1. Introduction
Carbon dioxide, more than 30 billion tons [
1] of which is annually released to the atmosphere, is believed to be responsible for global warming and climate change [
2,
3,
4,
5,
6,
7]. Meanwhile, it is also a nontoxic, abundant, inexpensive, nonflammable and sustainable C1 resource. From the viewpoint of environmental protection and resource utilization, it is important to transform CO
2 into useful chemicals efficiently, not only in academia but also for industrial applications. Cyclic carbonates have widely been used for various purposes, such as polar aprotic solvents, intermediates in organic synthesis, monomers for synthesizing polycarbonate, agricultural chemical, alkylating agents, electrolytic elements of lithium secondary batteries and chemical ingredients for preparing medicines [
8,
9,
10,
11]. As cycloaddition of CO
2 with epoxides are 100% atom-economical reactions, the synthesis of cyclic carbonates has received increasing attention [
3,
12,
13]. It is desirable to find a new type of material for catalyzing the cycloaddition of epoxides and CO
2 although numerous catalysts including both homogeneous and heterogeneous catalysts have already been developed [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. Ionic liquids (ILs) have only attracted considerable attention as promising catalysts in the last two decades, although they have been known for almost a century [
31]. In comparison with the traditional organic catalysts, a main advantage of the ILs is their structural diversity, which allows tuning of their properties. The tunable structures of the ILs by combination of different cations with anions make them highly promising candidates for tailored catalysts [
32]. In 2001, Peng
et al. first successfully employed ILs to catalyze cycloaddition of carbon dioxide for the synthesis of cyclic carbonates [
33]. Many scientists have made a concentrated effort to improve the performance of the ILs catalysts during the past decade [
2,
31,
34,
35,
36,
37,
38,
39,
40,
41]. Most research was focused on adding metal compounds into ILs catalytic system, synthesis of task-specific ionic liquids with functional groups and synthesis of supported ionic liquids. The performance of an IL can strongly depend on the catalytic system in which it is implemented. ILs combining with metal compounds can promote the cycloaddition reactions of epoxides. The combination of functional groups (such as -OH, -COOH) of cations with anions of ILs can accelerate cycloaddition reactions. Functional groups in cations or in the supports for ILs can combine with the anions of supported ILs resulting in a synergistic effect on the ring-opening of epoxides. Synergistic catalytic effects of ILs catalytic system play an important role of promoting the cycloaddition reactions of epoxides. However, due to the diversity of different anion–cation combinations and the diversity of metal compounds, solvents and supports it is difficult to make generalizations about synergistic effects, as there are homogeneous catalytic systems, supported ILs, multiphase catalytic systems and aqueous catalytic systems based on ILs. More importantly, it is necessary to move ahead to a rational design of catalytic systems based on ILs. Normally, ILs are tunable as they are generally composed of flexible ions with different sizes and shapes. In most cases, design of the catalytic ILs systems usually takes a trial-and-error approach because theoretical treatment and interpretations are complicated involving different types of dominant interactions. It is difficult to predict the performance of ILs in a given set of reaction conditions. Therefore, it is important to have a better understanding of synergistic catalytic effects of anions and cation of ILs, and ILs with other species such as metal ions, functional groups of solvents and various supports.
This review focuses on the most representative developments and progress on synergistic catalytic effects based on ionic liquids in the synthesis of cyclic carbonates. This review covers the different catalytic systems involving synergistic effects from homogenous catalytic system to heterogeneous catalytic system. More specifically, the following are reported: (i) metal-based ionic liquids: a synergistic effect of different metal compounds with ILs, (ii) hydrogen bond-promoted ionic liquids: a synergistic effect of functional groups of cations and anions, and (iii) supported ionic liquids: a synergistic effect of functional groups (in cations or supports) and anions.
2. Metal-Based Ionic Liquids
Many metal salts or complexes (such as Co, Ca, Fe, Ni) containing anions of ILs have been found to be very active for the coupling of CO
2 with epoxides. The activities of ILs could be greatly improved by adding metal salts or complexes for the synthesis of cyclic carbonates. Furthermore, synergistic effects on promoting the cycloaddition reactions of epoxides have been reported [
31,
34,
35,
36,
37,
38,
39,
40,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52].
Kossev
et al. [
53] studied the influence of CaCl
2 as a Lewis acid on the synthesis of cyclic carbonates from epoxides and CO
2. The reaction was carried out in the system consisting of tetraalkylammonium or phosphonium halide and CaCl
2. The cycloaddition reactions of different terminal epoxides with CO
2 were investigated. Using triethylbenzylammonium chloride (TEBACl) as a catalyst, the yields of the corresponding cyclic carbonates were very low (4.6%–36.1%). But in a catalytic system comprised of [TEBA][Cl] and CaCl
2 with a mole ratio of 2:1, above 95% yields of the corresponding cyclic carbonates can be achieved. For the cycloaddition reaction of CO
2 with propylene oxide (PO) under a pressure of 4MPa and 170 °C within 4 h, the highest yield of propylene carbonate (PC) reached 96.9% over a CaCl
2 based [TEBA][Cl] system, compared with a PC yield of 8.9% over a [TEBA][Cl] system alone. In comparison with the CaCl
2 or [TEBA][Cl] used as a sole catalyst, the [TEBA][Cl]—CaCl
2 catalytic system led to a sharp increase of the yields of carbonates. Thus, the CaCl
2 and [TEBA][Cl] system showed a synergistic effect on promoting the cycloaddition reactions of epoxides.
Sibaouih
et al. [
54] combined Co(II) salts with onium (ammonium, phosphonium, and imidazolium) salts as catalytic system for the coupling reaction of CO
2 and epoxides. The catalytic reactions were carried out at the conditions of 120 °C with 10 bar of CO
2 within 1 h. Activities of onium salts were moderate while CoCl
2 alone was inactive in the reaction. However, in a catalytic system comprised of tetrabutyl ammonium chloride ([TBA][Cl]) and CoCl
2, cyclic carbonates were produced at high turnover frequencies (TOF) and selectivity. For the coupling reaction of CO
2 and propylene oxide using [TBA][Cl] and CoCl
2 as a co-catalyst, the highest TOF was up to 2223 h
−1. Sibaouih
et al. [
54] and Kossev
et al. [
53] found a significant synergistic effect of the Co(II) salt together with the anions of onium salts. It was interesting that chloride as a IL anion was more advantageous than Br
− and I
− in terms of the tetrabutyl ammonium salts bearing different anions. It was found that phosphonium salts were more active than ammonium salts, while the imidazolium-based ILs were the least active.
Zn (II) halide complexes have been known to be active catalysts for cycloaddition reaction for a long time [
42,
43,
44,
45]. Kim reported a simple catalytic system consisting of ZnBr
2 and imidazolium-based ILs [
48]. Cycloaddition reactions were carried out at 100 °C and 3.5 MPa of CO
2 for 1 h. When IL (1-butyl-3-methylimidazolium chloride or 1-butyl-3-methylimidazolium bromide) was used alone as a catalyst for the coupling reaction of CO
2 and ethylene oxide (EO), the TOF value was less than 100 h
−1. Meanwhile ZnBr
2 was completely inactive. However, the TOF value was more than 3500 h
−1 when ZnBr
2 was added to the above imidazolium-based ILs. ZnBr
2 and imidazolium-based ILs turned out to be very active for the cycloaddition reaction under mild conditions. The dissociation of bromide ion and the following attack on the carbon atom of an epoxide may take place more easily than that of the chloride ion.
Palgunadi
et al. [
55] reported a series of bis(1-alkyl-3-methylimidazolium) tetrahalide Zn complexes for the coupling of CO
2 with various epoxides. The influence of halides on the reaction was investigated. It was found that a higher bromide content had a beneficial influence on the activity which is in agreement with the conclusions of Kim [
48]. The reason may be that the nucleophilicity of bromide is higher than that of the chloride. The results also showed that bulkier epoxides were more difficult to convert to the corresponding carbonates.
