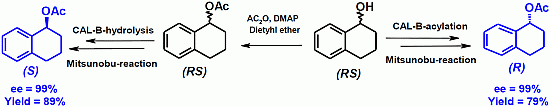
Enantiocomplementary Preparation of (S)- and (R)-Arylalkylcarbinols by Lipase-Catalysed Resolution and Mitsunobu Inversion: Impact of Lipase Amount
Abstract
:1. Introduction

2. Results and Discussion
2.1. Optimization of the Lipase Amount
| Entry | Substrate a | CAL-B (mg) | eeS (%) b-(S) d | eeP (%) b-(R) d | C % c | Ec |
|---|---|---|---|---|---|---|
| 1 | 1a | 150 | 44 | 96 | 32 | 76 |
| 2 | 12 | 98 | 99 | 50 | >500 | |
| 3 | 6 | 79 | 93 | 46 | 67 | |
| 4 | 2a | 150 | 83 | 98 | 46 | >200 |
| 5 | 50 | 99 | 99 | 50 | >500 | |
| 6 | 20 | 28 | 96 | 23 | 64 | |
| 7 | 3a | 150 | 98 | 99 | 50 | >500 |
| 8 | 20 | 99 | 98 | 50 | >500 | |
| 9 | 4a | 150 | 89 | 99 | 47 | >500 |
| 10 | 20 | 99 | 97 | 50 | >500 | |
| 11 | 5a | 150 | 7 | 99 | 6 | >200 |
| 12 | 60 | 10 | 96 | 9 | 54 | |
| 13 | 20 | - | - | - | - | |
| 14 | 6a | 150 | 75 | 87 | 46 | 32 |
| 15 | 60 | 51 | 99 | 34 | 70 | |
| 16 | 20 | 44 | 99 | 30 | >200 |
2.2. Deracemization by Combined Enzymatic Hydrolysis/Mitsunobu Stereoinversion
| Substrate a | CAL-B catalyzed hydrolysis b | Mitsunobu inversion | ||||
|---|---|---|---|---|---|---|
