Immunreconstitution and Infectious Complications After Rituximab Treatment in Children and Adolescents: What Do We Know and What Can We Learn from Adults?
Abstract
:1. Introduction
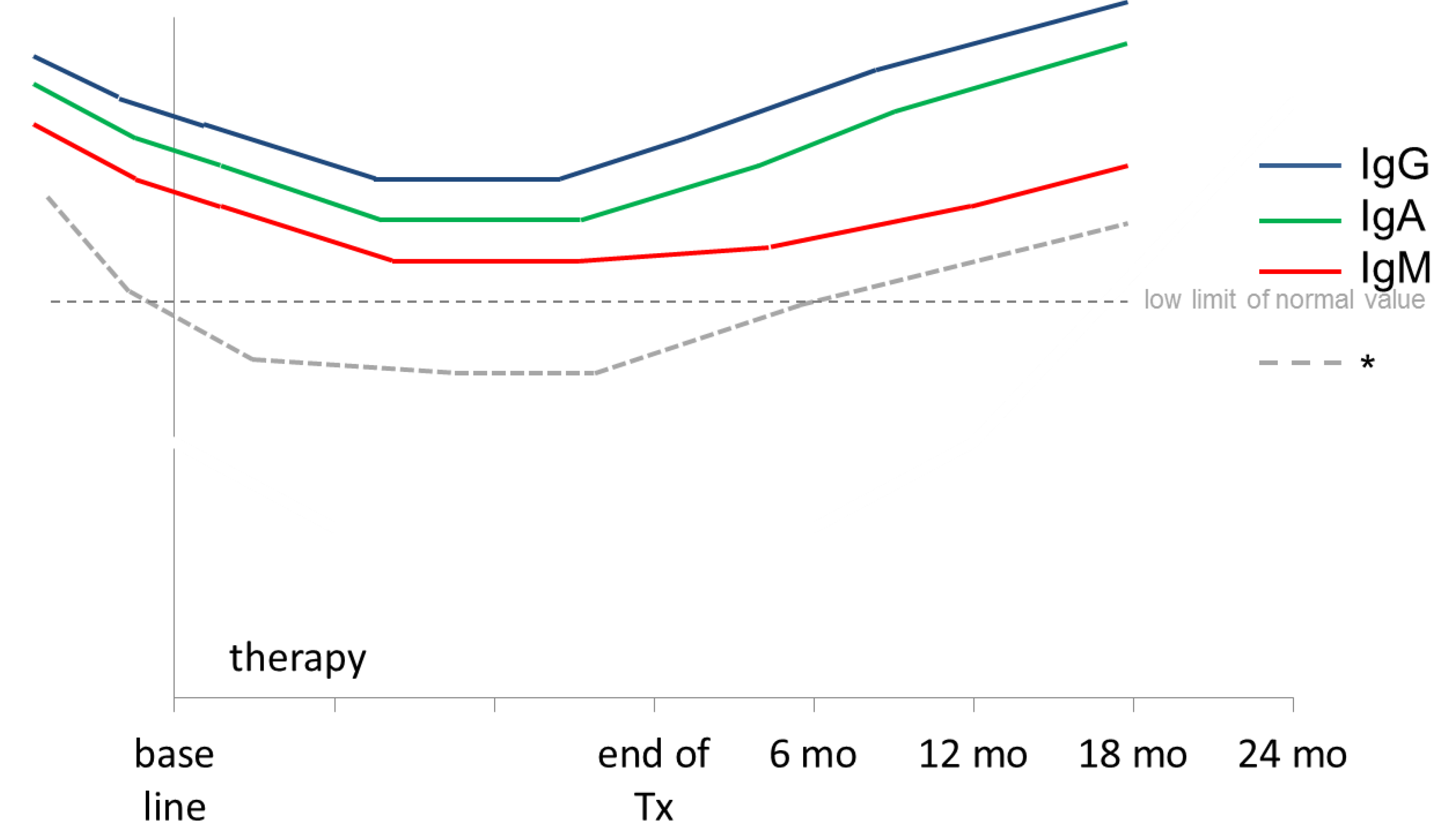
1.1. Immunreconstitution after Rituximab Treatment in Children and Adolescents
1.2. Immune Response and Vaccination Titres after Rituximab Treatment
| Diagnosis and Numbers | B-cell Reconstitution | Ig Level, Vaccination | Reference |
|---|---|---|---|
| chronic or refractory hematologic autoimmune cytopenias, rituximab 4 doses 375 mg/m2, non-responder after 3 doses: escalation to 3 × 750 mg/m², total 29 pts with 9 pts with dose escalation | B-cell recovery was noticed after 6 months. B cells were normalized after 1 year. Recovery was similar in pts that received 4 or 6 doses of rituxmab | IgM, IgA, IgG decreased but remained near normal range | [15] |
| autoimmune haemolytic anaemia, rituxmab 4 weekly doses 375 mg/m², 6 pts. 2 pts received 8 additional infusions | B cells did not reappear in blood 5–9 months after last infusion. Normal counts were then reached within the following months “A reduction to one or two injections, which would limit the duration of B-cell deficiency would be worth assessing” | Ig concentrations in serum fell below normal values for age. 5 pts were substituted 9–10 months after last ritux mab infusion | [16] |
| autoimmune hemolytic anemia (n = 9) and Evans syndrome (n = 6), ritux 375 mg/m² weekly with 2 doses (n = 3), 3 doses (n = 10), 4 doses (n = 2), 15 pts | B-cell undetectable after treatment in all pts. Normal B-cell count after 6 mo in 10/15 (67%) of pts | [51] | |
| nephrotic syndrome, single dose ritux 375 mg/m², 12 pts | Median time to B-cell recovery 119 days | Serum IgM levels gradually decreased | [52] |
| nephrotic syndrome, rituxmab 375 mg/m² weekly in 4 doses (n = 15), 3 doses (n = 2), 2 doses (n = 4) or 1 dose (n = 1), re-treatment single dose (n = 19), 22 pts | Duration of complete B-cell depletion 3 to 15 mths (mean 8 mths). Similar duration of B-cell depletion in pts with 1–2 doses and in pts with 3–4 doses of rituxmab | [53] | |
| acute rejection after renal transplantation, 4 doses 375 mg/m² weekly, 20 randomized pts | Correlation of age and B-cell recovery: children < 10years of age repopulated significantly faster than children > 10years of age (5 vs. 14 months) | IgM levels trended lower in rituximab group compared to control. Correlation between lowering of serum IgM, young age and B-cell repopulation > 10 months | [17] |
| severe chronic ITP, rituxmab 375 mg/m² weekly for 4 doses, 36 pts | B cell depletion in all pts, remaining unchanged at 2% between week 6 and week 12 | No significant HG: mean IgG falling only 0.7%/week but significant decrease of mean IgM levels. “it would appear that IVIG replacement therapy is unnecessary.” | [19] |
| Chronic ITP, rituxmab 375 mg/m² weekly for 4 doses, 24 pts | decreased IgG in 4 pts, decreased IgM in 5 pts | [54] | |
| Chronic ITP, rituxmab 375 mg/m² weekly for 4 doses, single pt | low CD19-positive cells (<400 × 109/L) | after begin of rituximab, IgG, IgM and IgA level were decreased for 3 years, with only increased IgG thereafter | [55] |