Fujita
et al. [
56] also reported that they employed the IL/Zn halide based catalyst systems where the cations of ILs were tetrabutylammonium cation([NBu
4]
+), 1-butyl-3-methylimidazolium cation ([BMIm]
+), 1-butylpyridinium cation ([BPy]
+ ) and choline cation ([Chol]
+). The general formula was IL
nZnX
2Y
n where X and Y are halides in the catalyst systems. The yield of styrene carbonate was 94% over [NBu
4]
2ZnBr
2Br
2 as catalyst at 80 °C and 50 bar of CO
2 for 30 min by using the substrate styrene oxide. The activity of different cations varied in the order: [NBu
4]
+ > [BPy]
+ > [BMIm]
+ >> [Chol]
+. The influence of the Zn halide salt on the activity was in the following order: ZnBr
2> ZnI
2> ZnCl
2. It was found that the halide originating from ZnX
2 had a higher effect on the activity than the halide originating from the IL ([IL]Y) in the synthesis. This effect was not clear although a possible reaction mechanism was discussed by the authors. The ratio of IL cation to Zn (
n = [IL]/Zn) had different effect on the activity based on the different cation used. The activity continued to increase linearly at least up to
n = 4 for [BMIm]
+, while the activity did not increase further at
n > 4 for[NBu
4]
+ under the given conditions. It was attributed to the cooperative activation of epoxide molecules by the bi-functionality of zinc based ionic liquid. Sun
et al. [
57] employed a series of metal bromide salts together with imidazolium-based ILs as cocatalysts for the synthesis of styrene carbonate from the corresponding epoxide and CO
2. The activities of different catalysts with different metal cations, imidazolium alkyl chain length and IL anion, were investigated. It was revealed that ZnBr
2/[BMIm][Cl] was the best catalyst for the reaction under the given conditions. The activity of the catalysts was found to be dependent on the Lewis-acidity of the metal cation to some extent. The catalytic activity with [BF
4]
− or [PF
6]
− as IL anions decreased compared to that of Cl
−. The activity was found to be dependent on temperature and CO
2 pressure. Higher yields were obtained at higher temperature and an optimal CO
2 pressure. The authors proposed a similar reaction mechanism (the acid attacks the oxygen atom of the epoxide ring, while the base attacks the carbon atom to open the ring), which is in good agreement with that published by Ramin and Sun previously [
58,
59].
Li
et al. [
60] reported zinc compounds based on [BMIm][Br] (1-butyl-3-methylimidazolium bromine) catalyst systems for the synthesis of cyclic carbonates from CO
2 and epoxides. Cycloaddition reactions were carried out at 100 °C and 1.5 MPa of CO
2 for 1 h. The catalytic activity of [BMIm][Br] by itself was very low (8% yield) (
Table 1, Entry 1). When ZnCl
2was added as a co-catalyst in the system, 95% yield of propylene carbonate was obtained and TOF value reached 5410 h
−1 (
Table 1, Entry 4). The molar ratio of [BMIm][Br]: ZnCl
2 had an influence on the TOF. A series of different anions with 1-butyl-3-methylimidazolium cation were investigated (
Table 1, Entries 5–7). It was found that the anions BF
4− and PF
6− were inactive in the reaction. The reaction with the Cl
− anion showed low activity and only 38% yield of propylene carbonate was obtained while Br
− exhibited good performance. These results illustrated that anions play an important role in the catalytic activity. Among the zinc compounds (
Table 1, Entries 4,8,9), the combination of ZnBr
2 and [BMIm]Br efficiently co-catalyzed the cycloaddition reactions.
Table 1.
Effects of different zinc compounds combined with [BMIm][X] on the coupling reaction of CO
2 and PO
a [
60].
Table 1.
Effects of different zinc compounds combined with [BMIm][X] on the coupling reaction of CO2 and PO a [60].
| Entry | Catalyst | Amount of [BMIm]X(mmol) | Yield b(%) | TOF |
|---|
| 1 | [BMIm][Br] | 0.3 | 8 | 45 |
| 2 | ZnCl2/[BMIm] [Br] | 0.1 | 60 | 3417 |
| 3 | ZnCl2/[BMIm] [Br] | 0.2 | 79 | 4500 |
| 4 | ZnCl2/[BMIm] [Br] | 0.3 | 95 | 5410 |
| 5 | ZnCl2/[BMIm][Cl] | 0.3 | 38 | 1564 |
| 6 | ZnCl2/[BMIm][BF4] | 0.3 | Tr | — |
| 7 | ZnCl2/[BMIm][PF6] | 0.3 | Tr | — |
| 8 | ZnBr2/[BMIm] [Br] | 0.3 | 98 | 5580 |
| 9 | Zn(OAc)2/[BMIm] [Br] | 0.3 | 64 | 3636 |
Phosphonium halides are more thermally stable [
61,
62,
63,
64,
65,
66] when compared with the ammonium halides. Moreover, they could be separated easily from the products since they have little potential interaction with the products [
67]. Sun
et al. [
68] studied the effect of the different zinc compounds combined with phosphonium salts as Lewis acid/base catalyst on the synthesis of cyclic carbonates from epoxides and CO
2. The catalytic reactions were carried out at the conditions of 120 °C with 1.5 MPa of CO
2 in 1 h. The effects of different phosphonium halides on the formation of PC were investigated (
Table 2). The activity of ZnCl
2/KBr was very low (
Table 2, Entry 2). In the ZnCl
2/[PPh
3C
6H
13][Br] catalytic system, 96% yield with over 99% selectivity of PC was obtained and the corresponding TOF value was 4718.4 h
−1 (
Table 2, Entry 1). The activity of phosphonium salts was affected by their structure, which probably also influences the behavior of anions. A possible role of cations was tested on this reaction. The catalytic activity increased with the increase in the phosphonium cations molecular weight ([PPh
3C
nH
2n+1]
+,
n = 2~10,
Table 2, Entries 1,3–6,9–11), and the selectivity kept constant for all the cases. In the ZnCl
2/[PPh
3C
10H
21][Br] catalytic system, a 98.5% yield and 4841.2 h
−1 TOF value, which were the highest among the tested catalysts were obtained. The reason was that the nucleophilic attack of halide anion on the PO is accelerated by the less electrostatic interaction between the halide anion and the larger phosphonium cation. The activity of different halide ions varied in the order: I
− ≥ Br
− > Cl
− (
Table 2, Entries 4,10,12). A possible reason may be that dissociation of I
− ion is more facile than Br
− and Cl
−. The effect of the different zinc compounds such as ZnSO
4, Zn(CH
3COO)
2 and Zn(NO
3)
2 on the catalytic activity was also investigated. The results showed that Zn(NO
3)
2/[PPh
3C
6H
13][Br] exhibited higher catalytic activity than the others (
Table 2, Entries 14–16). The number of moles of ZnCl
2 of the catalytic system was kept constant while the molar ratio of ZnCl
2: [PPh
3C
6H
13][Br] varied (
Table 2, Entries 1,17–19). The yield of PC increased with the increasing molar ratio of ZnCl
2: [PPh
3C
6H
13][Br]. The highest PC yield was obtained when the molar ratio of ZnCl
2:[PPh
3C
6H
13][Br] was 1:8.
Table 2.
Effects of different zinc compounds combined with phosphonium salts on the coupling reaction of CO
2 and PO
a [
68].
Table 2.
Effects of different zinc compounds combined with phosphonium salts on the coupling reaction of CO2 and PO a [68].
