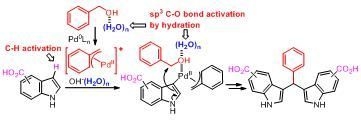
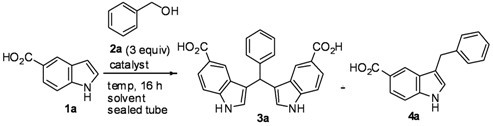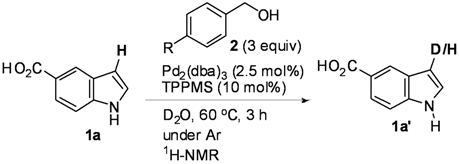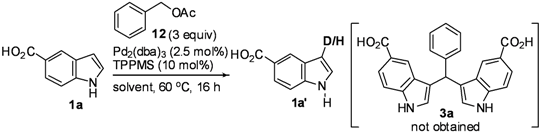Mechanistic Studies for Synthesis of Bis(indolyl)methanes: Pd-Catalyzed C–H Activation of Indole–Carboxylic Acids with Benzyl Alcohols in Water
Abstract
:1. Introduction

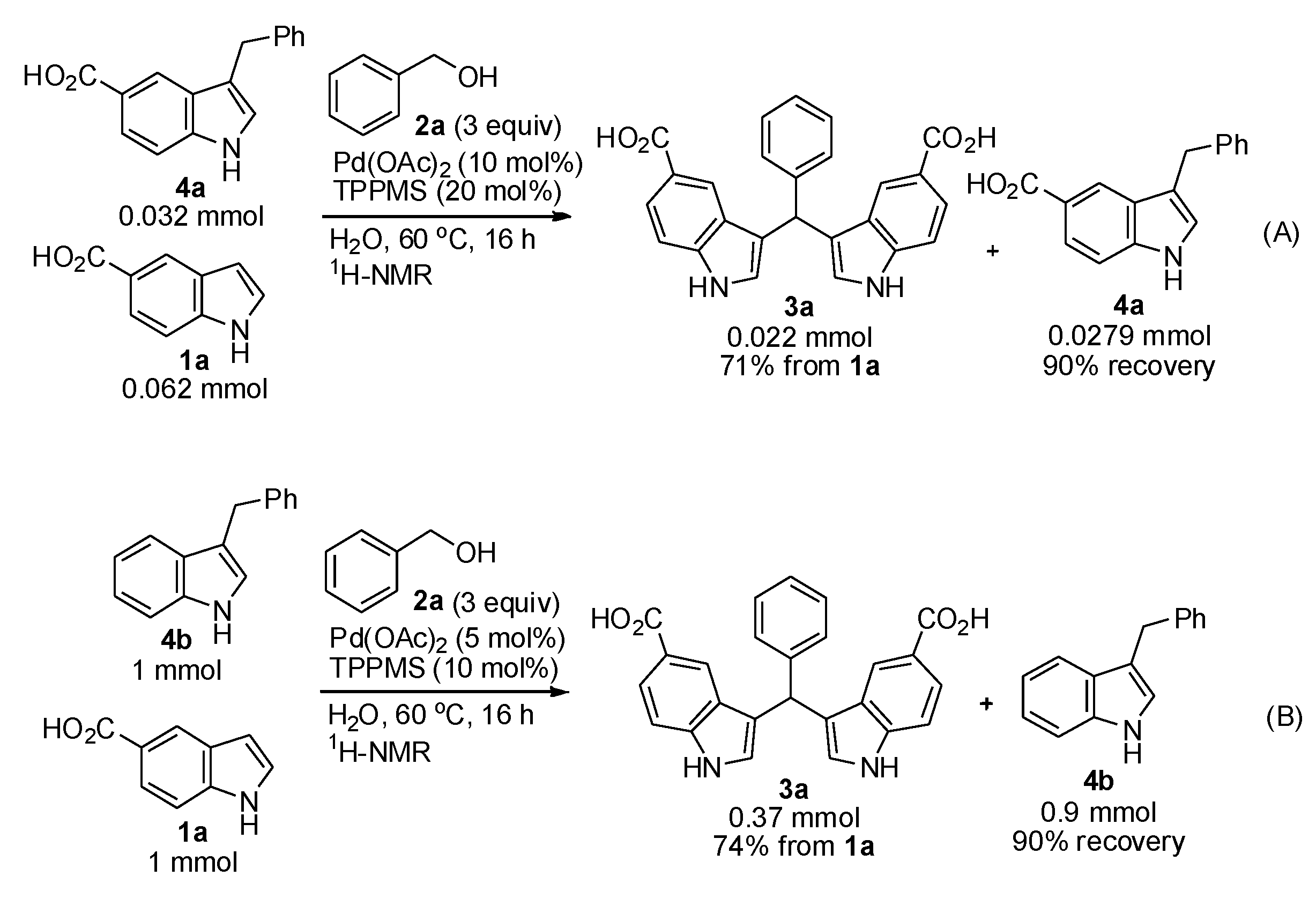
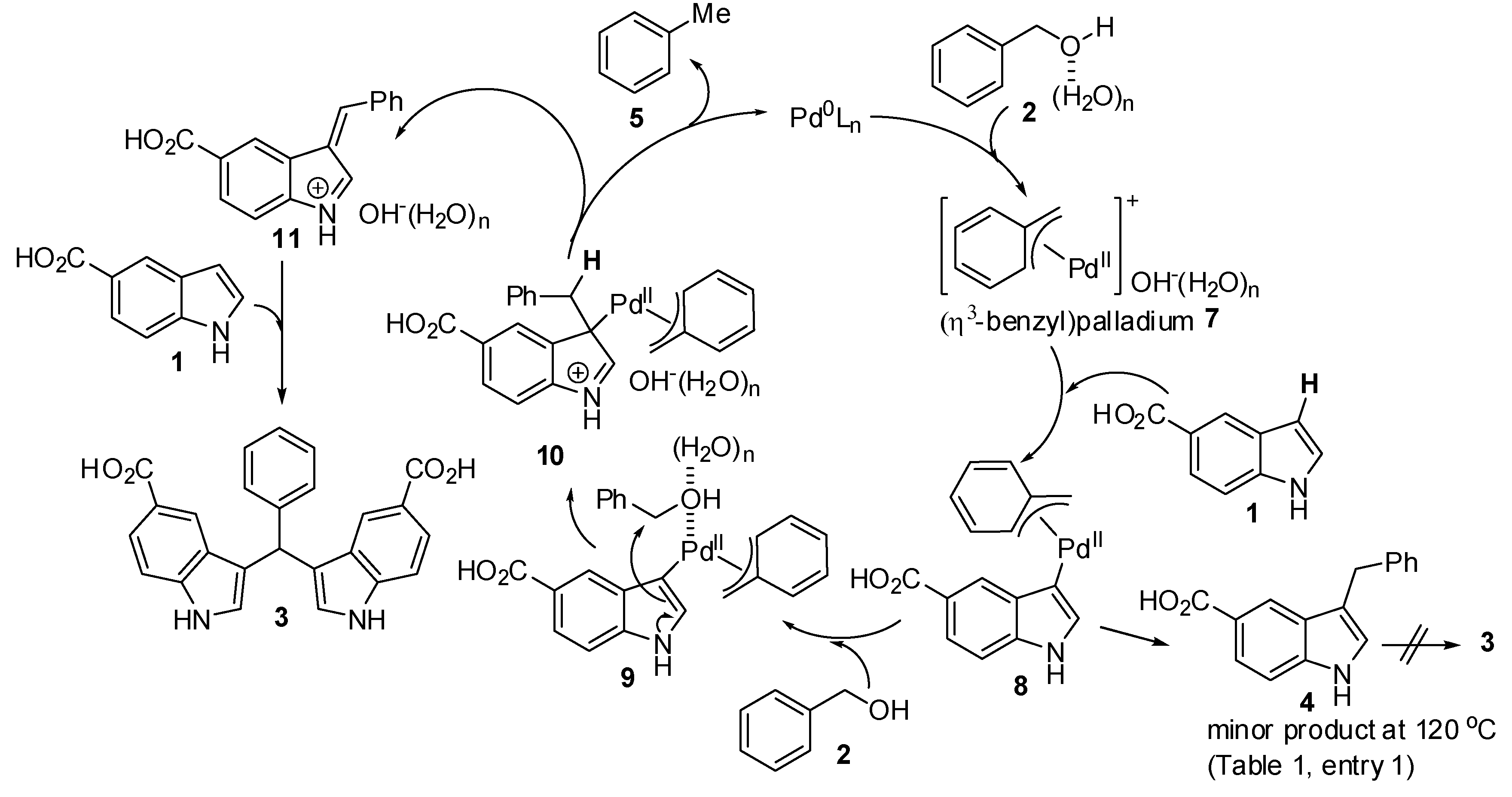
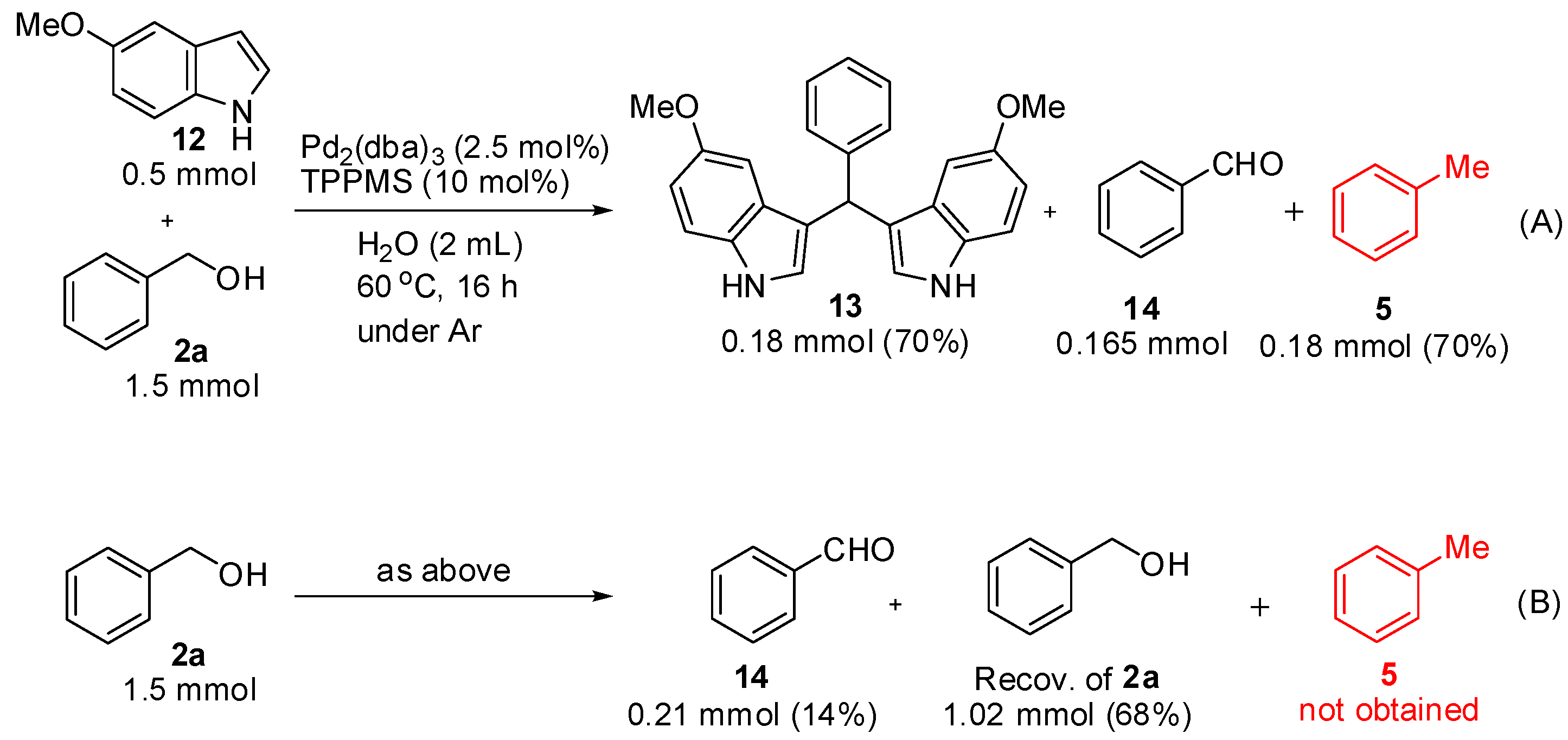
2. Results and Discussion
| Entry | Catalyst | Solvent | Temp | product (%) b | |
|---|---|---|---|---|---|
| (°C) | 3a | 4a | |||
| 1 | Pd(OAc)2/TPPMS | H2O | 120 | 84 | 15 |
| 2 | Pd(OAc)2/TPPMS | H2O | 60 | 91 (92) c | trace |
| 3 | Pd(OAc)2/TPPMS | H2O | rt | trace | 0 |
| 4 | none | H2O | 60 | 0 | 0 |
| 5 | PdCl2/TPPMS | H2O | 60 | 91 | trace |
| 6 | Pd2(dba)3 d/TPPMS | H2O | 60 | 93 | trace |
| 7 | PdCl2(PPh3)2 | H2O | 60 | trace | trace |
| 8 | Pd(OAc)2/TPPMS | DMSO, EtOH or THF | 60 | 0 | 0 |
| 9 | Pd(OAc)2/TPPMS | AcOH | 60 | 93 | trace |
| 10 | Pd(OAc)2/TPPMS | 1M NaOHaq.e | 60 | 63 | trace |

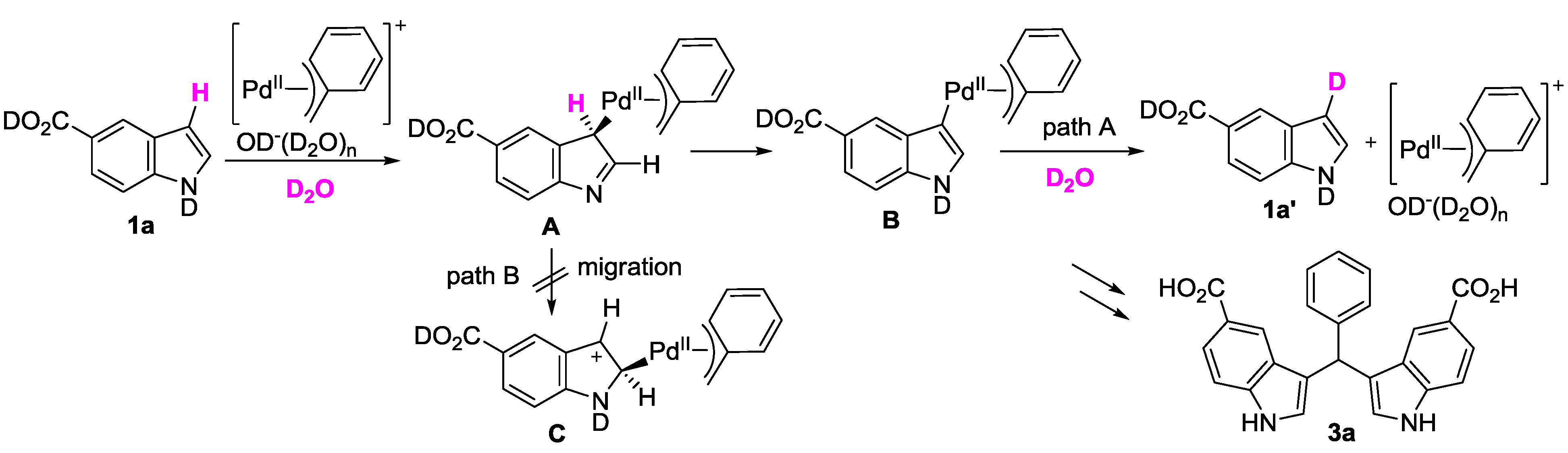
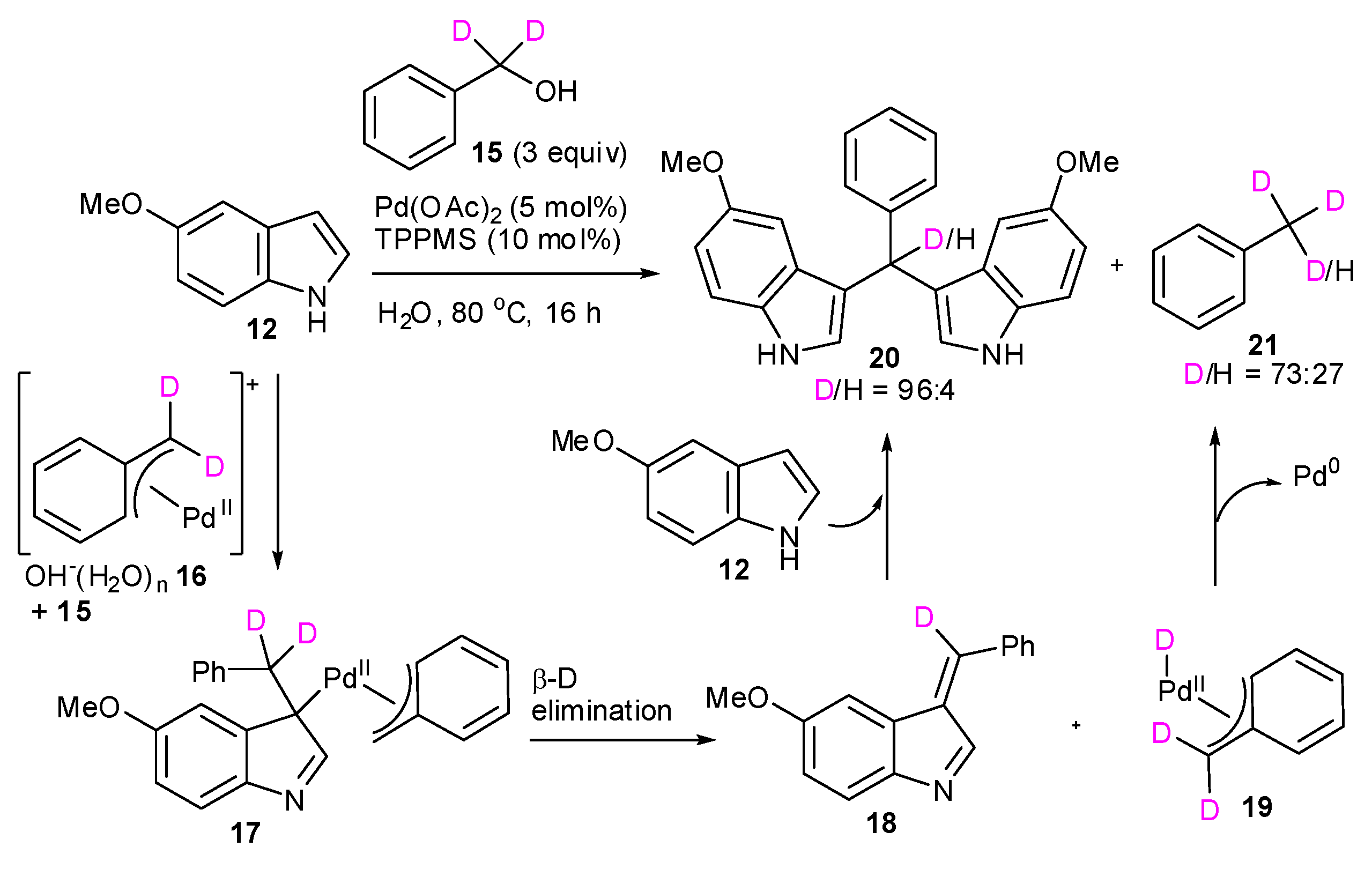
| Entry | Indoles 1 | Benzylic alcohols 2 | D incorporation (%) b |
|---|---|---|---|
| 1 | 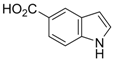 1a 1a | 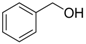 2a 2a | 65 |
| 2 | 1a | none | 12 |
| 3 | 1a |  instead of 2a instead of 2a | 14 |
| 4 | 1a |  2b 2b | 80 |
| 5 | 1a | 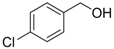 2c 2c | 70 |
| 6 | 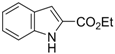 1b 1b |  2a 2a | 3 |