| SLE, rituxmab 750 mg/m² 1 dose (19 pts had 2 -6 doses), 63 pts | After a mean of 2.5 months, IgG, IgA and IgM levels were reduced, but only 2% with Ig replacement | [56] | |
| autoimmune and inflammatory CNS disease, 144 pts, rituximab 375 mg/m2 1–10 doses, the most common regimen weekly for 4 weeks (n = 57) | B-cell depletion in 119/24 (96%), >12mo in 12/124 (10%) | Hypogammaglobulinemia in 27/124 (22%) | [57] |
1.3. Immunereconstitution in Adult B-NHL Patients Treated with Rituximab and Chemotherapy: Lessons for the Pediatricians

2. Infectious Complications after Rituximab Treatment
| Diagnosis and Numbers | B-cell Reconstitution | Ig Level, Comments | Reference |
|---|---|---|---|
| relapsed FL, 4 doses ritux 375 mg/m² (n = 7), de novo FL 4 doses (n = 4) | B-cell reconstitution (>5 cells/µL) 6–9 months, recovery to pre-treatment levels by 12 months | delayed recovery of CD27+ memory B-cell pool; emerging B cells after rituxmab are functionally immature | [69] |
| FL and MCL, 4 weekly doses of rituximab mono 375 mg/m² (arm A) | 1 year after start median B-cell level 81% of baseline (n = 22) | IgG and IgM remained stable. IgM 100% recovered after 1 year (n = 30) | [61] |
| 4 weekly doses and 4 more doses at 2-month intervals (arm B) | 1 year after start median B-cell level 50% of baseline, 6 months longer to recover to baseline (n = 35) | IgG remained stable. IgM decreased to 73% of baseline after 1 year (n = 50) (p 0.007) | |
| relapsed FL, 4 weekly doses 375 mg/m² of rituximab mono (n = 166) | recovery of B cells started between 6 and 9 months; recovery to normal between 9 and 12 months | Mean serum IgA and IgG levels remained within normal. IgM recovered to normal at 8 mths | [58] |
| B-NHL (n = 66), 6× CHOP + rituxmab 375 mg/m² prior each cycle + 2 doses within 1 month (total 8 doses rituximab) | After 1 cycle R-CHOP CD19+ and CD20+ completely eliminated. One year after therapy B-cell levels same as at diagnosis and were almost double the level at diagnosis 2 years after therapy | After 6 cycles: IgG 68%, IgA 63%, IgM 56%. All Ig recovered gradually until 2 years after therapy IgG 94%, IgA 90%, IgM 76%. | [63] |
| FL or low grade Lymphoma, 8 doses rituximab 375 mg/m2 weekly, 37 pts | B-cell with counts returning to the lower limit of normal recovery 6–9 months after rituximab | Mean serum IgG, IgA and IgM levels within normal range. Decrease > 50% from baseline in IgG, IgM or IgA was observed in two, one and four pts, respectively. Within 1 year, levels recovered in all but one pt (low IgG) | [60] |
| review of different diagnoses | reconstitution of B lymphocytes usually takes several months; return to pre-treatment value after about one year | Reduction of serum Ig levels; unclear whether clinically significant | [70] |
| B-cell lymphoma (n = 211), median of 7× rituximab (monotherapy or combined with chemotherapy) | 39% of patient with initially normal IgG levels presented with hypogammaglobulinemia (mild for 77% of pt). | [59] | |