| Entry | Catalytic system | Yield (%) | Selectivity (%) | TOF c(h−1) |
|---|
| 1 | ZnCl2/[PPh3C6H13][Br] | 96.0 | >99.0 | 4718.4 |
| 2 b | ZnCl2/KBr | Trace | - | - |
| 3 | ZnCl2/[PPh3C2H5][Br] | 93.0 | >99.0 | 4570.9 |
| 4 | ZnCl2/[PPh3C4H9][Br] | 95.0 | >99.0 | 4669.2 |
| 5 | ZnCl2/[PPh3C8H17][Br] | 97.1 | >99.0 | 4767.5 |
| 6 | ZnCl2/[PPh3C10H21][Br] | 98.5 | >99.0 | 4841.2 |
| 7 | ZnCl2/[P(Bu)4][Br] | 79.0 | >99.0 | 3882.8 |
| 8 | ZnCl2/[PPh3iso-C4H9][Br] | 94.2 | >99.0 | 4629.8 |
| 9 | ZnCl2/[PPh3C3H7][Cl] | 65.0 | >99.0 | 3194.7 |
| 10 | ZnCl2/[PPh3C4H9][Cl] | 67.0 | >99.0 | 3293.1 |
| 11 | ZnCl2/[PPh3C5H11][Cl] | 70.0 | >99.0 | 3440.5 |
| 12 | ZnCl2/[PPh3C4H9][I] | 95.1 | >99.0 | 4674.1 |
| 13 | ZnCl2/[P(Bu)3C14H29][Cl] | 75.2 | >99.0 | 3696.1 |
| 14 | ZnSO4/[PPh3C6H13][Br] | 60.0 | >99.0 | 2949.0 |
| 15 | Zn(Ac)2/[PPh3C6H13][Br] | 67.1 | >99.0 | 3298.0 |
| 16 | Zn(NO3)2/[PPh3C6H13][Br] | 86.9 | >98.0 | 4271.1 |
| 17 d | ZnCl2/ [PPh3C6H13][Br] | 72.0 | >99.0 | 3538.8 |
| 18 e | ZnCl2/ [PPh3C6H13][Br] | 87.2 | >99.0 | 4285.9 |
| 19 f | ZnCl2/ [PPh3C6H13][Br] | 97.0 | >99.0 | 4767.6 |
Cheng
et al. [
69] explored the metal halide catalyst systems based on choline chloride (CH), which are simple, efficient and biodegradable for the synthesis of cyclic carbonates from CO
2 and epoxides under solventless condition. The performance of choline chloride with different metal halide catalysts was investigated (
Table 3). Choline chloride alone was inactive in the reaction. The behavior of CH with different Lewis acid catalysts varied considerably. The yield was unsatisfactory with CuCl
2∙2H
2O/CH, FeCl
3/CH, NaCl/CH or AlCl
3/CH (
Table 3, Entries 6–9), while the reaction did not take place over CH with K
+ or Mg
2+ (
Table 3, Entries 3–5). It is interesting to observe that the activities of zinc halide based on CH increased sharply (
Table 3, Entries 10,11). The highest yield of propylene carbonate reached 99% over ZnBr
2-based CH at 110 °C and 1.5 MPa of CO
2 for 1 h. When tetramethylammonium chloride (TMAC) without -OH group was substituted for CH, the corresponding catalyst system showed very low activity compared to the ZnBr
2-based CH system (
Table 3, Entry 12). This result indicated that the -OH group of CH promoted the reaction. The tentative mechanism was proposed and shown schematically in
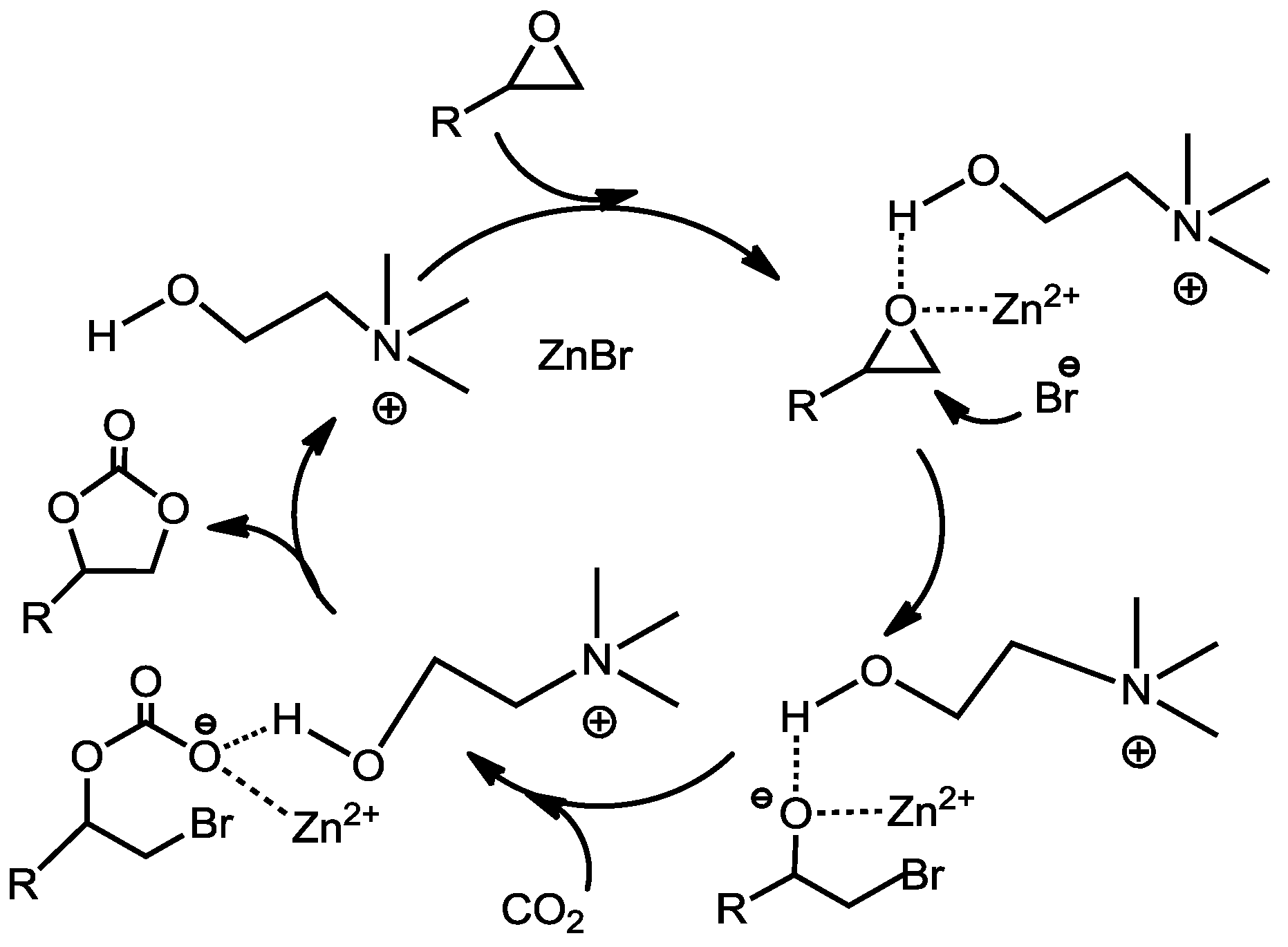
Scheme 1. Firstly the Lewis acid (Zn
2+) and -OH group of CH attack the oxygen atom of the epoxide ring, while the Lewis base (Br
−) attacks the carbon atom coordinatively. The coordination of the zinc atom and -OH group with the oxygen atom of epoxide through a donor-acceptor bond resulted in the polarization of C–O bonds, while the nucleophilic attack by Br
− on the less bulky β-carbon atom of the epoxide occurred at the same time. As a result, the ring of the epoxide was easily opened. Then the oxygen anion interacted with CO
2 to form the intermediate. Lastly, the intermediate would be transformed into a cyclic carbonate by the intramolecular substitution of bromide. The -OH group of CH and Zn
2+ play an important function on the ring-opening of epoxy, which indicates a synergistic effect on promoting the cycloaddition reactions of epoxides. The molar ratio of CH to ZnBr
2 also had significant influence on catalytic activity. The yield of PC increased with an increase of molar ratios of CH to ZnBr
2 from 1 to 5 (
Table 3, Entries 11,13–16), then decreased with a further increase of molar ratio (
Table 3, Entry 16). Moreover, the catalyst can be reused 5 times without the decrease in the yield of PC.
Table 3.
Effects of different metal compounds combined with choline chloride (CH) on the coupling reaction of CO
2 and PO
a [
69].
Table 3.
Effects of different metal compounds combined with choline chloride (CH) on the coupling reaction of CO2 and PO a [69].
| Entry | Catalyst | Ratio b | Yield c(%) | TOF d |
|---|
| 1 | CH | 0 | Trace | Trace |
| 2 | ZnBr2 | ∞ | 1 | 5 |
| 3 | KCl/CH | 1:5 | Trace | Trace |
| 4 | KBr/CH | 1:5 | Trace | Trace |
| 5 | MgCl2∙6H2O/CH | 1:5 | Trace | Trace |
| 6 | CuCl2∙2H2O/CH | 1:5 | 5 | 25 |
| 7 | FeCl3/CH | 1:5 | 14 | 70 |
| 8 | NaCl/CH | 1:5 | 21 | 106 |
| 9 | AlCl3/CH | 1:5 | 33 | 166 |
| 10 | ZnCl2/CH | 1:5 | 90 | 451 |
| 11 | ZnBr2/CH | 1:5 | 99 | 494 |
| 12 | ZnBr2/TMAC | 1:5 | 1 | 6 |
| 13 | ZnBr2/CH | 1:1 | 31 | 155 |
| 14 | ZnBr2/CH | 1:3 | 86 | 430 |
| 15 | ZnBr2/CH | 1:4 | 91 | 455 |
| 16 | ZnBr2/CH | 1:6 | 94 | 470 |
| 17 e | ZnBr2/CH | 1:5 | 99 | 494 |
| 18 f | ZnBr2/CH | 1:5 | 98 | 490 |
| 19 g | ZnBr2/CH | 1:5 | 99 | 494 |
| 20 h | ZnBr2/CH | 1:5 | 99 | 494 |
Scheme 1.