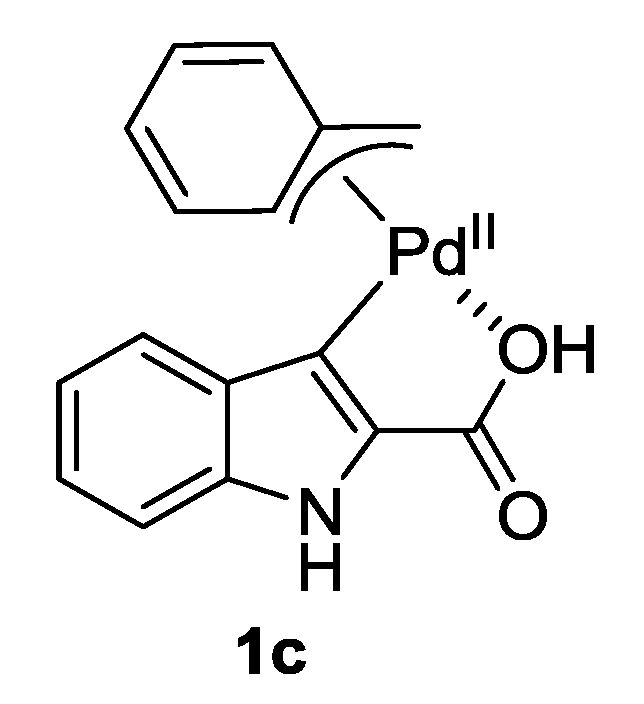
| 7 |  1c 1c | 2a | 81 |
| Entry | Solvent | Additive | D incorporation (%) b |
|---|---|---|---|
| 1 | D2O | none | 65 |
| 2 | 1,4-dioxane | benzyl alcohol
2a (3 equiv) | - |
| 3 | CD3OD | benzyl alcohol
2a (3 equiv) | 89 |

3. Experimental Section
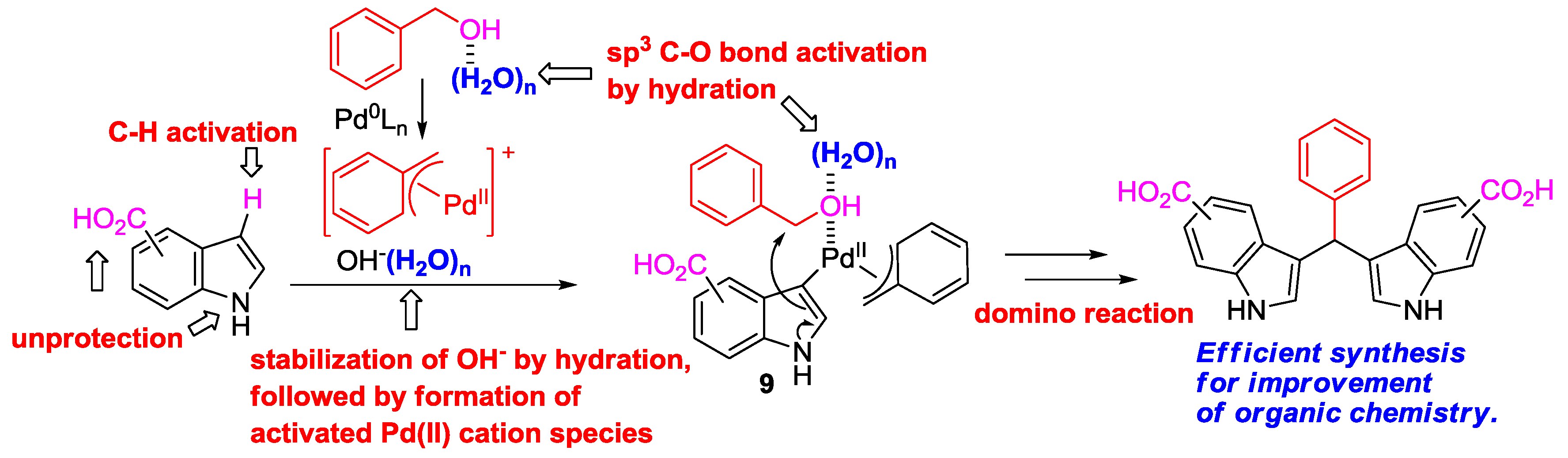
4. Conclusions
Conflict of Interest
References
- Ueno, S.; Komiya, S.; Tanaka, T.; Kuwano, R. Intramolecular SN'-Type Aromatic Substitution of Benzylic Carbonates at their Para-Position. Org. Lett. 2012, 14, 338–341. [Google Scholar]
- Xie, P.; Xie, Y.; Qian, B.; Zhou, H.; Xia, C.; Huang, H. Palladium-Catalyzed Oxidative Carbonylation of Benzylic C–H Bonds via Nondirected C(sp3)-H Activation. J. Am. Chem. Soc. 2012, 134, 9902–9905. [Google Scholar]
- Trost, B.M.; Czabaniuk, L.C. Benzylic Phosphates as Electrophiles in the Palladium-Catalyzed Asymmetric Benzylation of Azlactones. J. Am. Chem. Soc. 2012, 134, 5778–5781. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, S.; Yu, X.; Feng, X.; Bao, M. Nucleophilic Dearomatization of Chloromethyl Naphthalene Derivatives via η3-Benzylpalladium Intermediates: A New Strategy for Catalytic Dearomatization. Org. Lett. 2011, 13, 5402–5405. [Google Scholar] [CrossRef]
- Marquard, S.L.; Hartwig, J.F. C(sp3)-O Bond-forming reductive elimination of ethers from bisphosphine-ligated benzylpalladium(II) aryloxide complexes. Angew. Chem. Int. Ed. 2011, 50, 7119–7123. [Google Scholar] [CrossRef]