| Diffuse large B-cell lymphoma (n = 122) treated with CHOP (n = 24) or rituximab-CHOP (n = 98); 16 pts were excluded due to abnormal Ig levels before treatment; 6 cycles of treatment | No obvious changes of IgG, IgA and IgM in the CHOP group. Decreased levels of IgG, IgA and IgM by 20% of baseline in 85%, 85% and 88% respectively. IgG, IgA and IgM < low limit of normal value were observed in 48%, 49% and 52% of pts, respectively. | [68] | |
| B-cell NHL (n = 66). Standart treatment included R-CHOP, CHOP and CVP. Exact number of rituximab infusions is not indicated | Levels for IgA, Igg and IgM were decreased after treatment compared to pretreament levels. No occurrence of higher infection rates | [71] |
2.1. Infectious Complications after Rituximab Treatment in Children and Adolescents
2.2. Relevant Infectious Complications Reported in Adult Patients after Rituximab Treatment
| Indication | No. Pts | Age Years | Dosing | Toxicity | Serious Adverse Events | Reference |
|---|---|---|---|---|---|---|
| nephrotic syndrome | 12 | <20 | 1 × 375 | mild reactions (n = 5) 42%; fever and hypotension (n = 1), tachycardia (n = 1), hypertension (n = 1), facial flushing(n = 1), mild respiratory distress (n = 1) | no SAE during the patients clinical courses | [52] |
| nephrotic syndrome | 22 | 375, 4× (n = 15), 3× (n = 2), 2× (n = 4), 1× (n = 1), re-treatment 1× (n = 19) | dizziness, polypnea with dyspnea and tachycardia (n = 2), neutropenia (n = 19), peripheral vein thrombosis (n = 1), transient hepatic cytolysis (n = 1), transient thrombocytopenia (n = 1), gastroenteritis (n = 1), fever (n = 1) | no major side effects were observed | [53] | |
| acute rejection after renal transplant, randomized | 20 | 2–23 | 4 × 375 | mild hypotension and shortness of breath at the first dose only (n = 2) | no SAE | [17] |
| severe chronic ITP (n = 30) or Evans Syndrom (n = 6) | 36 | 375, 4× (n = 33), <4× (n = 3) | not all 4 doses (n = 3) due to serum sickness (n = 2), infusion related hypotension (n = 1) | [19] | ||
| autoimm. hemolytic anemia (n = 9), Evans syndrom (n = 6) | 15 | 0.3–14 | 375, 2× (n = 3), 3× (n = 10), 4× (n = 2) | infusion-related side effects: fever (n = 2) upper airway edema (n = 1) | primary varicella zoster virus infection two months after rituximab | [51] |
| Chronic or refractory hematologic autoimm. cytopenia | 29 | <21 | 4 × 375, non-responder after 3×, escalation to 3 × 750 (n = 9) | Mild infusion reactions including respiratory symptoms, fever, myalgia. No delayed infusion reactions, no early or delayed infectious complications | one patient (3%) did not tolerate the drug | [15] |
| autoimm. haemolytic anaemia | 6 | 0.6–2.9 | 375, 4× (n = 4), 12× (n = 12) | E. coli pyelonephriitis (n = 1), febrile bronchitis (n = 1) | no infusion-related side effects, low incidence of infections | [16] |
| Diamond-Blackfan anemia | 1 | 8 | 375, 2× weekly | no immediate serious side effect was observed | [84] | |