Mechanism for the coupling of epoxides with CO
2 catalyzed by ZnBr
2-based CH [
69].
Scheme 1.
Mechanism for the coupling of epoxides with CO
2 catalyzed by ZnBr
2-based CH [
69].
3. Hydrogen Bond-Promoted Ionic Liquids
Han
et al. [
70] reported several betaine(Bet)-based catalysts for the cycloaddition of CO
2 with epoxides. The catalyst systems were composed of betaine cation and Cl
−, Br
−, I
−, BF
4−, or PF
6−. Coupling of CO
2 and propylene oxide was carried out at 140 °C and 8 MPa of CO
2 for 8 h. The betaine (Bet) itself was inactive (
Table 4, Entry 1). The yields of PC increased when the anions were Cl
−, BF
4−, or PF
6− (
Table 4, Entries 2,5,6). The yields of PC were in the order of Cl
− > BF
4− > PF
6− which is consistent with the order of the nucleophilicity of the anions. The highest yield was achieved when [Hbet][I] was used as catalyst (
Table 4, Entry 6). But the order of activity of the catalysts containing halide elements was [Hbet][I] > [Hbet][Cl] > [Hbet][Br] (
Table 4, Entries 2–4), which was different from the order of their nucleophilicity. The catalytic activity of the betaine-based salts was compared with that of the conventional catalysts. The [Hbet][I] with a carboxylic acid group had higher catalytic activity than
n-tetrabutyl ammonium bromide (
Table 4, Entry 7). The carboxylic acid group, which is a Brønsted acid and a hydrogen bonding donor, could accelerate the ring opening of epoxides and showed a co-operative effect with halide anions.
Table 4.
Synthesis of propylene carbonate (PC) catalyzed by betaine(Bet)-based catalysts
a [
70].
Table 4.
Synthesis of propylene carbonate (PC) catalyzed by betaine(Bet)-based catalysts a [70].
| Entry | Catalyst | Yield b(%) |
|---|
| 1 | Bet | 0 |
| 2 | [Hbet][Cl] | 94 |
| 3 | [Hbet][Br] | 76 |
| 4 | [Hbet][I] | 98 |
| 5 | [Hbet][BF4] | 74 |
| 6 | [HbetP][F6] | 20 |
| 7 | [N(Bu)4][Br] | 88 |
| 8 | [N(Me)4][Cl] | l 2 |
| 9 b | N(Bu)4Br/CH3COOH | 91 |
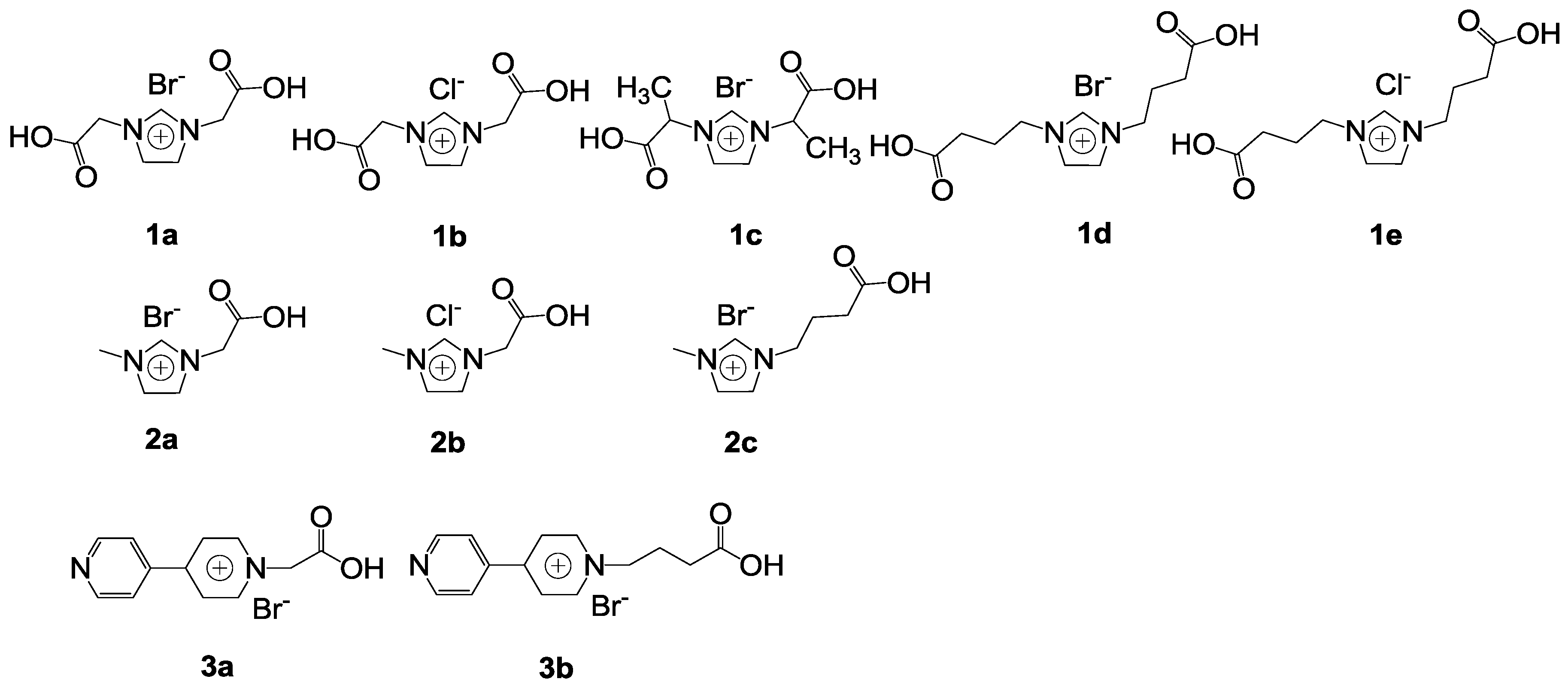
The acid-base bifunctional imidazolium(im) or methylimidazolium(mim) ILs with a carboxylic acid group were synthesized (
Scheme 2) [
71]. They were used to catalyze the reaction of propylene oxide and CO
2 (
Table 5). The 1-ethyl-3-methylimidazolium bromide ([emim][Br]) as a traditional ionic liquid is not efficient (
Table 5, Entry 1). However, most acid-base bifunctional imidazolium ILs with a carboxylic acid group exhibited high activities. Among the catalysts, [{(CH
2)
3CO
2H}
2im][Br] was the best one with 99% conversion and 98% yield. The structure of the cation has a strong effect on catalytic activity. For imidazolium, the activity orders were: [{(CH
2)
3CO
2H}
2im]
+ > [{CH(CH
3)CO
2H}
2im]
+ > [(CH
2CO
2H)
2im]
+ (
Table 5, Entries 5,7,8), and [{(CH
2)
3CO
2H}mim]
+ > [(CH
2CO
2H)mim]
+, respectively (
Table 5, Entries 10,12). For bipyridinium, the activity of cations were in the order: [{(CH
2)
3CO
2H}bpy]
+ > [(CH
2CO
2H)bpy]
+ (
Table 5, Entries 13–14). Hydrogen bonding has a positive effect on the ring-opening of epoxide to promote the synthesis of cyclic carbonate, while it may also weaken the nucleophilicity of the anions by limiting their movements to some extent. The anions also have a strong effect on catalytic activity. The activity order of anion is Br
− > Cl
−, which is consistent with the order of their nucleophilicity (
Table 5, Entries 5,6,8–11). Moreover, the activity of [emim][Br] could be greatly improved in the presence of equal amount of CH
3CO
2H (
Table 5, Entry 2). The carboxylic acid could effectively activate epoxide through a synergistic effect. Moreover, the catalyst exhibited good stability.
Table 5.
Synthesis of PC catalyzed by acid-base bifunctional catalysts catalyst
a [
71].
Table 5.