- Marquard, S.L.; Rosenfeld, D.C.; Hartwig, J.F. C(sp3)–N Bond-Forming Reductive Elimination of Amines: Reactions of Bisphosphine-Ligated Benzylpalladium(II) Diarylamido Complexes. Angew. Chem. Int. Ed. 2010, 49, 793–796. [Google Scholar]
- Trost, B.M.; Czabaniuk, L.C. Palladium-Catalyzed Asymmetric Benzylation of 3-Aryl Oxindoles. J. Am. Chem. Soc. 2010, 132, 15534–15536. [Google Scholar] [CrossRef]
- Kuwano, R. Catalytic transformations of benzylic carboxylates and carbonates. Synthesis 2009, 1049–1061. [Google Scholar] [CrossRef]
- Kuwano, R.; Konda, Y.; Matsuyama, Y. Palladium-Catalyzed Nucleophilic Benzylic Substitutions of Benzylic Esters. J. Am. Chem. Soc. 2003, 125, 12104–12105. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, J.; Li, M.; Gu, Y. Gluconic acid aqueous solution as a sustainable and recyclable promoting medium for organic reactions. Green Chem. 2011, 13, 2204–2211. [Google Scholar] [CrossRef]
- Shirakawa, S.; Kobayashi, S. Surfactant-Type Bronsted Acid Catalyzed Dehydrative Nucleophilic Substitutions of Alcohols in Water. Org. Lett. 2007, 9, 311–314. [Google Scholar] [CrossRef]
- Bisaro, F.; Prestat, G.; Vitale, M.; Poli, G. Alkylation of active methylenes via benzhydryl cations. Synlett 2002, 1823–1826. [Google Scholar]
- Yu, D.-G.; Wang, X.; Zhu, R.-Y.; Luo, S.; Zhang, X.-B.; Wang, B.-Q.; Wang, L.; Shi, Z.-J. Direct Arylation/Alkylation/Magnesiation of Benzyl Alcohols in the Presence of Grignard Reagents via Ni-, Fe-, or Co-Catalyzed sp3 C–O Bond Activation. J. Am. Chem. Soc. 2012, 134, 14638–14641. [Google Scholar]
- Ohshima, T.; Nakahara, Y.; Ipposhi, J.; Miyamotob, Y.; Mashima, K. Direct substitution of the hydroxy group with highly functionalized nitrogen nucleophiles catalyzed by Au(III). Chem. Commun. 2011, 8322–8324. [Google Scholar]
