Catalysts Supported on Carbon Materials for the Selective Hydrogenation of Citral
Abstract
:1. Introduction

- (i)
- Carbon surface is relatively inert, preventing the occurrence of unwanted reactions catalyzed by the support surface or reaction of the support with the active phase.
- (ii)
- The cost of conventional carbon materials is lower than other conventional supports.
- (iii)
- Carbon materials may be obtained in different forms (granules, pellets, fibers, foams, monoliths, fabrics, coatings, etc.).
- (iv)
- The active phase, usually expensive, can be easily recovered by simple calcination of the support.
- (v)
- They have a high surface area and its porous framework can be modified to obtain the pore size distribution (PSD) optimum for each particular reaction.
- (vi)
- They are stable at high temperatures in non-oxidizing atmospheres (even above 700 °C).
- (vii)
- Although carbon is usually a material with a hydrophobic nature, the chemical nature of their surface can be modified chemically to give them some hydrophilicity.
2. Hydrogenation Selectivity


3. The Type of Transition Metal as Catalytic Phase
| Metal | Support | T ª (°C) | P (Bar) | S (%) | C (%) | Ref. |
|---|---|---|---|---|---|---|
| Rh | TiO2 | 70 | 70 | 10 | 100 | [75] |
| Rh | TiO2 | 70 | 70 | 11 | 94 | [76] |
| Rh | SiO2 | 35 | 1 | 5.2 | 100 | [77] |