| autoimmune and inflammatory CNS disease | 144 | <18 | 375 mg/m2, 1–10 doses | Infectious any 11 (7.6%): 40: 2 (1.4%) CMV retinitis; shock and hypoxic brain injury, 30: 7 (5%): pneumonia (n = 2), empyema, bronchiectasis, salmonella enteritis, C. difficile enteritis, mastoiditis (all n = 1) | Infectious, 50: 2 (1.4), CMV colitis (complicated by fatal bowel perforation); staphylococcus toxic shock syndrome | [57] |
| Infection and Complication | Evidence and Diagnosis | Treatment Including Rituximab | Comments and/or Results | Reference |
|---|---|---|---|---|
| lympho-cytopenia without increased rates of infections | prospective randomized study in relapsed FL and MCL (n = 147) | fludarabine, cyclophosphamide, mitoxantrone with or without rituximab | lymphocytopenia grade III/IV more frequent with R-FCM (51%) compared to FCM (39%; p 0.006) without clinical relevance, i.e., no increased risk of infectious complications; WHO grade III/IV infections of 1.5% were not different between the two arms | [85] |
| overall infections and neutropenia including long term follow-up | randomized trial untreated DLBCL (n = 399) | CHOP (n = 197) versus R-CHOP (n = 202) | similar incidence of 65% for infectious events for all grades, grade III/IV infections were 12% for R-CHOP versus 20% for CHOP; neutropenia was not associated with an increase in episodes of infection, number of patients with infections in 5 year follow-up with trend of increase (12 R-CHOP versus 6 CHOP) | [86] |
| overall rates of infections | DLBCL randomized trial (n = 824) | CHOP (n = 411) versus R-CHOP (n = 413) | Similar rates of infections for R-CHOP (30%) versus CHOP (31%) | [87] |
| overall rates of infections | DLBCL randomized trial | CHOP (n = 314) vs. R-CHOP (n = 318) | Similar rates of grade III/IV infections for R-CHOP versus CHOP (17% vs. 16%) and neutropenia (78% vs. 78%) | [88] |
| overall rates of infections including fever of unknown origin and hemato-logical toxicities | FL, randomized trial (n = 428) | CHOP (n = 205) vs. R-CHOP (n = 223) | Similar rates of grade III/IV infections for R-CHOP vs. CHOP (5% vs. 7%) despite higher frequency of granulocytopenia grade III/IV with R-CHOP vs. CHOP (63% vs. 53%)similar numbers of infection-related deaths with both treatment arms | [89] |
| WHO grade infections | MCL, randomized trial (n = 122) | CHOP (n = 60) vs. R-CHOP (n = 62) | Similar grade III/IV infectious complications comparing R-CHOP with CHOP (5% vs. 6%) | [90] |
| hematological and non-hematological toxicity | MCL; phase III randomized study (n = 156) | fludarabine and cyclophosphamide with (n = 78) or without rituximab (n = 78) | toxicity data (n = 139): non-hemotological toxicity similar; more hematological events III/IV with 58% (FCR) leukopenia compared to 41% (FC) leukopenia (p 0.024); this did not translate into increased rates of febrile episodes or infections | [91] |
| Infection and Complication | Evidence and Diagnosis | Treatment Including Rituximab | Comments and/or Results | Reference |
|---|---|---|---|---|
| PML | MCL (n = 2) splenic MZL (n = 1) | Rituximab combine d with hyperCVAD and DHAP (n = 1), CVP (n = 1) or CHOP (n = 1) | 3 case reports of PML in HIV negative patients | [92] |
