–155. [Google Scholar] [CrossRef]
- Yadav, G.D.; George, G. Friedel-Crafts acylation of anisole with propionic anhydride over mesoporous superacid catalyst UDCaT-5. Microporous Mesoporous Mater. 2006, 96, 36–43. [Google Scholar] [CrossRef]
- Jain, S.K.; Meena, S.; Singh, V.P.; Bharate, J.B.; Joshi, P.; Singh, V.P.; Vishwakarma, R.A.; Bharate, S.B. KF/alumina catalyzed regioselective benzylation and benzoylation using solvent-free grind-stone chemistry. RSC Adv. 2012, 2, 8929–8933. [Google Scholar] [CrossRef]
- Rahmatpour, A. Polystyrene-supported GaCl3: A new, highly efficient and recyclable heterogeneous Lewis acid catalyst for acetylation and benzoylation of alcohols and phenols. Comptes Rendus Chim. 2012, 15, 1048–1054. [Google Scholar] [CrossRef]
- Ramani, T.; Umadevi, P.; Prasanth, K.L.; Sreedhar, B. Synthesis of ortho -Hydroxybenzophenones Catalyzed by Magnetically Retrievable Fe3O4 Nanoparticles under Ligand-Free Conditions. European J. Org. Chem. 2013, 6021–6026. [Google Scholar] [CrossRef]
- Tamaddon, F.; Amrollahi, M.A.; Sharafat, L. A green protocol for chemoselective O-acylation in the presence of zinc oxide as a heterogeneous, reusable and eco-friendly catalyst. Tetrahedron Lett. 2005, 46, 7841–7844. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Saeidian, H. Controlled microwave-assisted synthesis of ZnO nanopowder and its catalytic activity for O-acylation of alcohol and phenol. Mater. Sci. Eng. B. 2007, 139, 265–269. [Google Scholar] [CrossRef]
- Farhadi, S.; Jahanara, K. ZnAl2O4@SiO2 nanocomposite catalyst for the acetylation of alcohols, phenols and amines with acetic anhydride under solvent-free conditions. Chin. J. Catal. 2014, 35, 368–375. [Google Scholar] [CrossRef]
- Prabhu, A.; Kumaresan, L.; Palanichamy, M.; Murugesan, V. Synthesis and characterization of aluminium incorporated mesoporous KIT-6: Efficient catalyst for acylation of phenol. Appl. Catal. A 2009, 360, 59–65. [Google Scholar] [CrossRef]
- Kumar, P.; Pandey, R.K.; Bodas, M.S.; Dagade, S.P.; Dongare, M.K.; Ramaswamy, A.V. Acylation of alcohols, thiols and amines with carboxylic acids catalyzed by yttria-zirconia-based Lewis acid. J. Mol. Catal. A 2002, 181, 207–213. [Google Scholar] [CrossRef]
- Signoretto, M.; Ghedini, E.; Menegazzo, F.; Cerrato, G.; Crocellà, V.; Bianchi, C.L. Aerogel and xerogel WO3/ZrO2 samples for fine chemicals production. Microporous Mesoporous Mater. 2013, 165, 134–141. [Google Scholar] [CrossRef]
- Alam, M.; Rahman, A.; Alandis, N.M.; Shaik, M.R. Ni/Silica catalyzed acetylation of phenols and naphthols: An eco-friendly approach. Arab. J. Chem. 2014, 7, 53–56. [Google Scholar] [CrossRef]
- Zakrzewski, J.; Szymanowski, J. 2-Hydroxybenzophenone UV-absorbers containing 2,2,6,6-tetramethylpiperidine (HALS) group—benzoylation of corresponding phenol derivatives. Polym. Degrad. Stab. 2000, 67, 279–283. [Google Scholar] [CrossRef]
- Singh, R.P.; Kamble, R.M.; Chandra, K.L.; Saravanan, P.; Singh, V.K. An efficient method for aromatic Friedel-Crafts alkylation, acylation, benzoylation, and sulfonylation reactions. Tetrahedron 2001, 57, 241–247. [Google Scholar] [CrossRef]
- Saravanan, P.; Singh, V.K. An efficient method for acylation reactions. Tetrahedron Lett. 1999, 40, 2611–2614. [Google Scholar] [CrossRef]
- Mohammadpoor-baltork, I.; Aliyan, H.; Khosropour, A.R. Bismuth (III) salts as convenient and efficient catalysts for the selective acetylation and benzoylation of alcohols and phenols. Tetrahedron 2001, 57, 5851–5854. [Google Scholar] [CrossRef]
- Kobayashi, S.; Moriwaki, M.; Hachiya, I. Hafnium trifluoromethanesulfonate (Hf(OTf)4) as an efficient catalyst in the Fries rearrangement and direct acylation of phenol and naphthol derivatives. Tetrahedron Lett. 1996, 37, 2053–2056. [Google Scholar] [CrossRef]
- Kobayashi, S.; Moriwaki, M.; Hachiya, I. Catalytic direct C-acylation of phenol and naphthol derivatives using carboxylic acids as acylating reagents. Tetrahedron Lett. 1996, 37, 4183–4186. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Shimizu, K.; Satsuma, A. Toward a rational control of solid acid catalysis for green synthesis and biomass conversion. Energy Environ. Sci. 2011, 4, 3140–3153. [Google Scholar] [CrossRef]
- Phung, T.K.; Busca, G. On the Lewis acidity of protonic zeolites. Appl. Catal. A 2015, 504, 151–157. [Google Scholar] [CrossRef]
- Hoefnagel, A.J.; Van Bekkum, H. Direct Fries reaction of resorcinol with benzoic acids catalyzed by zeolite H-beta. Appl. Catal. A 1993, 97, 87–102. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gliozzi, G.; Passeri, S.; Bortolani, F.; Ardizzi, M.; Mangifesta, P.; Cavani, F. Zeolite Catalysts for Phenol Benzoylation with Benzoic Acid: Exploring the Synthesis of Hydroxybenzophenones. Catalysts 2015, 5, 2223-2243. https://doi.org/10.3390/catal5042223
Gliozzi G, Passeri S, Bortolani F, Ardizzi M, Mangifesta P, Cavani F. Zeolite Catalysts for Phenol Benzoylation with Benzoic Acid: Exploring the Synthesis of Hydroxybenzophenones. Catalysts. 2015; 5(4):2223-2243. https://doi.org/10.3390/catal5042223
Chicago/Turabian StyleGliozzi, Gherardo, Sauro Passeri, Francesca Bortolani, Mattia Ardizzi, Patrizia Mangifesta, and Fabrizio Cavani. 2015. "Zeolite Catalysts for Phenol Benzoylation with Benzoic Acid: Exploring the Synthesis of Hydroxybenzophenones" Catalysts 5, no. 4: 2223-2243. https://doi.org/10.3390/catal5042223