- Hirashita, T.; Kuwahara, S.; Okochi, S.; Tsuji, M.; Araki, S. Direct benzylation and allylic alkylation in high-temperature water without added catalysts. Tetrahedron Lett. 2010, 51, 1847–1851. [Google Scholar] [CrossRef]
- Cozzi, P.G.; Zoli, L. A rational approach towards the nucleophilic substitutions of alcohols “on water”. Angew. Chem. Int. Ed. 2008, 47, 4162–4166. [Google Scholar] [CrossRef]
- Cozzi, P.G.; Zoli, L. Nucleophilic substitution of ferrocenyl alcohols “on water”. Green Chem. 2007, 9, 1292–1295. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Shi, W.; Lei, A. Bond formations between two nucleophiles: Transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 2011, 111, 1780–1824. [Google Scholar]
- Bras, J.L.; Muzart, J. Intermolecular Dehydrogenative Heck Reactions. Chem. Rev. 2011, 111, 1170–1214. [Google Scholar] [CrossRef]
- Yeung, C.S.; Dong, V.M. Catalytic Dehydrogenative Cross-Coupling: Forming Carbon-Carbon Bonds by Oxidizing Two Carbon-Hydrogen Bonds. Chem. Rev. 2011, 111, 1215–1292. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Pd-catalyzed oxidative coupling with organometallic reagents via C-H activation. Chem. Commun. 2010, 677–685. [Google Scholar]
- Chen, X.; Engle, K.M.; Wang, D.-H.; Yu, J.-Q. Palladium(II)-Catalyzed C–H Activation/C-C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar]
- Ackermann, L.; Vicente, R.; Kapdi, A.R. Transition metal-catalyzed direct arylation of (hetero)arenes by C-H bond cleavage. Angew. Chem. Int. Ed. 2009, 48, 9792–9826. [Google Scholar] [CrossRef]