| PML | NHL (n = 976) | rituximab (n = 517), no rituximab (n = 459) | Retrospective cohort study in HIV negative patients | [93] |
| PML | FL (n = 1), ALL (n = 1), DLBCL (n = 1) | Case 1: CHOP, R-DHAP and ASCT, Case 2: Rituximab, R-P-VABEC, CIP, Case 3: Hyper-CVAD + imatinib, HSCT rituximab | 3 case reports of PML in HIV negative patients | [94] |
| PML | B-cell lympho-proliferative disorders (n = 52); autoimmune disorders (n = 5) | rituximab combined with HSCT (n = 7), purine analogs (n = 26), alkylating agents (n = 39) | clinical characteristics of 52 HIV negative patients with PML, median time from last rituximab dose to PML diagnosis was 5.5 months, overall incidence of fatality 90% | [13] |
| PML and CMV | mediastinal thymic B-NHL, DLBCL, MZ | HSCT and rituximab | PML (n = 2); CMV retinitis (n = 1); CMV pneumonitis (n = 1) | [95] |
| PML | DLBCL | R-CHOP | Case report of PML | [96] |
| PML | electronic medical records from the Veteran’s Administration, 2003–2011, 57,041 non-HIV NHL pts | rituximab, cyclophosphamide, hydroxydaunorubicin vincrisitne | PML: 14/57,041 (0.025%): 7/8895 (7.8 per 10,000) NHL patients who received rituximab 7/48,146 (1.5 per 10,000) NHL patients who did not receive rituximab | [97] |
3. Summary, Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Okroj, M.; Osterborg, A.; Blom, A.M. Effector mechanisms of anti-CD20 monoclonal antibodies in B cell malignancies. Cancer Treat. Rev. 2013, 39, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Golay, J.; Bologna, L.; Andre, P.A.; Buchegger, F.; Mach, J.P.; Boumsell, L.; Introna, M. Possible misinterpretation of the mode of action of therapeutic antibodies in vitro: Homotypic adhesion and flow cytometry result in artefactual direct cell death. Blood 2010, 116. [Google Scholar] [CrossRef]
- Golay, J.; Semenzato, G.; Rambaldi, A.; Foa, R.; Gaidano, G.; Gamba, E.; Pane, F.; Pinto, A.; Specchia, G.; Zaja, F.; et al. Lessons for the clinic from rituximab pharmacokinetics and pharmacodynamics. MABS 2013, 5, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Boross, P.; Leusen, J.H. Mechanisms of action of CD20 antibodies. Am. J. Cancer Res. 2012, 2, 676–690. [Google Scholar] [PubMed]
- Shan, D.; Ledbetter, J.A.; Press, O.W. Signaling events involved in anti-CD20-induced apoptosis of malignant human B cells. Cancer Immunol. Immunother. 2000, 48, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Stolz, C.; Hess, G.; Hahnel, P.S.; Grabellus, F.; Hoffarth, S.; Schmid, K.W.; Schuler, M. Targeting Bcl-2 family proteins modulates the sensitivity of B-cell lymphoma to rituximab-induced apoptosis. Blood 2008, 112, 3312–3321. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Terui, Y.; Mishima, Y.; Kuniyoshi, R.; Matsusaka, S.; Mikuniya, M.; Kojima, K.; Hatake, K. High reproducible ADCC analysis revealed a competitive relation between ADCC and CDC and differences between FcγRllla polymorphism. Int. Immunol. 2012, 24, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Racila, E.; Taylor, R.P.; Weiner, G.J. Nk-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood 2008, 111, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Daikos, G.; Boumpas, D.; Tsiodras, S. Does rituximab increase the incidence of infectious complications? A narrative review. Int. J. Infect. Dis. 2011, 15, e2–e16. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Chiou, T.J.; Hsiao, L.T.; Yang, M.H.; Lin, P.C.; Poh, S.B.; Yen, C.C.; Liu, J.H.; Teng, H.W.; Chao, T.C.; et al. Rituximab therapy increased post-transplant cytomegalovirus complications in non-Hodgkin’s lymphoma patients receiving autologous hematopoietic stem cell transplantation. Ann. Hematol. 2008, 87, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kavcic, M.; Fisher, B.T.; Seif, A.E.; Li, Y.; Huang, Y.S.; Walker, D.; Aplenc, R. Leveraging administrative data to monitor rituximab use in 2875 patients at 42 freestanding children’s hospitals across the united states. J. Pediatr. 2012, 162, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S.; Harputluoglu, H.; Kilickap, S.; Dede, D.S.; Dizdar, O.; Altundag, K.; Barista, I. Rituximab-related viral infections in lymphoma patients. Leuk. Lymphoma 2007, 48, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Carson, K.R.; Bennett, C.L. Rituximab and progressive multi-focal leukoencephalopathy: The jury is deliberating. Leuk. Lymphoma 2009, 50, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.; Haddad, E.; Niaudet, P.; Kone-Paut, I.; Bensman, A.; Cochat, P.; Deschenes, G.; Fakhouri, F.; Leblanc, T.; Llanas, B.; et al. Rituximab therapy for childhood-onset systemic lupus erythematosus. J. Pediatr. 2006, 148, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Kelly, M.; Musselman, M.; Ramadas, J.; Wilson, D.; Grossman, W.; Shenoy, S. Safety, efficacy, and immune reconstitution after rituximab therapy in pediatric patients with chronic or refractory hematologic autoimmune cytopenias. Pediatr. Blood Cancer 2008, 50, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Quartier, P.; Brethon, B.; Philippet, P.; Landman-Parker, J.; le Deist, F.O.; Fischer, A. Treatment of childhood autoimmune haemolytic anaemia with rituximab. Lancet 2001, 358, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Zarkhin, V.; Li, L.; Kambham, N.; Sigdel, T.; Salvatierra, O.; Sarwal, M.M. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am. J. Transplant. 2008, 8, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, L.; Gao, J.; Hu, D.; Ai, Y. Rituximab for children with immune thrombocytopenia: A systematic review. PLOS ONE 2012, 7, e36698. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.M.; Rogers, Z.R.; Kinnamon, D.D.; Bussel, J.B.; Mahoney, D.H.; Abshire, T.C.; Sawaf, H.; Moore, T.B.; Loh, M.L.; Glader, B.E.; et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood 2006, 107, 2639–2642. [Google Scholar] [CrossRef] [PubMed]
- Mellgren, K.; Hedegaard, C.J.; Schmiegelow, K.; Muller, K. Plasma cytokine profiles at diagnosis in pediatric patients with non-Hodgkin lymphoma. J. Pediatr. Hematol. Oncol. 2012, 34, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, A.; Schadwill, S.; Zimmermann, M.; Gruhn, B.; Behnisch, W.; Becker, M.; Reiter, A. Serum immunoglobulin levels in children and adolescents after B-NHL BFM chemotherapy with and without one dose of rituximab. 2009, 3, Abstract 036. [Google Scholar]