| CAL-B (mg/mmole) | (R)-Alcohol (%ee) c | (S)-Acetate (%ee) c | E d | (S)-Acetate (%ee) | Yield e (%) | |
| 1a | 25 | 99 | 98 | >500 | 94 | 78 |
| 2a | 100 | 99 | 99 | >500 | 99 | 89 |
| 3a | 40 | 98 | 99 | >500 | 71 | 82 |
| 4a | 40 | 97 | 99 | >500 | 92 | 76 |
| Substrate a | CAL-B Catalyzed Acylation/Mitsunobu Stereoinversion | Substrate b | CAL-B Catalyzed Hydrolysis/Mitsunobu Stereoinversion | ||||
|---|---|---|---|---|---|---|---|
| CAL-B (mg) | (R)-Acetate (%ee) | Yield (%) | CAL-B (mg) | (S)-Acetate (%ee) | Yield (%) | ||
| 1 | 12 | >99 | 82 | 1a | 25 | 94 | 78 |
| 2 | 100 | >99 | 79 | 2a | 100 | 99 | 89 |
| 3 | 40 | >99 | 74 | 3a | 40 | 71 | 82 |
| 4 | 50 | 91 | 76 | 4a | 40 | 92 | 76 |

3. Experimental Section
3.1. General
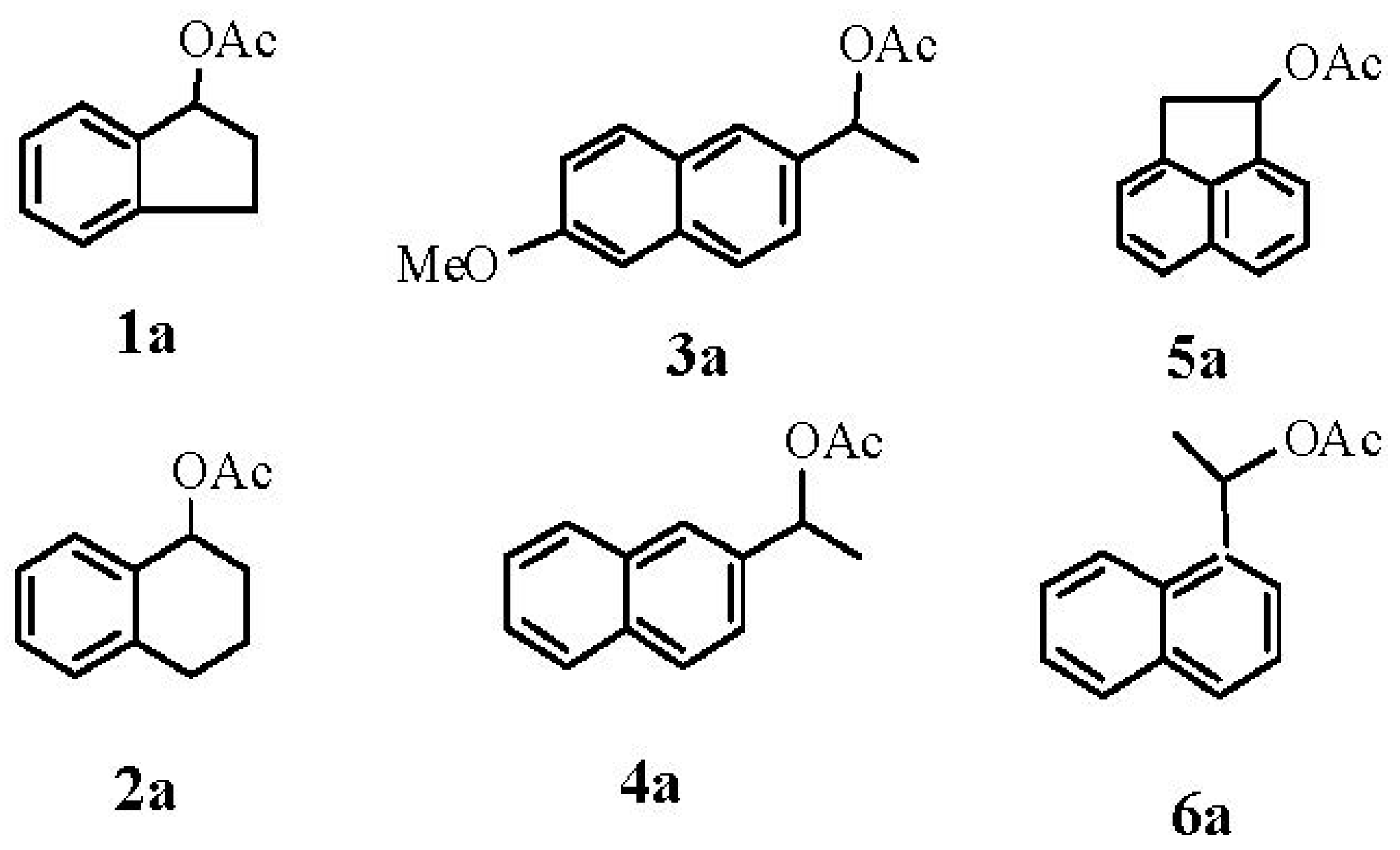
3.2. General Procedure for the Synthesis of Racemic Acetates 1a–6a
3.3. General Procedure for the Hydrolysis of Racemic Acetates 1a–6a with CAL-B
3.4. Typical Procedure for Synthesis of Enantiomerically Pure Acetates (S)-1a–4a
3.5. The Conditions for the Analysis of Alcohols (R)-1–6 Are Reported Below
- 1:
- (R)-(−)-Indan-1-ol: HPLC (Chiralcel® OD-H), tR = 37.02 min; tS = 43.24 min; (Hexane/i-PrOH 98:2 flow: 0.5 mL/min). [α]D = −16.7 (c 1, MeOH).