| Pt | TiO2 | 70 | 70 | 58 | 98 | [75] |
| Pt | TiO2 | 90 | 100 | 68 | 95 | [78] |
| Pt | SiO2 | 100 | 20 | 56 | 30 | [79] |
| Ir | TiO2 | 90 | 6.2 | 100 | 11.2 | [80] |
| Ir | SiO2 | 90 | 6.2 | 47 | 5 | [81] |
| Ir | Nb2O5 | 90 | 6.2 | 82 | 15 | [82] |
| Ir | SiO2 | 90 | 6.2 | 44 | 3 | [82] |
| Ir | TiO2 | 90 | 6.2 | 91 | 10 | [81] |
| Ir | SiO2 | 70 | 4 | 100 | 10 | [83] |
| Ir | TiO2 | 70 | 4 | 100 | 10 | [83] |
| Ir | SiO2 | 27 | 1 | 15 | 5 | [44] |
| Os | SiO2 | 27 | 1 | 88 | 5 | [44] |
| Co | TiO2 | 90 | 70 | 50 | 80 | [42] |
| Co | C | 120 | 10 | 60 | 17 | [84] |
| Au | TiO2 | 80 | 40 | 16 | 6 | [85] |
| Ru | TiO2 | 80 | 40 | 22 | 15 | [85] |
| Ru | TiO2 | 126 | 50 | 42 | 76.8 | [86] |
| Ru | Al2O3 | 126 | 50 | 48 | 12.2 | [86] |
| Pd | TiO2 | 80 | 40 | 8 | 27 | [85] |
| Pd | SiO2 | 130 | 70 | 0 | 100 | [87] |
| Ni | Al2O3 | 70 | 1 | 0 | n.d. | [88] |
| Ni | Cr2O3 | 120 | 40 | 0 | n.d. | [89] |
| Ni | Graphite | 50 | 50 | 0 | 100 | [90] |
4. Carbon Materials as Supports
4.1. Activated Carbons

4.2. Graphite
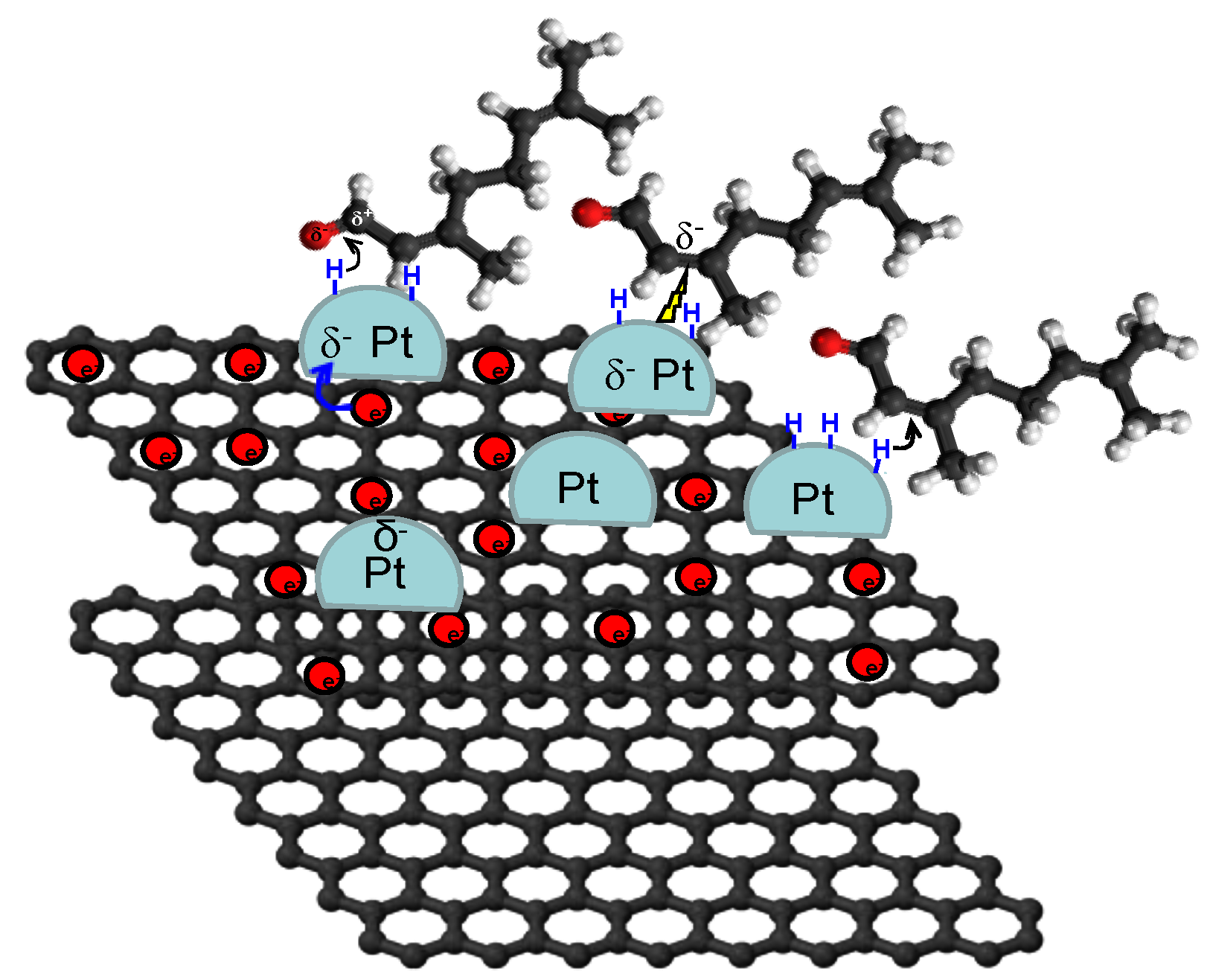
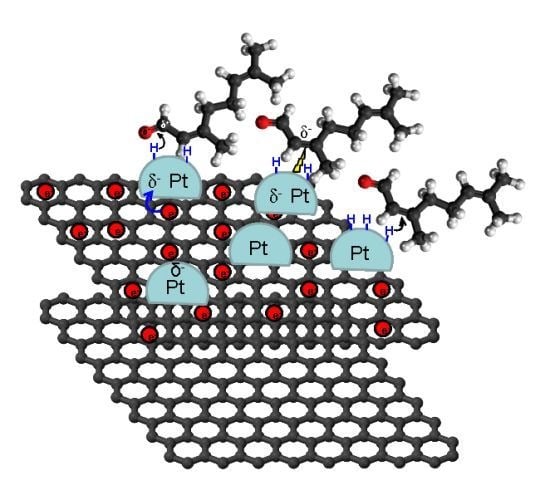
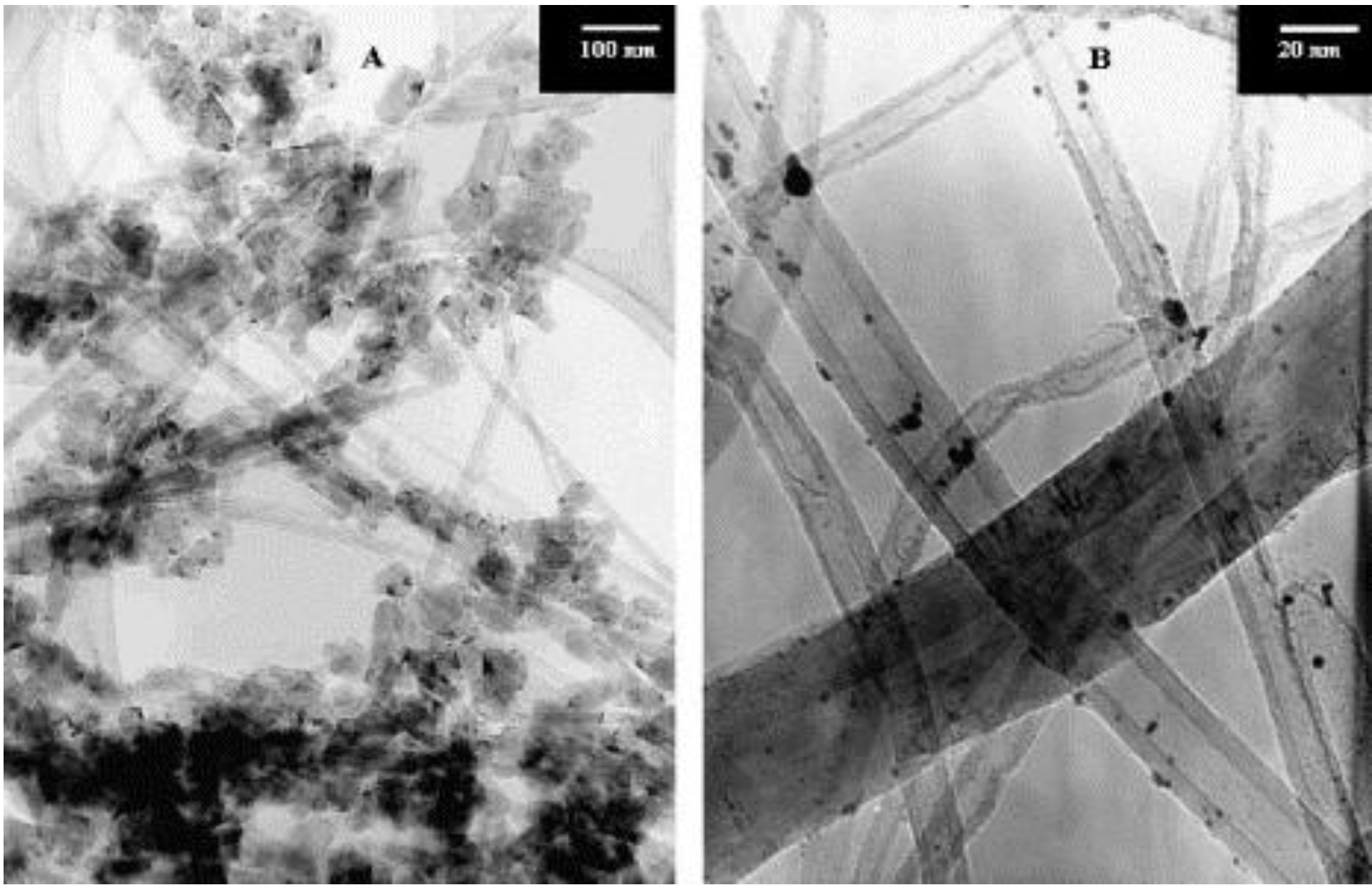
4.3. Carbon Nanotubes
4.4. Composite Materials
 Ti3+ or Ti4+ species and ☐ oxygen vacancies and
Ti3+ or Ti4+ species and ☐ oxygen vacancies and  C=O bond [75,129].