Synthesis of PC catalyzed by acid-base bifunctional catalysts catalyst a [71].
| Entry | Catalysts | Conversion (%) | Yield b (%) |
|---|
| 1 | [emim][Br] | 60 | 59 |
| 2 c | CH3CO2H/[emim][Br] | 80 | 78 |
| 3 | 2CH3CO2H/[emim] [Br] | 91 | 88 |
| 4 d | 2CH3CH2OH /[emim] [Br] | 84 | 83 |
| 5 | [(CH2CO2H)2im] [Br] (1a) | 78 | 77 |
| 6 | [(CH2CO2H)2im][Cl] (1b) | 12 | 11 |
| 7 | [{CH(CH3)CO2H}2im] [Br] (1c) | 89 | 88 |
| 8 | [{(CH2)3CO2H}2im]Br (1d) | 99 | 98 |
| 9 | [{(CH2)3CO2H}2im][Cl] (1e) | 30 | 29 |
| 10 | [(CH2CO2H)mim] [Br] (2a) | 66 | 65 |
| 11 | [(CH2CO2H)mim][Cl] (2b) | 10 | 9 |
| 12 | [{(CH2)3CO2H}mim] [Br] (2c) | 92 | 91 |
| 13 | [(CH2CO2H)bpy] [Br] (3a) | 60 | 59 |
| 14 | [{(CH2)3CO2H}bpy] [Br] (3b) | 84 | 83 |
| 15 e | [{(CH2)3CO2H}2im] [Br] (1d) | 99 | 98 |
| 16 f | [{(CH2)3CO2H}2im] [Br] (1d) | 98 | 98 |
| 17 g | [{(CH2)3CO2H}2im] [Br] (1d) | 98 | 97 |
| 18 h | [{(CH2)3CO2H}2im] [Br] (1d) | 98 | 97 |
Scheme 2.
Acid-base bifunctional type catalysts [
71].
Scheme 2.
Acid-base bifunctional type catalysts [
71].
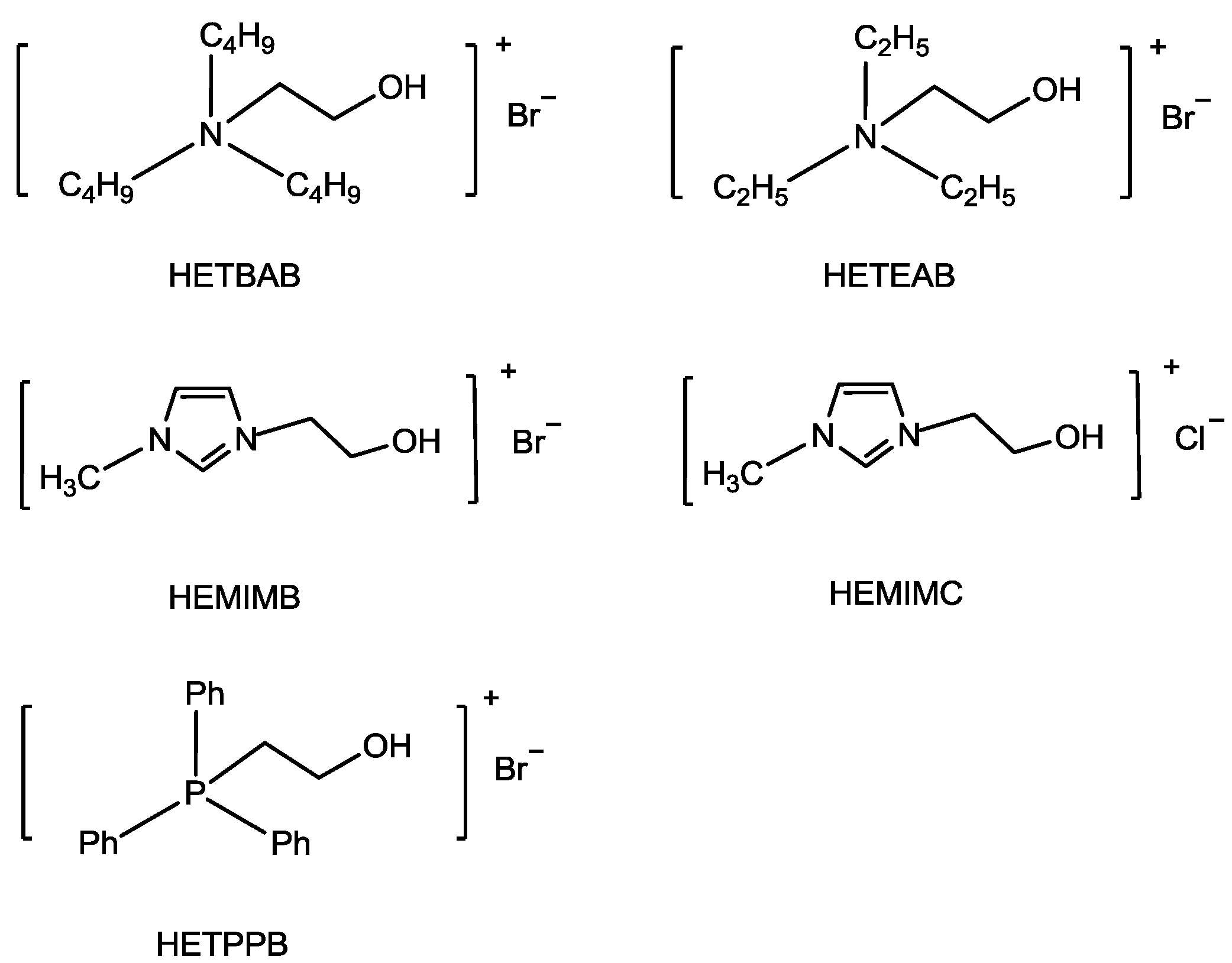
Compared to the traditional ILs, functionalized ionic liquids (FILs) containing ether or alcohol on the alkyl chains, show additional advantages such as tunable polarity, lower viscosity, higher solubility of inorganic salts and have received much attention in the fields of organic synthesis and catalysis [
72,
73,
74,
75,
76]. Sun
et al. [
77,
78] explored the hydroxyl-functionalized ionic liquids (HFILs) as catalysts for the synthesis of cyclic carbonate as the OH group has a positive effect on the ring-opening of epoxy (
Scheme 3). The HFILs showed higher reactivity (
Table 6, Entries 1,7,8,10) in comparison with ionic salts which did not have a OH group, such as [EMIM][Br] (1-ethyl-3-methylimidazolium bromine), TBAB (tetrabutylammonium bromide), and PPh
3EtBr (triphenyl(ethyl)phosphonium bromide) (
Table 6, Entries 2,9,11). Among the HFILs catalysts, 1-(2-hydroxyl-ethyl)-3-methylimdazolium bromide (HEMIMB) (
Table 6, Entry 1) was the most effective catalyst. Interestingly, [EMIM][Br] could also show high activity in the presence of OH group containing chemicals such as H
2O or C
2H
5OH (
Table 6, Entries 3,4), but exhibited low activity in the presence of non-OH group containing chemicals such as DMC (dimethyl carbonate) and DMF (
N,
N-dimethylformamide) (
Table 6, Entries 5,6). A synergistic effect from the OH group and Lewis basic site of the ionic liquid apparently played an important role in accelerating the cycloaddition reactions CO
2 with of epoxides. The HEMIMB catalyst, which was a functionalized ionic liquid, could be used to catalyze reactions to a variety of terminal epoxides (
Table 7, epoxides 1a–f). Aromatic epoxide 1
e and aliphatic epoxides 1
a, 1
b, 1
d, except 1
f which has the higher hindrance originated from the two rings, were the preferred substrates for the reaction.
Reaction medium such as water had a significant effect on the activities of ILs for the synthesis of cyclic carbonate (
Table 8) [
79]. Activities of ILs are considerably increased in the presence of water compared with the results without water. The reaction ratio could be about 5–6 times higher in the presence than that in the absence of water. The activity order of cations is PPh
3Bu
+ > Bu
4N
+, BMIM
+ (
Table 8, Entries 1,4,9) in the presence of water. The activity of the anions varied in the order I
− > Br
− > Cl
− > PF
6−, BF
4− (
Table 8, Entries 4–8). Low PO conversion and PC selectivity were obtained by using catalysts such as 1-butyl-3-methyl-imidazolium hexafluorophosphate ([BMIM][PF
6]), and 1-buthyl-3-methyl-imidazolium tetrafluoroborate ([BMIM][BF
4]) (
Table 8, Entries 2,5,7,8,10) due to the low nucleophilic nature of PF
6− and BF
4− anions [
80]. In aqueous system, 100% conversion of PO with 96.8% selectivity toward PC was obtained by using the best catalyst [PPh
3Bu][I] after 1 h of reaction time at 125 °C and 2.0 MPa.