- 2:
- (R)-(−)-1,2,3,4-Tetrahydro-1-naphthalenol: HPLC (Chiralcel® OD-H), tR = 15.82 min; tS = 17.64 min; (hexane/i-PrOH 95:5, flow: 0.5 mL/min). [α]D = −28.1(c 2, MeOH).
- 3:
- (R)-(+)-1-(6-Methoxy-2-naphthyl) ethanol: HPLC (Chiralcel® OD-H), tR = 19.47 min; tS = 26.69 min; (hexane/i-PrOH 90:10, flow: 0.5 mL/min). [α]D = +36.4 (c 0.8, EtOH).
- 4:
- (R)-(+)-1-(2-Naphthyl) ethanol: HPLC (Chiralcel® OD-H), tR = 30.54 min; tS = 33.71 min; (hexane/i-PrOH 95:5, flow: 0.5 mL/min). [α]D = +36.5 (c 1, MeOH).
- 5:
- (R)-(−)-1-Acenaphthenol: HPLC (Chiralcel® OD-H), tR = 28.80 min; tS = 34.67 min; (hexane/i-PrOH 95:5, flow: 0.5 mL/min). [α]D = −1.4 (c 2.6, CHCL3).
- 6:
- (R)-(+)-1-(1-Naphthyl) ethanol: HPLC (Chiralcel® OD-H), tR = 20.05 min; tS = 28.90; (hexane/i-PrOH 90:10, flow: 0.5 mL/min). [α]D = +66.5 (c 1, MeOH).
3.6. The Conditions for the Analysis of Acetates (S)-1a–6a Are Reported Below
- 1a:
- (S)-(+)- Indan-1-yl acetate: HPLC (Chiralcel® OD-H), tS = 10.14 min; tR = 11.18 min; (Hexane/i-PrOH 98:2 flow: 0.5 mL/min). [α]D = −110.1 (c 2, CHCl3).
- 2a:
- (S)-(+)-1,2,3,4-Tetrahydro-1-naphthalenol acetate: HPLC (Chiralcel® OD-H), tS = 8.62 min; tR = 9.05 min; (hexane/i-PrOH 95:5, flow: 0.5 mL/min). [α]D = −112.8 (c 2, CHCl3).
- 3a:
- (S)-(−)-1-[2-(6-Methoxynaphthyl)] ethyl acetate: HPLC (Chiralcel® OD-H), tS = 10.78 min; tR = 12.05 min; (hexane/i-PrOH 90:10, flow: 0.5 mL/min). [α]D = −110 (c 1, EtOH).
- 4a:
- (S)-(−)-1-(2-Naphthyl) ethyl acetate: HPLC (Chiralcel® OD-H), tS = 10.47 min; tR = 11.98 min; (hexane/i-PrOH 95:5, flow: 0.5 mL/min). [α]D = −110.2 (c 1, CHCl3).
- 5a:
- (S)-(+)-1-Acenaphthylenol-1,2-dihydro acetate: HPLC (Chiralcel® OD-H), tS = 11.88 min; tR = 12.52 min; (hexane/i-PrOH 95:5, flow: 0.5 mL/min). [α]D = −85.9 (c 2.4, CHCL3).
- 6a:
- (S)-(−)-1-(1-Naphthyl)ethyl acetate: HPLC (Chiralcel® OD-H), tS = 9.87 min; tR = 13.50 min; (hexane/i-PrOH 90:10, flow: 0.5 mL/min). [α]D = −49.5 (c 1, CHCl3).