C=O bond [75,129].
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Baser, K.H.C.; Kürkçüoglu, M.; Demirci, B. Ninde Oil (Aeollanthus myrianthus Taylor) Revisited: Analysis of a Historical Oil. J. Essent. Oil Res. 2005, 17, 137–138. [Google Scholar] [CrossRef]
- Baydar, H.; Baydar, N.G. The effects of harvest date, fermentation duration and Tween 20 treatment on essential oil content and composition of industrial oil rose (Rosa damascena Mill.). Ind. Crop. Prod. 2005, 21, 251–255. [Google Scholar] [CrossRef]
- Dubey, V.S.; Luthra, R. Biotransformation of geranyl acetate to geraniol during palmarosa (Cymbopogon martinii, Roxb. wats. var. motia) inflorescence development. Phytochemistry 2001, 57, 675–680. [Google Scholar] [CrossRef]
- Simon, D.Z.; Beliveau, J.; Aube, C. Extraction by hydrodiffusion of the essential oil of Monarda fistulosa grown in the province of Quebec: Assay of geraniol in the hydrodiffused oil. Int. J. Crude Drug Res. 1986, 24, 120–122. [Google Scholar]
- Rajeswara Rao, B.R.; Bhattacharya, A.K.; Mallavarapu, G.R.; Ramesh, S. Yellowing and crinkling disease and its impact on the yield and composition of the essential oil of citronella (Cymbopogon winterianus Jowitt.). Flavour Frag. J. 2004, 19, 344–350. [Google Scholar] [CrossRef]
- Bedoukian, P.Z. Geraniol and Nerol. Perfumery and Flavoring Synthetics; Allured Publishing Corporation: Wheaton, State, USA, 1986. [Google Scholar]
- Clark, G.S. Geraniol. Perfum. Flavorist 1998, 23, 19–25. [Google Scholar]
- Rastogi, S.C.; Johansen, J.D.; Frosch, P.; Menne, T.; Bruze, M.; Lepoittevin, J.P.; Dreier, B.; Andersen, K.E.; White, I.R. Deodorants on the European market: Quantitative chemical analysis of 21 fragrances. Contact Dermatitis 1998, 38, 29–35. [Google Scholar] [CrossRef]
- Rastogi, S.C.; Heydorn, S.; Johansen, J.D.; Basketter, D.A. Fragrance chemicals in domestic and occupational products. Contact Dermatitis 2001, 45, 221–225. [Google Scholar] [CrossRef]
- Rastogi, S.C.; Johansen, J.D.; Menné, T. Natural ingredients based cosmetics. Content of selected fragrance sensitizers. Contact Dermatitis 1996, 34, 423–426. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol: A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Sato, K.; Krist, S.; Buchbauer, G. Antimicrobial effect of vapours of geraniol, (R)-(−)-linalool, terpineol, γ-terpinene and 1,8-cineole on airborne microbes using an airwasher. Flavour Frag. J. 2007, 22, 435–437. [Google Scholar] [CrossRef]
- Bugband. Available online: http://www.bugband.net (accessed on 4 August 2013).
- Fulltec. Available online: http://www.fulltec.org (accessed on 4 August 2013).
- Papachristos, D.P.; Karamanoli, K.I.; Stamopoulos, D.C.; Menkissoglu-Spiroudi, U. The relationship between the chemical composition of three essential oils and their insecticidal activity against Acanthoscelides obtectus (Say). Pest Manag. Sci. 2004, 60, 514–520. [Google Scholar] [CrossRef]
- Ji, P.; Si, M.S.; Podnos, Y.; Imagawa, D.K. Monoterpene geraniol prevents acute allograft rejection. Transplant. Proc. 2002, 34, 1418–1419. [Google Scholar] [CrossRef]
- Hierro, I.; Valero, A.; Pérez, P.; González, P.; Cabo, M.M.; Montilla, M.P.; Navarro, M.C. Action of different monoterpenic compounds against Anisakis simplex s.l. L3 larvae. Phytomedicine 2004, 11, 77–82. [Google Scholar] [CrossRef]
- Navarro, M.C.; Noguera, M.A.; Romero, M.C.; Montilla, M.P.; González de Selgas, J.M.; Valero, A. Anisakis simplex s.l.: Larvicidal activity of various monoterpenic derivatives of natural origin against L3 larvae in vitro and in vivo. Exp. Parasitol 2008, 120, 295–299. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants-Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol Vitro 2009, 23, 295–301. [Google Scholar] [CrossRef]
- Shoji, Y.; Ishige, H.; Tamura, N.; Iwatani, W.; Norimatsu, M.; Shimada, J.; Mizushima, Y. Enhancement of anti-herpetic activity of antisense phosphorothioate oligonucleotides 5' end modified with geraniol. J. Drug Targeting 1998, 5, 261–273. [Google Scholar] [CrossRef]
- Ahmad, S.T.; Arjumand, W.; Seth, A.; Nafees, S.; Rashid, S.; Ali, N.; Sultana, S. Preclinical renal cancer chemopreventive efficacy of geraniol by modulation of multiple molecular pathways. Toxicology 2011, 290, 69–81. [Google Scholar] [CrossRef]
- Kim, S.H.; Bae, H.C.; Park, E.J.; Lee, C.R.; Kim, B.J.; Lee, S.; Park, H.H.; Kim, S.J.; So, I.; Kim, T.W.; Jeon, J.H. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem. Biophys. Res. Commun. 2011, 407, 129–134. [Google Scholar] [CrossRef]
- Madankumar, A.; Jayakumar, S.; Asokkumar, S.; Raghunandhakumar, S.; Naveenkumar, C.; Devaki, T. Chemopreventive potential of geraniol on 4-Nitroquinoline-1 oxide induced oral carcinogenesis in rats. Int. J. Res. Pharm. Sci. 2011, 2, 531–536. [Google Scholar]
- Polo, M.P.; de Bravo, M.G. Effect of geraniol on fatty-acid and mevalonate metabolism in the human hepatoma cell line Hep G2. Biochem. Cell B 2006, 84, 102–111. [Google Scholar] [CrossRef]