Wang
et al. [
81] investigated the hydrogen bond donors mechanism (HBD) through density functional theory (DFT) studies. The reaction mechanisms of [Bu
4N][Br]/H
2O-catalyzed process, non-catalytic process and Bu
4NBr-catalyzed process for the fixation of CO
2 with ethylene oxide respectively were showed in
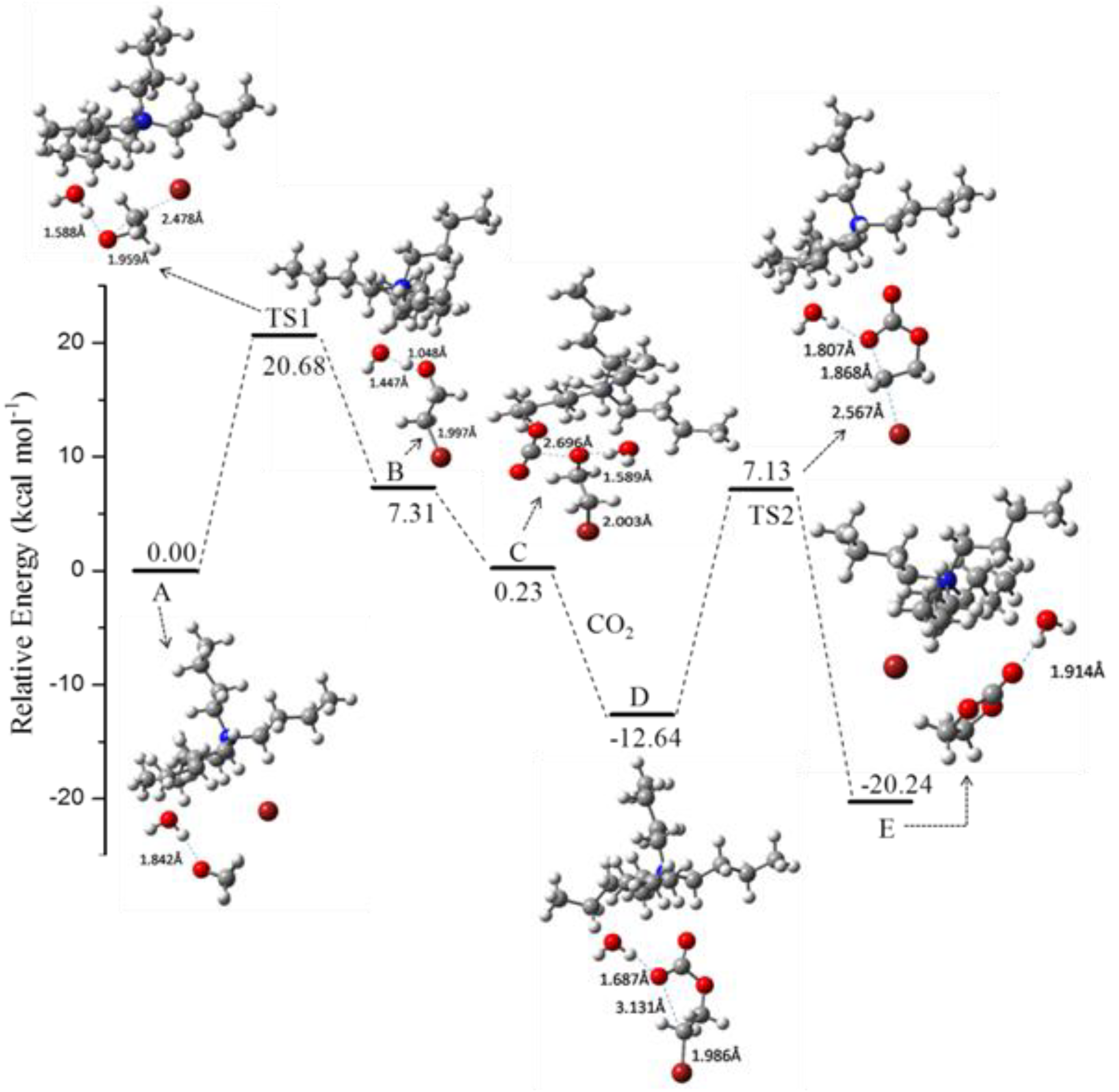
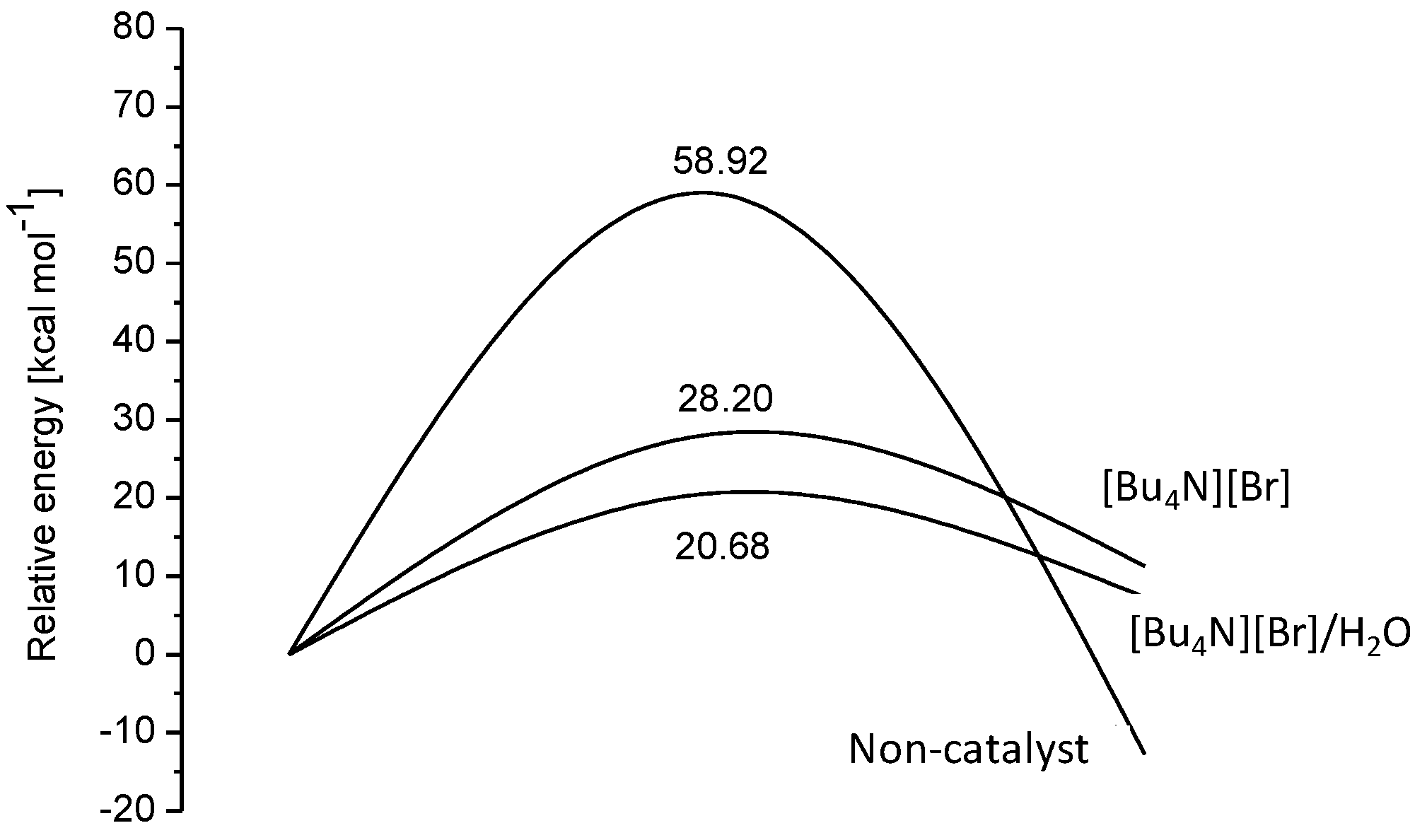
Figure 1. The hydrogen bond indeed played an important role in all the intermediates and transition states. The [Bu
4N][Br]/H
2O-catalyzed process via hydrogen bond interaction has a much lower energy barrier compared to a non-catalytic process or a Bu
4NBr-catalyzed process for the rate-determining step (
Figure 2). The results showed that H
2O improved the cycloaddition process compared with only [Bu
4N][Br] (
Table 9, Entry 1 versus 2). For different chain length of alcohols or halogen substituted alcohols as HBD, the PO conversion was nearly at the same level (
Table 9, Entries 2–10). For a stronger HBD such as phenol, acid, PC selectivity decreased sharply due to the side products though the PO conversion was remarkably enhanced (
Table 9, Entries 11,12). DMF was not effective in this catalytic system. 1,2-benzenediol was found to have excellent catalytic activity (
Table 9, Entry 16), which was due to the appropriate hydrogen bond strength. Therefore, the presence of HBD could remarkably reduce the activation energy and the proper choice of HBD was crucial for the reaction.