- Hikawa, H.; Yokoyama, Y. Pd-catalyzed C-H activation in water: synthesis of bis(indolyl)methanes from indoles and benzyl alcohols. RSC Adv. 2013, 3, 1061–1064. [Google Scholar] [CrossRef]
- Hikawa, H.; Ino, Y.; Suzuki, H.; Yokoyama, Y. Pd-Catalyzed Benzylic C-H Amidation with Benzyl Alcohols in Water: A Strategy To Construct Quinazolinones. J. Org. Chem. 2012, 77, 7046–7051. [Google Scholar] [CrossRef]
- Hikawa, H.; Yokoyama, Y. Palladium-catalyzed S-benzylation of unprotected mercaptobenzoic acid with benzyl alcohols in water. Org. Biomol. Chem. 2012, 10, 2942–2945. [Google Scholar] [CrossRef]
- Hikawa, H.; Yokoyama, Y. Palladium-Catalyzed Benzylation of Unprotected Anthranilic Acids with Benzyl Alcohols in Water. Org. Lett. 2011, 13, 6512–6515. [Google Scholar] [CrossRef]
- Hikawa, H.; Yokoyama, Y. Palladium-catalyzed mono-N-allylation of unprotected anthranilic acids with allylic alcohols in aqueous media. J. Org. Chem. 2011, 76, 8433–8439. [Google Scholar] [CrossRef]
- Hikawa, H.; Yokoyama, Y. Palladium-catalyzed mono-N-allylation of unprotected amino acids with 1,1-dimethylallyl alcohol in water. Org. Biomol. Chem. 2011, 9, 4044–4050. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Hikawa, H.; Mitsuhashi, M.; Uyama, A.; Hiroki, Y.; Murakami, Y. Total synthesis without protection: three-step synthesis of optically active clavicipitic acids by a biomimetic route. Eur. J. Org. Chem. 2004, 1244–1253. [Google Scholar]