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Itoh, N.; Isotani, K.; Nakamura, M.; Inoue, K.; Isogai, Y.; Makino, Y. Efficient synthesis of optically pure alcohols by asymmetric hydrogen-transfer biocatalysis: Application of engineered enzymes in a 2-propanol-water medium. Appl. Microbiol. Biotechnol. 2012, 93, 1075–1085. [Google Scholar] [CrossRef]
- Ahmed, M.; Kelly, T.; Ghanem, A. Applications of enzymatic and non-enzymatic methods to access enantiomerically pure compounds using kinetic resolution and racemisation. Tetrahedron 2012, 68, 6781–6802. [Google Scholar] [CrossRef]
- Lee, Y.S.; Murphy, J.M.; Ukai, A.; Fu, G.C. Nanoenzymatic dynamic kinetic resolution of Secondary alcohols via acylation: Synthetic and mechanistic studies. J. Am. Chem. Soc. 2012, 134, 15149–15153. [Google Scholar] [CrossRef]
- Adams, T.B.; McGowen, M.M.; Williams, M.C. The FEMA GRAS assessment of aromatic substituted secondary alcohols, ketones, and related esters used as flavor ingredients. Food Chem. Toxicol. 2007, 45, 171–201. [Google Scholar] [CrossRef]
- Pollard, D.J.; Woodley, M.J. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef]
- Athawale, V.; Manrekar, N.; Athawale, M. Effect of Reaction Parameters on Synthesis of Citronellyl Methacrylate by Lipase-Catalyzed Transesterification. Biotechnol. Prog. 2003, 19, 298–302. [Google Scholar] [CrossRef]
- Turner, N.J. Deracemisation methods. Curr. Opin. Chem. Biol. 2010, 14, 115–121. [Google Scholar] [CrossRef]
- Bouzemi, N.; Debbeche, H.; Aribi-Zouioueche, L.; Fiaud, J.C. On the use of succinic anhydride as acylating agent for practical resolution of aryl-alkyl alcohols through lipase-catalyzed acylation. Tetrahedron Lett. 2004, 45, 627–630. [Google Scholar] [CrossRef]
- Escorcia, A.M.; Molina, D.; Daza, M.C.; Doerr, M. Acetylation of (R,S)-propranolol catalyzed by Candida antarctica lipase B: An experimental and computational study. J. Mol. Catal. B 2013, 98, 21–29. [Google Scholar] [CrossRef]
- Salezadeh-Asl, R.; Lee-Ruff, E. Enantiomeric resolution of cyclobutanones and related derivatives by enzyme-catalyzed acylation and hydrolysis. Tetrahedron 2005, 16, 3986–3991. [Google Scholar] [CrossRef]
- Strauss, U.T.; Felfer, U.; Faber, K. Biocatalytic transformation of racemates into chiral building blocks in 100% chemical yield and 100% enantiomeric excess. Tetrahedron 1999, 10, 107–117. [Google Scholar] [CrossRef]
- Larissegger-Schnell, B.; Glueck, S.M.; Kroutil, W.; Faber, K. Enantio-complementary deracemization of (±)-2-hydroxy-4-phenylbutanoic acid and (±)-3-phenyllactic acid using lipase-catalyzed kinetic resolution combined with biocatalytic racemization. Tetrahedron 2006, 62, 2912–2916. [Google Scholar]
- Kamaruddin, A.H.; Uzir, M.H.; Aboul-enein, H.Y.; hairul, N.A. Chemoenzymatic and microbial dynamic kinetic resolutions. Chirality 2009, 21, 449–467. [Google Scholar] [CrossRef]
- Traff, A.; Lihammar, R.; Backvall, J.E. A Chemoenzymatic Dynamic Kinetic Resolution Approach to Enantiomerically Pure (R)- and (S)-Duloxetine. J. Org. Chem. 2011, 76, 3917–3921. [Google Scholar] [CrossRef]