Scheme 3.
Hydroxyl-functionalized ionic liquids (HFILs) [
77,
78].
Scheme 3.
Hydroxyl-functionalized ionic liquids (HFILs) [
77,
78].
Table 6.
Synthesis of PC catalyzed by Hydroxyl-functionalized ionic liquids (HFILs) catalyst
a [
77,
78].
Table 6.
Synthesis of PC catalyzed by Hydroxyl-functionalized ionic liquids (HFILs) catalyst a [77,78].
| Entry | Catalysts | Conversion (%) | Yield b(%) |
|---|
| 1 | HEMIMB | 99.2 | 99.0 |
| 2 | [EMIM][Br] | 83 | 82.4 |
| 3 | [EMIM][Br]/H2O c | 93 | 92.1 |
| 4 | [EMIM][Br]/C2H5OH c | 92 | 91.3 |
| 5 | [EMIM][Br]/DMC c | 84 | 83.8 |
| 6 | [EMIM][Br]/DMF c | 85 | 84.7 |
| 7 | HETBAB | 95.8 | 95.0 |
| 8 | HETEAB | 87.8 | 87.1 |
| 9 | TBAB | 73.6 | 73.1 |
| 10 | HETPPB | 96.2 | 96.0 |
| 11 | [PPh3Et][Br] | 50.1 | 50.0 |
| 12 | HEMIMC | 78 | 77.5 |
Table 7.
Synthesis of different cyclic carbonates catalyzed by HEMIMB
a [
77,
78].
Table 8.
Comparison of cycloaddition of PO to PC catalyzed by ILs with and without water
a [
79].
Table 8.
Comparison of cycloaddition of PO to PC catalyzed by ILs with and without water a [79].
| Entry | Catalyst | With water | Without water |
|---|
| Conversion (%) | Selectivity (%) | TOF b(h−1) | Conversion (%) | Selectivity (%) | TOF (h−1) |
|---|
| 1 | [Bu4N][Br] | 94.5 | 93.2 | 175 | 56 | 99.5 | 111.4 |
| 2 | [Bu4N][Cl] | 69.9 | 70 | 97.8 | 63.9 | 99 | 126.5 |
| 3 | [Bu4N][I] | 95.1 | 94.5 | 185.2 | 26.7 | 99.5 | 53.1 |
| 4 | [BMIM][Br] | 93.9 | 95.4 | 180.4 | 52.3 | 99.5 | 104.1 |
| 5 | [BMIM][Cl] | 64 | 70.6 | 74.8 | 45.6 | 98.8 | 90.1 |
| 6 | [BMIM][I] | 96.5 | 94.6 | 184.4 | 52.9 | 99.5 | 105.3 |
| 7 | [BMIM][BF4] | 3.3 | 10 | 0.66 | trace | 99.5 | trace |
| 8 | [BMIM][PF6] | 10 | 6.3 | 1.2 | 2.7 | 99.5 | 5.4 |
| 9 | [PPh3Bu][Br] | 95.9 | 94.1 | 176.6 | 54.2 | 99.6 | 107.9 |
| 10 | [PPh3Bu][Cl] | 71.6 | 74.8 | 89.8 | 65.3 | 99.9 | 130.5 |
| 11 | [PPh3Bu][I] | 100 | 96.8 | 193.6 | 24.5 | 99.6 | 48.8 |
| 12 | [PPh3Et][Br] | 94.7 | 91.6 | 173.4 | 50 | 100 | 100 |
| 13 | [PPh3He][Br] | 97 | 93.7 | 181.6 | 60.8 | 99.9 | 121.5 |
Figure 1.
Potential energy surface profiles of [Bu
4N][Br]/H
2O-catalyzed process [
81]. Acknowledgements to be used by RSC authors.
Figure 1.
Potential energy surface profiles of [Bu
4N][Br]/H
2O-catalyzed process [
81]. Acknowledgements to be used by RSC authors.
Figure 2.
Comparison of relative energy for the rate-determining step for the fixation of CO
2 with ethylene oxide [
81]. Acknowledgements to be used by RSC authors.
Figure 2.
Comparison of relative energy for the rate-determining step for the fixation of CO
2 with ethylene oxide [
81]. Acknowledgements to be used by RSC authors.
Table 9.
Cycloaddition of PO to PC catalyzed by [Bu
4N][Br] with different HBDs
a [
81].
Table 9.
Cycloaddition of PO to PC catalyzed by [Bu4N][Br] with different HBDs a [81].
| Entry | HBD | Conversion b(%) | Selectivity b(%) |
|---|
| 1 | None | 24 | 96 |
| 2 | H2O | 49 | 92 |
| 3 | CH3OH | 49 | 98 |
| 4 | CH3CH2OH | 58 | 100 |
| 5 | CH3CH2CH2OH | 50 | 100 |
| 6 | CH3(CH2)3OH | 53 | 98 |
| 7 | CH3(CH2)5OH | 47 | 98 |
| 8 | OHCH2CH2OH | 46 | 98 |
| 9 | BrCH2CH2OH | 45 | 100 |
| 10 | ClCH2CH2CH2OH | 46 | 98 |
| 11 | PhOH | 95 | 41 |
| 12 | CH3COOH | 81 | 1 |
| 13 | HCON(CH3)2 | 38 | 97 |
| 14 | NH2CONH2 | 17 | 100 |
| 15 | Methyl salicylate | 62 | 100 |
| 16 c | 1,2-Benzenediol | 92 | 100 |
| 17 c | 2-t-Butyl-1,4-di-hydroxybenzene | 84 | 100 |
4. Supported Ionic Liquids
Immobilized IL-based catalysts offer a promising approach for the cycloaddition of CO
2 to epoxides due to the advantages of easy product separation by filtration and the high dispersion of active species. As the design of functional ILs for the synthesis of immobilized IL is possible, such catalysts show much potential application in industry. The catalytic species consisted of ammonium, phosphonium, and imidazolium-based ILs. The support materials included silicas [
50,
51,
52,
82,
83,
84,
85], oxides [
51], resins [
82,
83,
84,
85,
86], zeolites [
87,
88], and chitosan [
89].
The first report of immobilized IL-based catalysts was reported by Wang
et al., who employed tetrabutylammonium halides immobilized on SiO
2 by a simple adsorption method [
90]. The activity of the catalyst was comparatively low, as good PC yield could be obtained only after 10 h at 150 °C and 8 MPa CO
2 pressure. Different halides anions and various ammonium cations were screened as catalysts. The halides except iodide increased the activity in agreement with their nucleophilicity. However, the alkyl chain length of the cations had little effect on the activity. Thereafter, Wang et al. extended tetrabutylammonium halides catalysts to imidazolium-based ILs supported on SiO
2 [
91]. No obvious difference in the activity of catalysts was observed among [BMIm][BF
4]/SiO
2, [BMIm][ PF
6] /SiO
2 and [BMIm] [Br]/SiO
2. The optimized reaction conditions (10 h at 150
oC and 8MPa) were obtained after parametric studies including temperature, pressure, and reaction time. Various carbonates were synthesized using [BMIm][BF
4]/SiO
2.
Sakai et al. [
51] reported a Al
2O
3 supported phosphonium bromide for the cycloaddition of CO
2 and 1,2-epoxyhexane. The activity of Al
2O
3 supported phosphonium bromide catalyst was very low (52% yield) compared to that of SiO
2 supported phosphonium bromide catalyst after 6 h at 90
oC and 1MPa CO
2 pressure. The reason was that the OH groups on Al
2O
3 are much less than that on SiO
2, as there is the synergistic effect of the OH group and phosphonium bromide on the surface of the supports on catalytic activity.