- Wiseman, D.A.; Werner, S.R.; Crowell, P.L. Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21 Cip1 and p27 Kip1 in human pancreatic adenocarcinoma cells. J. Pharm. Exp. Ther. 2007, 320, 1163–1170. [Google Scholar] [CrossRef]
- Groling, J. Ullmann’s Encyclopedia of Industrial Chemistry; Willey-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Eisenacher, M.; Beschnitt, S.; Hölderich, W. Novel route to a fruitful mixture of terpene fragrances in particular phellandrene starting from natural feedstock geraniol using weak acidic boron based catalyst. Catal. Commun. 2012, 26, 214–217. [Google Scholar]
- Somogyi, L.P.; Kishi, A. Aroma Chemicals and the Flavour and Fragrance Industry; Chemical Economics Handbook (CEH) Product Review; Technical Report for SRI International: Menlo Park, CA, USA, August 2001. [Google Scholar]
- Weiss, R. Hydrochlorination of Myrcene. U.S. patent 28823223, 14 April 1959. [Google Scholar]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; WILEY-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Pérez-Cadenas, A.F.; Ros, C.H.; Morales-Torres, S.; Pérez-Cadenas, M.; Kooyman, P.J.; Moreno-Castilla, C.; Kapteijn, F. Metal-doped carbon xerogels for the electro-catalytic conversion of CO2 to hydrocarbons. Carbon 2013, 56, 324–331. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Design of low-temperature Pt-carbon combustion catalysts for VOC's treatments. J. Hazard. Mater. 2010, 183, 814–822. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J.; Moreno-Castilla, C.; Pérez-Cadenas, A.F. Catalytic combustion of toluene on platinum-containing monolithic carbon aerogels. Applied Catalysis B: Environmental 2004, 54, 217–224. [Google Scholar]
- Duarte, F.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Madeira, L.M. Fenton-like degradation of azo-dye Orange II catalyzed by transition metals on carbon aerogels. Appl. Catal. B 2009, 85, 139–147. [Google Scholar] [CrossRef]
- Samant, P.V.; Pereira, M.F.R.; Figueiredo, J.L. Mesoporous carbon supported Pt and Pt-Sn catalysts for hydrogenation of cinnamaldehyde. Catal. Today 2005, 102–103, 183–188. [Google Scholar]
- Mahata, N.; Gonçalves, F.; Pereira, M.F.; Figueiredo, J.L. Selective hydrogenation of cinnamaldehyde to cinnamyl alcohol over mesoporous carbon supported Fe and Zn promoted Pt catalyst. Appl. Catal. A 2008, 339, 159–168. [Google Scholar] [CrossRef]
- Machado, B.F.; Morales-Torres, S.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L. Preparation of carbon aerogel supported platinum catalysts for the selective hydrogenation of cinnamaldehyde. Appl. Catal. A 2012, 425–426, 161–169. [Google Scholar]
- Giroir-Fendler, A.; Richard, D.; Gallezot, P. Heterogeneus Catalysis and Fine Chemicals. Studies in Surface Science and Catalysis.; Elsevier: Amsterdam, Netherlands, 1988. [Google Scholar]
- Kouachi, K.; Lafaye, G.; Especel, C.; Cherifi, O.; Marecot, P. Effects of support and metal loading on the characteristics of Co based catalysts for selective hydrogenation of citral. J. Mol. Catal. A 2008, 280, 52–60. [Google Scholar] [CrossRef]
- Neri, G.; Mercadante, L.; Donato, A.; Visco, A.M.; Galvagno, S. Influence of Ru precursor, support and solvent in the hydrogenation of citral over ruthenium catalysts. Catal. Lett. 1994, 29, 379–386. [Google Scholar] [CrossRef]
- Singh, U.K.; Vannice, M.A. Liquid-phase citral hydrogenation over SiO2-supported group VIII metals. J. Catal. 2001, 199, 73–84. [Google Scholar] [CrossRef]
- Coq, B.; Kumbhar, P.S.; Moreau, C.; Moreau, P.; Warawdekar, M.G. Liquid phase hydrogenation of cinnamaldehyde over supported ruthenium catalysts: Influence of particle size, bimetallics and nature of support. J. Mol. Catal. 1993, 85, 215–228. [Google Scholar] [CrossRef]
- Giroir-Fendler, A.; Richard, D.; Gallezot, P. Chemioselectivity in the catalytic hydrogenation of cinnamaldehyde. Effect of metal particle morphology. Catal. Lett. 1990, 5, 175–181. [Google Scholar] [CrossRef]
- Plomp, A.J.; Vuori, H.; Krause, A.O.; de Jong, K.P.; Bitter, J.H. Particle size effects for carbon nanofiber supported platinum and ruthenium catalysts for the selective hydrogenation of cinnamaldehyde. Appl. Catal. A 2008, 351, 9–15. [Google Scholar] [CrossRef]
- Neri, G.; Milone, C.; Donato, A.; Mercadante, L.; Visco, A.M. Selective Hydrogenation of Citral Over Pt-Sn Supported on Activated Carbon. J. Chem. Technol. Biotechnol. 1994, 60, 83–88. [Google Scholar] [CrossRef]
- Neri, G.; Mercadante, L.; Milone, C.; Pietropaolo, R.; Galvagno, S. Hydrogenation of citral and cinnamaldehyde over bimetallic Ru-Me/Al2O3 catalysts. J. Mol. Catal. A 1996, 108, 41–50. [Google Scholar] [CrossRef]
- Ponec, V. On the role of promoters in hydrogenations on metals; α,β-unsaturated aldehydes and ketones. Appl. Catal. A 1997, 149, 27–48. [Google Scholar] [CrossRef]