- Yokoyama, Y.; Hikawa, H.; Mitsuhashi, M.; Uyama, A.; Murakami, Y. Syntheses without protection: a three-step synthesis of optically active clavicipitic acid by utilizing biomimetic synthesis of 4-bromotryptophan. Tetrahedron Lett. 1999, 40, 7803–7806. [Google Scholar]
- Afagh, N.A.; Yudin, A.K. Chemoselectivity and the Curious Reactivity Preferences of Functional Groups. Angew. Chem. Int. Ed. 2010, 49, 262–310. [Google Scholar] [CrossRef]
- Basset, J.-M.; Bouchu, D.; Godard, G.; Karame´, I.; Kuntz, E.; Lefebvre, L.; Legagneux, N.; Lucas, C.; Michelet, D.; Tommasino, J.B. Heterolytic Splitting of Allylic Alcohols with Palladium(0)-TPPTS in Water. Stabilities of the Allylphosphonium Salt of TPPTS and of the Ionic Complex [Pd(η3-allyl)(TPPTS)2]+. Organometallics 2008, 27, 4300–4309. [Google Scholar]
- Potavathri, S.P.; Pereira, K.C.; Gorelsky, S.I.; Pike, A.; LeBris, A.P.; DeBoef, B. Regioselective Oxidative Arylation of Indoles Bearing N-Alkyl Protecting Groups: Dual C-H Functionalization via a Concerted Metalation-Deprotonation Mechanism. J. Am. Chem. Soc. 2010, 132, 14676–14681. [Google Scholar]
- Zhao, J.; Zhang, Y.; Cheng, K. Palladium-Catalyzed Direct C-2 Arylation of Indoles with Potassium Aryltrifluoroborate Salts. J. Org. Chem. 2008, 73, 7428–7431. [Google Scholar] [CrossRef]
- Lane, B.S.; Brown, M.A.; Sames, D. Direct Palladium-Catalyzed C-2 and C-3 Arylation of Indoles: A Mechanistic Rationale for Regioselectivity. J. Am. Chem. Soc. 2005, 127, 8050–8057. [Google Scholar] [CrossRef]