- Merabet-Khelassi, M.; Vriamont, N.; Riant, O.; Aribi-Zouioueche, L. Racemization of secondary alcohols catalyzed by ruthenium: Application to chemoenzymatic dynamic resolution. Tetrahedron 2011, 22, 1790–1796. [Google Scholar]
- Hoyos, P.; Pace, V.; Alcántara, A.R. Dynamic Kinetic Resolution via Hydrolase-Metal Combo Catalysis in Stereoselective Synthesis of Bioactive Compounds. Adv. Synth. Catal. 2012, 354, 2585–2611. [Google Scholar] [CrossRef]
- Mantovani, S.M.; Angolini, C.F.; Marsaioli, A.J. Mechanistic investigation of the Candida albicans CCT 0776 stereoinversion system and application to obtain enantiopure secondary alcohols. Tetrahedron 2009, 20, 2635–2638. [Google Scholar] [CrossRef]
- Koszelewski, D.; Pressnitz, D.; Clay, D.; Kroutil, K. Deracemization of mexiletine biocatalyzed by ω-Transaminases. Org. Lett. 2009, 11, 4810–4812. [Google Scholar] [CrossRef]
- Danda, H.; Maehara, A.; Umemura, T. Preparation of (4S)-4-hydroxy-3-methyl-2-(2edirect.yl)-2-cyclopentenone by combination of enzymatic hydrolysis and chemical transformation. Tetrahedron Lett. 1991, 32, 5119–5122. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, I.S. Combined Chemical and Enzymatic Synthesis of (S,S)-2,5-Dimethylpyrrolidine. Synlett 1993, 1993, 767–768. [Google Scholar]
- Turner, N.J. Controlling chirality. Curr. Opin. Biotechnol. 2003, 14, 401–406. [Google Scholar] [CrossRef]
- Mitsunobu, O.; Yamada, M. Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382. [Google Scholar] [CrossRef]
- Wallner, A.; Mang, H.; Glueck, S.M.; Steinreiber, A.; Mayer, S.F.; Faber, K. Chemo-enzymatic enantio-convergent asymmetric total synthesis of (S)-(+)-dictyoprolene using a kinetic resolution-stereoinversion protocol. Tetrahedron 2003, 14, 2427–2432. [Google Scholar] [CrossRef]
- Shimada, Y.; Usuda, K.; Okabe, H.; Suzuki, T.; Matsumoto, K. Deracemization of 1,2-diol monotosylate derivatives by a combination of enzymatic hydrolysis with the Mitsunobu inversion using polymer-bound triphenylphosphine. Tetrahedron 2009, 20, 2802–2808. [Google Scholar] [CrossRef]
- Bouzemi, N.; Aribi-Zouioueche, L.; Fiaud, J.C. Combined lipase-catalyzed resolution/Mitsunobu esterification for the production of enantiomerically enriched arylalkyl carbinols. Tetrahedron 2006, 17, 797–800. [Google Scholar] [CrossRef]
- Chênevert, R.; Gravil, S.; Bolte, J. Enzymatic resolution of 1,1-dimethoxybut-3-en-2-ol and 1,1-dimethoxypent-4-en-2-ol, α-hydroxyaldehyde precursors for aldol-type reactions. Tetrahedron 2005, 16, 2081–2086. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.L.; Wang, F.; Mun, H.S.; Suh, M.; Jeong, J.H. Resolution of α-methylbenzylamine via diastereomeric salt formation using the naturally based reagent N-tosyl-(S)-phenylalanine together with a solvent switch technique. Tetrahedron 2008, 19, 1647–1653. [Google Scholar] [CrossRef]
- Steinreiber, A.; Stadler, A.; Mayer, S.F.; Faber, K.; Kappe, C.O. High-speed microwave-promoted Mitsunobu inversions: Application toward the deracemization of sulcatol. Tetrahedron Lett. 2001, 42, 6283–6286. [Google Scholar] [CrossRef]