- Barbaro, P.; Liguori, F. Heterogenized Homogeneous Catalysts for Fine Chemicals Production: Materials and Processes; Springer: London, UK, 2010. [Google Scholar]
- Claus, P.; Önal, Y. Regioselective hydrogenations. Handbook of Heterogeneous Catalysis. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; WILEY-VCH: Weinheim, Germany, 2008; pp. 3311–3312. [Google Scholar]
- Vannice, A.; Singh, U.K. Citral hydrogenation over Pt and other group VIII metals. Abstr. Pap. Amer. Chem. Soc. 2002, 223, 437–437. [Google Scholar]
- Delbecq, F.; Sautet, P. A density functional study of adsorption structures of unsaturated aldehydes on Pt(III): a key factor for hydrogenation Selectivity. J. Catal. 2002, 211, 398–406. [Google Scholar]
- Claus, P. Selective hydrogenation ofα,β-unsaturated aldehydes and other C=O and C=C bonds containing compounds. Topic. Catalysis 1998, 5, 51–62. [Google Scholar] [CrossRef]
- Ekou, T.; Vicente, A.; Lafaye, G.; Especel, C.; Marecot, P. Bimetallic Rh-Ge and Pt-Ge catalysts supported on TiO2 for citral hydrogenation II. Catalytic properties. Appl. Catal. A 2006, 314, 73–80. [Google Scholar] [CrossRef]
- Delbecq, F.; Sautet, P. Competitive C=C and C=O Adsorption ofα,β-Unsaturated Aldehydes on Pt and Pd Surfaces in Relation with the Selectivity of Hydrogenation Reactions: A Theoretical Approach. J. Catal. 1995, 152, 217–236. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Tiainen, L.P.; Neyestanaki, A.K.; Sjoholm, R.; Rantakyla, T.K.; Laine, E.; Salmi, T.; Murzin, D.Y. Liquid phase hydrogenation of citral: Suppression of side reactions. Appl. Catal. A 2002, 237, 181–200. [Google Scholar] [CrossRef]
- Hájek, J.; Kumar, N.; Mäki-Arvela, P.; Salmi, T.; Murzin, D.Y.; Paseka, I.; Heikkilä, T.; Laine, E.; Laukkanen, P.; Väyrynen, J. Ruthenium-modified MCM-41 mesoporous molecular sieve and Y zeolite catalysts for selective hydrogenation of cinnamaldehyde. Appl. Catal. A 2003, 251, 385–396. [Google Scholar] [CrossRef]
- Berty, T.E.; Reamer, H.H.; Sage, B.H. Phase behavior in the hydrogen-cyclohexane system. J. Chem. Eng. Data 1966, 11, 25–30. [Google Scholar] [CrossRef]
- Kun, I.; Szöllösi, G.; Bartók, M. Crotonaldehyde hydrogenation over clay-supported platinum catalysts. J. Mol. Catal. A 2001, 169, 235–246. [Google Scholar] [CrossRef]
- Yamada, H.; Goto, S. The effect of solvents polarity on selective hydrogenation of unsaturated aldehyde in gas-liquid-solid three phase reactor. J. Chem. Eng. Jpn. 2003, 36, 586–589. [Google Scholar] [CrossRef]
- Burgener, M.; Furrer, R.; Mallat, T.; Baiker, A. Hydrogenation of citral over Pd/alumina: comparison of “supercritical” CO2 and conventional solvents in continuous and batch reactors. Appl. Catal. A 2004, 268, 1–8. [Google Scholar] [CrossRef]
- Zhao, F.; Fujita, S.i.; Akihara, S.; Arai, M. Hydrogenation of benzaldehyde and cinnamaldehyde in compressed co2 medium with a pt/c catalyst: A study on molecular interactions and pressure effects. J. Phys. Chem. A 2005, 109, 4419–4424. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, F.; Fujita, S.i.; Arai, M. Selective hydrogenation of citral with transition metal complexes in supercritical carbon dioxide. Appl. Catal. A 2007, 316, 127–133. [Google Scholar] [CrossRef]
- Jiang, H.J.; Jiang, H.B.; Zhu, D.M.; Zheng, X.L.; Fu, H.Y.; Chen, H.; Li, R.X. Cooperation between the surface hydroxyl groups of the support and organic additives in the highly selective hydrogenation of citral. Appl. Catal. A 2012, 445–446, 351–358. [Google Scholar]
- Fujita, S.i.; Sano, Y.; Bhanage, B.M.; Arai, M. Supported liquid-phase catalysts containing ruthenium complexes for selective hydrogenation of α,β-unsaturated aldehyde: Importance of interfaces between liquid film, solvent, and support for the control of product selectivity. J. Catal. 2004, 225, 95–104. [Google Scholar] [CrossRef]
- Strohmeier, W.; Graser, B.; Mar-çec, R.; Holke, K. Comparison of the activity of homogeneous catalysts in liquid phase without solvent and as supported liquid phase catalysts (SLPC). J. Mol. Catal. 1981, 11, 257–262. [Google Scholar] [CrossRef]
- Sokolskii, D.; Anisimova, N.; Zharmagambetova, A.; Mukhamedzhanova, S.; Edygenova, L. Pt-Fe2O3 catalytic system for hydrogenation reactions. React. Kinet. Catal. Lett. 1987, 33, 399–403. [Google Scholar] [CrossRef]
- Sokolskii, D.V.; Pak, A.M.; Ginzburg, M.A.; Vozdvizhenskii, V.F. Hydrogenation of citral on group-VIII metals. Dokl. Akad. Nauk Sssr 1978, 239, 897–900. [Google Scholar]
- Sautet, P. Theoretical chemistry as a tool for interpreting catalysts selectivities. Topic. Catal. 2000, 13, 213–219. [Google Scholar] [CrossRef]
- Davis, J.L.; Barteau, M.A. Vinyl substituent effects on the reactions of higher oxygenates on Pd(111). J. Mol. Catal. 1992, 77, 109–124. [Google Scholar] [CrossRef]
- Fadley, C.S.; Shirley, D.A. Electronic Density of States. In Proceedings of the Third Materials Research Symposium, Gaithersburg, MD, USA, 1969.