- Abrahamsson, J.; Marky, I.; Mellander, L. Immunoglobulin levels and lymphocyte response to mitogenic stimulation in children with malignant disease during treatment and follow-up. Acta Paediatr. 1995, 84, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.K.; Woods, A.; Buckstein, R.; Mangel, J.; Pennell, N.; Zhang, L.; Imrie, K.; Spaner, D.; Cheung, M.C.; Boudreau, A.; et al. Rituximab purging and maintenance combined with auto-sct: Long-term molecular remissions and prolonged hypogammaglobulinemia in relapsed follicular lymphoma. Bone Marrow Transplant. 2009, 43, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Shortt, J.; Spencer, A. Adjuvant rituximab causes prolonged hypogammaglobulinaemia following autologous stem cell transplant for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2006, 38, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Fujimoto, K.; Yamamoto, S.; Endo, T.; Sakai, T.; Obara, M.; Kumano, K.; Yamaguchi, K.; Takeda, Y.; Goto, H.; et al. Delayed redistribution of CD27, CD40 and CD80 positive B cells and the impaired in vitro immunoglobulin production in patients with non-Hodgkin lymphoma after rituximab treatment as an adjuvant to autologous stem cell transplantation. Br. J. Haematol. 2007, 137, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Fujimoto, K.; Yamamoto, S.; Endo, T.; Sakai, T.; Obara, M.; Kumano, K.; Minauchi, K.; Yamaguchi, K.; Takeda, Y.; et al. Hypogammaglobulinemia with a selective delayed recovery in memory B cells and an impaired isotype expression after rituximab administration as an adjuvant to autologous stem cell transplantation for non-Hodgkin lymphoma. Eur. J. Haematol. 2006, 77, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Negrin, R.S.; Blume, K.G.; Breslin, S.; Stuart, M.J.; Stockerl-Goldstein, K.E.; Johnston, L.J.; Wong, R.M.; Shizuru, J.A.; Horning, S.J. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood 2004, 103, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Kano, G.; Nakatani, T.; Yagi, K.; Sakamoto, I.; Imamura, T. Complicated pathophysiology behind rituximab-induced persistent hypogammaglobulinemia. Immunol. Lett. 2014, 159, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Van Esser, J.W.; Niesters, H.G.; van der Holt, B.; Meijer, E.; Osterhaus, A.D.; Gratama, J.W.; Verdonck, L.F.; Lowenberg, B.; Cornelissen, J.J. Prevention of epstein-barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 2002, 99, 4364–4369. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Dallorso, S.; Faraci, M.; Morreale, G.; di Martino, D.; Cristina, E.; Scarso, L.; Lanino, E. Long-lasting hypogammaglobulinemia following rituximab administration for epstein-barr virus-related post-transplant lymphoproliferative disease preemptive therapy. J. Hematother. Stem Cell Res. 2003, 12, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Masjosthusmann, K.; Ehlert, K.; Eing, B.R.; Roth, J.; Koehler, G.; Juergens, H.; Fruehwald, M.; Groll, A.H. Delay in B-lymphocyte recovery and function following rituximab for EBV-associated lymphoproliferative disease early post-allogeneic hematopoietic SCT. Bone Marrow Transplant. 2009, 43, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Kuehnle, I.; Huls, M.H.; Liu, Z.; Semmelmann, M.; Krance, R.A.; Brenner, M.K.; Rooney, C.M.; Heslop, H.E. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood 2000, 95, 1502–1505. [Google Scholar] [PubMed]