- Grimster, N.P.; Gauntlett, C.; Godfrey, C.R.A.; Gaunt, M.J. Palladium-catalyzed intermolecular alkenylation of indoles by solvent-controlled regioselective C-H functionalization. Angew. Chem. Int. Ed. 2005, 44, 3125–3129. [Google Scholar]
- Yokogi, M.; Kuwano, R. Use of acetate as a leaving group in palladium-catalyzed nucleophilic substitution of benzylic esters. Tetrahedron Lett. 2007, 48, 6109–6112. [Google Scholar] [CrossRef]
- Kuwano, R.; Yokogi, M. Cross-coupling of benzylic acetates with arylboronic acids: One-pot transformation of benzylic alcohols to diarylmethanes. Chem. Commun. 2005, 5899–5901. [Google Scholar] [CrossRef]
- Carter, M.D.; Hadden, M.; Weaver, D.F.; Jacobo, S.M.H.; Lu, E. Preparation of bis-hetero/aryls, particularly bis-indoles, for treatment of protein folding disorders. WO2006/125324, 30 November 2006. [Google Scholar]
- Nobuta, T.; Fujiya, A.; Tada, N.; Miura, T.; Itoh, A. One-pot synthesis of bisindolylmethanes from benzyl alcohols and indoles using catalytic iodine and molecular oxygen under visible light irradiation. Synlett 2012, 2975–2979. [Google Scholar]
- Anilkumar, G.N.; Lesburg, C.A.; Selyutin, O.; Rosenblum, S.B.; Zeng, Q.; Jiang, Y.; Chan, T.-Y.; Pu, H.; Vaccaro, H.; Wang, L.; et al. Novel HCV NS5B polymerase inhibitors: Discovery of indole 2-carboxylic acids with C3-heterocycles. Bioorg. Med. Chem. Lett. 2011, 21, 5336–5341. [Google Scholar] [CrossRef]
- Bovens, S.; Elfringhoff, S.A.; Kaptur, M.; Reinhardt, D.; Schäfers, M.; Lehr, M. 1-(5-Carboxyindol-1-yl)propan-2-one Inhibitors of Human Cytosolic Phospholipase A2α: Effect of Substituents in Position 3 of the Indole Scaffold on Inhibitory Potency, Metabolic Stability, Solubility, and Bioavailability. J. Med. Chem. 2010, 53, 8298–8308. [Google Scholar] [CrossRef]