- Manikandan, D.; Divakar, D.; Sivakumar, T. Selective hydrogenation of citral over noble metals intercalated montmorillonite catalysts. Catal. Lett. 2008, 123, 107–114. [Google Scholar] [CrossRef]
- Ekou, T.; Ekou, L.; Vicente, A.; Lafaye, G.; Pronier, S.; Especel, C.; Marecot, P. Citral hydrogenation over Rh and Pt catalysts supported on TiO2: Influence of the preparation and activation protocols of the catalysts. J. Mol. Catal. A 2011, 337, 82–88. [Google Scholar] [CrossRef]
- Vicente, A.; Ekou, T.; Lafaye, G.; Especel, C.; Marecot, P.; Williams, C.T. Influence of the nature of the precursor salts on the properties of Rh-Ge/TiO2 catalysts for citral hydrogenation. J. Catal. 2010, 275, 202–210. [Google Scholar] [CrossRef]
- Sordelli, L.; Psaro, R.; Vlaic, G.; Cepparo, A.; Recchia, S.; Dossi, C.; Fusi, A.; Zanoni, R. EXAFS studies of supported Rh-Sn catalysts for citral hydrogenation. J. Catal. 1999, 182, 186–198. [Google Scholar] [CrossRef]
- Ananthan, S.A.; Narayanan, V. Liquid Phase Selective Hydrogenation of Citral over Ru/Tio2 and Pt/Tio2 Nano Catalysts. In Proceedings of the International Conference on Nanoscience, Engineering and Technology, athyabama University, Chennai, Tamilnadu, India, November 2011; pp. 23–29.
- Mukherjee, S.; Vannice, M.A. Solvent effects in liquid-phase reactions: I. Activity and selectivity during citral hydrogenation on Pt/SiO2 and evaluation of mass transfer effects. J. Catal. 2006, 243, 108–130. [Google Scholar]
- Diaz, G.; Gomez-Cortes, A.; Hernandez-Cristobal, O.; Murcia, J.J.; Borda, G.; Rojas, H. Hydrogenation of citral over irau/tio2 catalysts. effect of the preparation method. Topic. Catal. 2011, 54, 467–473. [Google Scholar] [CrossRef]
- Rojas, H.; Borda, G.; Reyes, P.; Martinez, J.J.; Valencia, J.; Fierro, J.L.G. Citral hydrogenation over Ir/TiO2 and Ir/TiO2/SiO2 catalysts. Catal. Today 2008, 133, 699–705. [Google Scholar]
- Borda, G.; Rojas, H.; Murcia, J.; Fierro, J.L.G.; Reyes, P.; Oportus, M. Hydrogenation of citral on Ir/SiO2 catalysts. Effect of the addition of Nb2O5 on surface and catalytic properties. React. Kinet. Catal. Lett. 2007, 92, 369–376. [Google Scholar] [CrossRef]
- Reyes, P.; Rojas, H.; Pecchi, G.; Fierro, J.L.G. Liquid-phase hydrogenation of citral over Ir-supported catalysts. J. Mol. Catal. A 2002, 179, 293–299. [Google Scholar] [CrossRef]
- Bertero, N.M.; Trasarti, A.F.; Moraweck, B.; Borgna, A.; Marchi, A.J. Selective liquid-phase hydrogenation of citral over supported bimetallic Pt-Co catalysts. Appl. Catal. A 2009, 358, 32–41. [Google Scholar] [CrossRef]
- Liu, R.X.; Zhao, F.Y. Selective hydrogenation of citral over Au-based bimetallic catalysts in supercritical carbon dioxide. Sci. China Chem. 2010, 53, 1571–1577. [Google Scholar]
- Silva, A.M.; Santos, O.A.A.; Mendes, M.J.; Jordao, E.; Fraga, M.A. Hydrogenation of citral over ruthenium-tin catalysts. Appl. Catal. A 2003, 241, 155–165. [Google Scholar] [CrossRef]
- Vicente, A.; Lafaye, G.; Especel, C.; Marecot, P.; Williams, C.T. The relationship between the structural properties of bimetallic Pd-Sn/SiO2 catalysts and their performance for selective citral hydrogenation. J. Catal. 2011, 283, 133–142. [Google Scholar] [CrossRef]
- Tiainen, L.P.; Maki-Arvela, P.; Salmi, T. Modelling of citral hydrogenation kinetics on an Ni/Al2O3 catalyst. Catal. Today 1999, 48, 57–63. [Google Scholar] [CrossRef]
- Pak, A.M.; Konuspaev, S.R.; Zakumbaeva, G.D.; Sokolskii, D.V. Hydrogenation of Citral to Citronellol Over Ni-Cr2O3. React. Kinet. Catal. Lett. 1981, 16, 339–343. [Google Scholar] [CrossRef]
- Asedegbega-Nieto, E.; Bachiller-Baeza, B.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Modification of catalytic properties over carbon supported Ru-Cu and Ni-Cu bimetallics: I. Functional selectivities in citral and cinnamaldehyde hydrogenation. Appl. Catal. A 2006, 300, 120–129. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Zieverink, M.M.P.; Kapteijn, F.; Moulijn, J.A. High performance monolithic catalysts for hydrogenation reactions. Catal. Today 2005, 105, 623–628. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Kapteijn, F.; Zieverink, M.M.P.; Moulijn, J.A. Selective hydrogenation of fatty acid methyl esters over palladium on carbon-based monoliths: Structural control of activity and selectivity. Catal. Today 2007, 128, 13–17. [Google Scholar] [CrossRef]
- Galvagno, S.; Milone, C.; Donate, A.; Neri, G.; Pietropaolo, R. Influence of metal particle size in the hydrogenation of citral over Ru/C. Catal. Lett. 1993, 18, 349–355. [Google Scholar] [CrossRef]