- Zaks, A.; Tamarez, M.; Li, T. Convergent Synthesis of Both Enantiomers of 4-Hydroxypent-2-ynoic Acid Diphenylamide for a Thrombin Receptor Antagonist Sch 530348 and Himbacin Analogues. Adv. Synth. Catal. 2009, 351, 2351–2357. [Google Scholar] [CrossRef]
- Aribi-Zouioueche, L.; Fiaud, J.C. Kinetic resolution of 1-acenaphthenol and 1-acetoxynaphthene through lipase-catalyzed acylation and hydrolysis. Tetrahedron Lett. 2000, 41, 4085–4088. [Google Scholar] [CrossRef]
- Merabet-khelassi, M.; Bouzemi, N.; Fiaud, J.-C.; Riant, O.; Aribi-Zouioueche, L. Effet de la quantité de lipase sur la sélectivité du dédoublement cinétique par acylation enzymatique des arylalkylcarbinols. Comptes Rendus Chim. 2011, 14, 978–986. (In French) [Google Scholar] [CrossRef]
- Paizs, C.; Tosa, M.; Majdik, C.; Tahtinen, P.; Irimie, F.D.; Kanerva, L.T. Candida antarcticalipase A in the dynamic resolution of novel furylbenzotiazol-based cyanohydrin acetates. Tetrahedron 2003, 14, 619–627. [Google Scholar] [CrossRef]
- Rotticci, D.; Norin, T.; Hult, K. Mass Transport Limitations Reduce the Effective Stereospecificity in Enzyme-Catalyzed Kinetic Resolution. Org. Lett. 2000, 2, 1373–1376. [Google Scholar] [CrossRef]
- Warmerdam, E.; Brussee, J.; Kruse, C.G.; van der Gen, A. Inversion of the configuration of cyanohydrins by a mitsunobu esterification reaction. Tetrahedron 1993, 49, 1063–1070. [Google Scholar] [CrossRef]
- Hillier, M.C.; Desrosiers, J.N.; Marcoux, J.F.; Grabowski, E.J.J. Stereoselective Carbon–Carbon Bond Formation via the Mitsunobu Displacement of Chiral Secondary Benzylic Alcohols. Org. Lett. 2004, 9, 573–576. [Google Scholar]
- Thvedt, T.H.K.; Fuglseth, E.; Sundby, E.; Hoff, B.H. Enantioenriched 1-aryl-2-fluoroethylamines. Efficient lipase-catalysed resolution and limitations to the Mitsunobu inversion protocol. Tetrahedron 2010, 66, 6733–6743. [Google Scholar] [CrossRef]
- Houiene, Z.; Merabet-Khelassi, M.; Bouzemi, N.; Riant, O.; Aribi-Zouioueche, L. A green route to enantioenriched (S)-arylalkyl carbinols by deracemization via combined lipase alkaline-hydrolysis/Mitsunobu esterification. Tetrahedron 2013, 24, 290–296. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bouzemi, N.; Grib, I.; Houiene, Z.; Aribi-Zouioueche, L. Enantiocomplementary Preparation of (S)- and (R)-Arylalkylcarbinols by Lipase-Catalysed Resolution and Mitsunobu Inversion: Impact of Lipase Amount. Catalysts 2014, 4, 215-225. https://doi.org/10.3390/catal4030215
Bouzemi N, Grib I, Houiene Z, Aribi-Zouioueche L. Enantiocomplementary Preparation of (S)- and (R)-Arylalkylcarbinols by Lipase-Catalysed Resolution and Mitsunobu Inversion: Impact of Lipase Amount. Catalysts. 2014; 4(3):215-225. https://doi.org/10.3390/catal4030215
Chicago/Turabian StyleBouzemi, Nassima, Ismahane Grib, Zahia Houiene, and Louisa Aribi-Zouioueche. 2014. "Enantiocomplementary Preparation of (S)- and (R)-Arylalkylcarbinols by Lipase-Catalysed Resolution and Mitsunobu Inversion: Impact of Lipase Amount" Catalysts 4, no. 3: 215-225. https://doi.org/10.3390/catal4030215