- Ludwig, J.; Bovens, S.; Brauch, C.; Elfringhoff, S.A.; Lehr, M. Design and synthesis of 1-Indol-1-yl-propan-2-ones as inhibitors of human cytosolic phospholipase A2α. J. Med. Chem. 2006, 49, 2611–2620. [Google Scholar] [CrossRef]
- Mazzei, M.; Miele, M.; Nieddu, E.; Barbieri, F.; Bruzzo, C.; Alama, A. Unsymmetrical methylene derivatives of indoles as antiproliferative agents. Eur. J. Med. Chem. 2001, 36, 915–923. [Google Scholar]
- Kinoshita, H.; Shinokubo, H.; Oshima, K. Water Enables Direct Use of Allyl Alcohol for Tsuji-Trost Reaction without Activators. Org. Lett. 2004, 6, 4085–4088. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hikawa, H.; Suzuki, H.; Yokoyama, Y.; Azumaya, I. Mechanistic Studies for Synthesis of Bis(indolyl)methanes: Pd-Catalyzed C–H Activation of Indole–Carboxylic Acids with Benzyl Alcohols in Water. Catalysts 2013, 3, 486-500. https://doi.org/10.3390/catal3020486
Hikawa H, Suzuki H, Yokoyama Y, Azumaya I. Mechanistic Studies for Synthesis of Bis(indolyl)methanes: Pd-Catalyzed C–H Activation of Indole–Carboxylic Acids with Benzyl Alcohols in Water. Catalysts. 2013; 3(2):486-500. https://doi.org/10.3390/catal3020486
Chicago/Turabian StyleHikawa, Hidemasa, Hideharu Suzuki, Yuusaku Yokoyama, and Isao Azumaya. 2013. "Mechanistic Studies for Synthesis of Bis(indolyl)methanes: Pd-Catalyzed C–H Activation of Indole–Carboxylic Acids with Benzyl Alcohols in Water" Catalysts 3, no. 2: 486-500. https://doi.org/10.3390/catal3020486