- Galvagno, S.; Milone, C.; Neri, G.; Donato, A.; Pietropaolo, R. Hydrogenation of cinnamaldehyde and citral over ru supported catalysts. Stud. Surf. Sci. Catal. 1993, 78, 163–170. [Google Scholar] [CrossRef]
- Neri, G.; Milone, C.; Galvagno, S.; Pijpers, A.P.J.; Schwank, J. Characterization of Pt-Sn/carbon hydrogenation catalysts. Appl. Catal. A 2002, 227, 105–115. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Sepulveda-Escribano, A.; Rodriguez-Reinoso, F.; Duprez, D. Pt-Sn catalysts supported on highly-dispersed ceria on carbon—Application to citral hydrogenation. J. Mol. Catal. A 2007, 268, 227–234. [Google Scholar] [CrossRef]
- Vilella, I.M.; Borbath, I.; Somodi, F.; Margitfalvi, J.L.; de Miguel, S.R.; Scelza, O.A. The influence of the preparation method on the behaviour of PtGe catalysts supported on activated carbon in citral hydrogenation. Catal. Lett. 2008, 125, 254–263. [Google Scholar] [CrossRef]
- Vilella, I.M.J.; de Miguel, S.R.; Scelza, O.A. Hydrogenation of citral on Pt and PtSn supported on activated carbon felts (ACF). Latin Amer. Appl. Res. 2005, 35, 51–57. [Google Scholar]
- Vilella, I.M.J.; de Miguel, S.R.; Scelza, O.A. Pt, PtSn and PtGe catalysts supported on granular carbon for fine chemistry hydrogenation reactions. J. Mol. Catal. A 2008, 284, 161–171. [Google Scholar] [CrossRef]
- Vilella, I.M.J.; Miguel, S.R.; Salinas-Martínez de Lecea, C.; Linares-Solano, Á.; Scelza, O.A. Catalytic performance in citral hydrogenation and characterization of PtSn catalysts supported on activated carbon felt and powder. Appl. Catal. A 2005, 281, 247–258. [Google Scholar]
- Aumo, J.; Oksanen, S.; Mikkola, J.P.; Salmi, T.; Murzin, D.Y. Hydrogenation of citral over activated carbon cloth catalyst. Ind. Eng. Chem. Res. 2005, 44, 5285–5290. [Google Scholar] [CrossRef]
- Aumo, J.; Oksanen, S.; Mikkola, J.P.; Salmi, T.; Murzin, D.Y. Novel woven active carbon fiber catalyst in the hydrogenation of citral. Catal. Today 2005, 102, 128–132. [Google Scholar]
- Giroir-Fendler, A.; Richard, D.; Gallezot, P. Selectivity in cinnamaldehyde hydrogenation of group-viii metals supported on graphite and carbon. Stud. Surf. Sci. Catal. 1988, 41, 171–178. [Google Scholar]
- Steffan, M.; Klasovsky, F.; Arras, J.; Roth, C.; Radnik, J.; Hofmeister, H.; Claus, P. Carbon-carbon double bond versus carbonyl group hydrogenation: Controlling the intramolecular selectivity with polyaniline-supported platinum catalysts. Adv. Synth. Catal. 2008, 350, 1337–1348. [Google Scholar] [CrossRef]
- Gallezot, P.; Richard, D. Selective Hydrogenation of α,β-Unsaturated Aldehydes. Catal. Rev. 1998, 40, 81–126. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Guerrero-Ruiz, A.; Wang, P.; Rodríguez-Ramos, I. Hydrogenation of citral on activated carbon and high-surface-area graphite-supported ruthenium catalysts modified with iron. J. Catal. 2001, 204, 450–459. [Google Scholar] [CrossRef]
- Court, J.; Jablonski, J.; Hamarthibault, S. Hydrogenation of citral in the liquid-phase over new bimetallic Ni-M catalysts supported on graphite. Stud. Surf. Sci. Catal. 1993, 78, 155–162. [Google Scholar] [CrossRef]
- Cerro-Alarcón, M.; Bachiller-Baeza, B.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Effect of the reduction-preparation method on the surface states and catalytic properties of supported-nickel particles. J. Mol. Catal. A 2006, 258, 221–230. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Huang, C.; Chen, S.; Yu, B.; Xu, J.; Liu, Z. Pd nanoparticles immobilized on graphite oxide modified with a base: Highly efficient catalysts for selective hydrogenation of citral. Sci. China Chem. 2013, 56, 203–209. [Google Scholar]
- Satagopan, V.; Chandalia, S.B. Selectivity aspects in the multiphase hydrogenation of alpha,beta-unsaturated aldehydes over supported noble-metal catalysts 1. J. Chem. Technol. Biotechnol. 1994, 59, 257–263. [Google Scholar] [CrossRef]
- Tin, K.C.; Wong, N.B.; Li, R.X.; Li, Y.Z.; Li, X.J. Studies on catalytic hydrogenation of citral by water-soluble palladium complex. J. Mol. Catal. A 1999, 137, 113–119. [Google Scholar] [CrossRef]
- Yang, Q.H.; Hou, P.X.; Bai, S.; Wang, M.Z.; Cheng, H.M. Adsorption and capillarity of nitrogen in aggregated multi-walled carbon nanotubes. Chem. Phys. Lett. 2001, 345, 18–24. [Google Scholar] [CrossRef]