The molecular sieve such as SBA-15, which has special pore structures, high surface area and abundant surface hydroxyl groups [
92,
93], is a good support with high thermal stability. Meanwhile, ideal candidates of ILs should have groups such as tertiary nitrogen, which could activate CO
2. Cheng
et al. [
94] explored the activities of a series of SBA-15 supported1,2,4-triazolium-based ILs (TRILs) (
Scheme 4) in the cycloadditon reaction of CO
2 with PO (
Table 10). Performances of the SBA-15 supported TRILs with -OH groups or -COOH groups were compared with that of the catalysts without functional groups (
Table 10, Entries 3,5,6). The activity of SBA-15-IL3Br without functional group was much lower than that of SBA-15-IL1Br and SBA-15-IL2Br, which has -OH group or -COOH group. The activity of anion of SBA-15 supported TRILs was in the order of I
− > Br
− > Cl
−. The possible reason may be that anions are further away from the 1,2,4-triazolium cation in the order of I
− > Br
− > Cl
− as a result of their radius [
68]. For the same Br
−, the TOF value was in the order of SBA-15-IL2Br > SBA-15-IL1Br > SBA-15-IL3Br. In comparison SBA-15 with polystyrene (PS), the catalytic activity of SBA-15-IL1Br was significantly higher than that of PS-IL1Br (
Table 10, Entry 7), although the loading amount of TRILs on SBA-15 was less than that of PS. The main reason was the abundant surface hydroxyl groups on SBA-15, which provided a synergistic catalysis role in the reaction. Moreover, solvents containing -OH group such as water, ethanol and acetic acid also play a synergistic catalytic role. The activity of SBA-15-IL3Br without -OH group could be remarkably enhanced using water, ethanol or acetic acid as co-catalyst (
Table 10, Entries 8–10).
Table 10.
Synthesis of PC catalyzed by SBA-15 supported1,2,4-triazolium-based ILs
a [
94].
Table 10.
Synthesis of PC catalyzed by SBA-15 supported1,2,4-triazolium-based ILs a [94].
| Entry | Catalyst | Conversion (%) | Selectivity (%) | TOF b(h−1) |
|---|
| 1 | SBA-15 | 13 | 99 | - |
| 2 | SBA-15-IL1Cl | 74 | 97 | 42.5 |
| 3 | SBA-15-IL1Br | 85 | 99 | 53.9 |
| 4 | SBA-15-IL1I | 88 | 99 | 59.4 |
| 5 | SBA-15-IL2Br | 70 | 98 | 104.1 |
| 6 | SBA-15-IL3Br | 71 | 97 | 29.5 |
| 7 | PS-IL1Br | 65 | 99 | 17.5 |
| 8 c | SBA-15-IL3Br-H2O | 84 | 92 | 34.7 |
| 9 c | SBA-15-IL3Br-C2H5OH | 82 | 98 | 33.9 |
| 10 c | SBA-15-IL3Br-CH3COOH | 85 | 97 | 35.1 |
| 11 d | SBA-15-IL4Br | 71 | 97 | 39.0 |
Scheme 4.
Structure of SBA-15 supported 1,2,4-triazolium-based ILs [
94].
Scheme 4.
Structure of SBA-15 supported 1,2,4-triazolium-based ILs [
94].
The mechanism for the reaction catalyzed by SBA-15 supported TRILs was proposed (
Scheme 5). The ethylene oxide was activated by hydrogen bond interaction, which facilitated the ring opening step (step I). The epoxide was polarized after hydrogen bond formation with the -OH group of ILs because the length of C-O bond was increased from 1.435 Å to 1.445 Å through density functional theory calculation. Then, the ring of the epoxide opened via nucleophilic attack at the less sterically hindered carbon atom (step II). Next, the activated CO
2 by SBA-15 supported triazolium-based ionic liquids was inserted (step III). The bond angle of CO
2 was changed to 179
oC from a linear molecule after interaction with triazolium, which polarized and activate the CO
2 to a certain extent. Lastly, cyclization via an intramolecular nucleophilic attack (step IV) led to the cyclic carbonate and the regeneration of the catalyst. The -OH or -COOH group and N atom in the TRIL cation combining with the halide anions have a synergistic effect on accelerating the reactions.
Scheme 5.
The proposed mechanism for fixation of CO
2 into ethylene carbonate [
94].
Scheme 5.
The proposed mechanism for fixation of CO
2 into ethylene carbonate [
94].
The polymer-supported hydroxyl-functionalized ionic liquids (PSHFILs) were developed by Sun
et al. [
86] as catalysts for the synthesis of cyclic carbonate. Hydroxyl-imidazolium based ILs were covalently anchored on highly crosslinked polystyrene (PS) resin. The PSHFILs showed higher reactivity in comparison with PS-supported ILs, which did not have the -OH group. Among the PSHFILs catalysts, 1-(2-hydroxyl-ethyl)-imidazolium bromide (PS-HEIMBr) was the most effective. At the conditions of 120 °C with 2.5 MPa of CO
2 within 4 h, 98% PO conversion with 99% PC selectivity was obtained. The PS-HEIMBr catalyst could be reused five times without obvious loss of its catalytic activity and was found to be applicable to a variety of terminal epoxides to produce the corresponding cyclic carbonates. The -OH group of PSHFILs could substitute for Lewis acid to accelerate the reactions and showed a synergistic effect with halide anions. Shortly thereafter, Chen
et al. [
95] improved the performance of PSHFILs. The supported diethanolamine based ILs such as diethanolamine ethyl bromide (PS-DHEEAB) and trithanolamine ethyl bromide (PS-THEEAB) were synthesized for the synthesis of cyclic carbonates (
Scheme 6). The catalytic activities of PS supported diethanolamine based ILs with -OH groups were much higher than that without -OH group (
Table 11, Entries 2,5–7). PS-DHEEAB and PS-THEAB showed higher activities than PS-HEIMB due to the presence of more -OH groups (
Table 11, Entries 2,5,6). PS-THEAB exhibited a little lower activity than PS-DHEEAB. The reason was that the two -OH groups in the cation of PS-DHEEAB could form two hydrogen bonding effect with the O atom of the epoxide which promoted the ring opening of epoxide, but the excess -OH group in the cation of PS-THEAB might prefer to form intramolecular hydrogen bond with halide anion, by which the nucleophilic behavior of the anion was weakened [
96,
97,
98]. These results showed the synergistic catalysis role of -OH groups. Moreover, the catalyst can be reused five times and the conversion of PC did not decrease.
Scheme 6.
Structures of the PS supported diethanolamine based ILs catalysts [
95].
Scheme 6.
Structures of the PS supported diethanolamine based ILs catalysts [
95].
Table 11.
Synthesis of PC catalyzed by PS supported diethanolamine based ILs catalyst
a [
95].
Table 11.
Synthesis of PC catalyzed by PS supported diethanolamine based ILs catalyst a [95].
| Entry | Catalyst | Conversion b(%) | Selectivity b(%) |
|---|
| 1 | DHEDEAB | 86 | 99 |
| 2 | PS-DHEEAB | 69 | 99 |
| 3 c | PS-DHEEAB | 92 | 99 |
| 4 d | PS-DHEEAB | 99 | 99 |
| 5 | PS-THEAB | 65 | 99 |
| 6 | PS-HEIMB | 52 | 99 |
| 7 | PS-EIMB | 35 | 99 |
| 8 e | PS-DHEEAB | 91 | 99 |
| 9 f | PS-DHEEAB | 91 | 99 |
| 10 g | PS-DHEEAB | 92 | 99 |
| 11 h | PS-DHEEAB | 91 | 99 |
Chitosan (CS), one of the most abundant natural biopolymers with many -OH groups, was used to synthesize chemically supported ILS catalysts [
84,
99,
100,
101,
102,
103,
104]. A series of CS chemically supported 1-ethyl-3-methyl imidazolium halide catalysts (CS-[EMIm][X], X = Cl, Br) were investigated for the synthesis of cyclic carbonates [
105]. The CS-[EMIm][Br] catalyst showed high activity, which was comparable to that of the homogeneous EMImBr catalyst. The reason was the synergistic catalysis effect of hydroxyl groups and tertiary amine groups in CS on the reaction. The CS-[EMIm][Br] catalyst could be reused five times with high activity and selectivity. In summary, CS not only played the role of the hydrogen bond-assisted ring-opening of epoxide but also the nucleophilic tertiary nitrogen-induced activation of CO
2.
 1a
1a 1b
1b 1c
1c 1d
1d 1e
1e 1f
1f