- Nhut, J.M.; Pesant, L.; Tessonnier, J.P.; Winé, G.; Guille, J.; Pham-Huu, C.; Ledoux, M.J. Mesoporous carbon nanotubes for use as support in catalysis and as nanosized reactors for one-dimensional inorganic material synthesis. Appl. Catal. A 2003, 254, 345–363. [Google Scholar] [CrossRef]
- Li, Y.; Lai, G.H.; Zhou, R.X. Carbon nanotubes supported Pt-Ni catalysts and their properties for the liquid phase hydrogenation of cinnamaldehyde to hydrocinnamaldehyde. Appl. Surf. Sci. 2007, 253, 4978–4984. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Chen, L.; Dong, C.; Yu, W.; Huang, T.; Qian, Y. Pt nanoparticles deposited over carbon nanotubes for selective hydrogenation of cinnamaldehyde. Catal. Commun. 2007, 8, 452–456. [Google Scholar] [CrossRef]
- Abbaslou, R.M.M.; Tavassoli, A.; Soltan, J.; Dalai, A.K. Iron catalysts supported on carbon nanotubes for Fischer-Tropsch synthesis: Effect of catalytic site position. Appl. Catal. A 2009, 367, 47–52. [Google Scholar] [CrossRef]
- Bligaard, T. Linear energy relations and the computational design of selective hydrogenation/dehydrogenation catalysts. Angew. Chem. Int. Ed. 2009, 48, 9782–9784. [Google Scholar] [CrossRef]
- Asedegbega-Nieto, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Modification of the stereo selectivity in the citral hydrogenation by application of carbon nanotubes as support of the Pt particles. Carbon 2006, 44, 804–806. [Google Scholar] [CrossRef]
- Qin, F.; Shen, W.; Wang, C.C.; Xu, H.L. Selective hydrogenation of citral over a novel platinum/MWNTs nanocomposites. Catalysis Communications 2008, 9, 2095–2098. [Google Scholar] [CrossRef]
- Guo, G.Q.; Qin, F.; Yang, D.; Wang, C.C.; Xu, H.L.; Yang, S. Synthesis of platinum nanoparticles supported on poly(acrylic acid) grafted MWNTs and their hydrogenation of citral. Chem. Mater. 2008, 20, 2291–2297. [Google Scholar] [CrossRef]
- Zgolicz, P.D.; Stassi, J.P.; Yañez, M.J.; Scelza, O.A.; de Miguel, S.R. Influence of the support and the preparation methods on the performance in citral hydrogenation of Pt-based catalysts supported on carbon nanotubes. J. Catal. 2012, 290, 37–54. [Google Scholar] [CrossRef]
- Ananthan, S.A.; Vengidusamy, N.; Giribabu, K.; Suresh, R. Carbon nanotunes supported Pt and Pt-Ru catalysts for selective hydrogenation of citral: Effect of promoters and thermal activation of catalysts. Adv. Mater. Res. 2012, 584, 229–233. [Google Scholar] [CrossRef]
- Ananthan, S.A.; Narayanan, V. MWCNT supported Pt-Au nanocatalysts for liquid phase selective hydrogenation of citral. Int. J.Modern Chem. 2012, 1, 45–58. [Google Scholar]
- Bachiller-Baeza, B.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Influence of Mg and Ce addition to ruthenium based catalysts used in the selective hydrogenation of alpha,beta-unsaturated aldehydes. Appl. Catal. A 2001, 205, 227–237. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, M.H.; Li, M.S.; Zhu, J.J.; Shan, Y.H. Selective Hydrogenation of citral over a carbon-titania composite supported palladium catalyst. Chinese J. Chem. 2011, 29, 655–660. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Lu, M. Synthesis of carbon nanofiber-titania-cordierite monolith composite and its application as catalyst support on citral hydrogenation. Adv. Mater. Res. 2012, 535–537, 178–185. [Google Scholar]
- Zhu, J.; Lu, M.; Li, M.; Zhu, J.; Shan, Y. Synthesis of carbon-titania composite and its application as catalyst support. Mater. Chem. Phys. 2012, 132, 316–323. [Google Scholar] [CrossRef]
- Ananthan, S.A.; Narayanan, V. Liquid-Phase Hydrogenation of citral over Pt/TiO2 and Pt-Fe/TiO2 catalysts. Asian J. Chem. 2011, 23, 183–188. [Google Scholar]
- Li, D.; Ichikuni, N.; Shimazu, S.; Uematsu, T. Hydrogenation of CO2 over sprayed Ru/TiO2 fine particles and strong metal support interaction. Appl. Catal. A 1999, 180, 227–235. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bailón-García, E.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Catalysts Supported on Carbon Materials for the Selective Hydrogenation of Citral. Catalysts 2013, 3, 853-877. https://doi.org/10.3390/catal3040853
Bailón-García E, Maldonado-Hódar FJ, Pérez-Cadenas AF, Carrasco-Marín F. Catalysts Supported on Carbon Materials for the Selective Hydrogenation of Citral. Catalysts. 2013; 3(4):853-877. https://doi.org/10.3390/catal3040853
Chicago/Turabian StyleBailón-García, Esther, Francisco J. Maldonado-Hódar, Agustín F. Pérez-Cadenas, and Francisco Carrasco-Marín. 2013. "Catalysts Supported on Carbon Materials for the Selective Hydrogenation of Citral" Catalysts 3, no. 4: 853-877. https://doi.org/10.3390/catal3040853