- Looney, R.J.; Srinivasan, R.; Calabrese, L.H. The effects of rituximab on immunocompetency in patients with autoimmune disease. Arthritis Rheum. 2008, 58, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bearden, C.M.; Agarwal, A.; Book, B.K.; Vieira, C.A.; Sidner, R.A.; Ochs, H.D.; Young, M.; Pescovitz, M.D. Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage phiX174. Am. J. Transplant. 2005, 5, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Stawinski, G.V.; Yu, P.B.; Love, S.D.; Parker, W.; Davis, R.D., Jr. Hapten-induced primary and memory humoral responses are inhibited by the infusion of anti-CD20 monoclonal antibody (IDEC-C2B8, Rituximab). Clin. Immunol. 2001, 98, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Oren, S.; Mandelboim, M.; Braun-Moscovici, Y.; Paran, D.; Ablin, J.; Litinsky, I.; Comaneshter, D.; Levartovsky, D.; Mendelson, E.; Azar, R.; et al. Vaccination against influenza in patients with rheumatoid arthritis: The effect of rituximab on the humoral response. Ann. Rheum. Dis. 2008, 67, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Bingham, C.O., 3rd; Looney, R.J.; Deodhar, A.; Halsey, N.; Greenwald, M.; Codding, C.; Trzaskoma, B.; Martin, F.; Agarwal, S.; Kelman, A. Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial. Arthritis Rheum. 2010, 62, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Bedognetti, D.; Zoppoli, G.; Massucco, C.; Zanardi, E.; Zupo, S.; Bruzzone, A.; Sertoli, M.R.; Balleari, E.; Racchi, O.; Messina, M.; et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin’s lymphoma patients treated with rituximab-containing regimens. J. Immunol. 2011, 186, 6044–6055. [Google Scholar] [CrossRef] [PubMed]
- Yri, O.E.; Torfoss, D.; Hungnes, O.; Tierens, A.; Waalen, K.; Nordoy, T.; Dudman, S.; Kilander, A.; Wader, K.F.; Ostenstad, B.; et al. Rituximab blocks protective serologic response to influenza a (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood 2011, 118, 6769–6771. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, D.; Sticchi, L.; Racchi, O.; Mangerini, R.; Ferraris, A.M.; Gaetani, G.F. Influenza vaccine in chronic lymphoproliferative disorders and multiple myeloma. Eur. J. Haematol. 2003, 70, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.; Loulergue, P.; Mir, O.; Krivine, A.; Kotti, S.; Viel, E.; Simon, T.; de Gramont, A.; Goldwasser, F.; Launay, O.; et al. Immunogenicity and safety of the influenza a H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: The vacance study. Ann. Oncol. 2012, 23, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Feery, B.J.; Sullivan, J.R.; Hurley, T.H.; Evered, M.G. Immunization with influenza vaccine in patients with haematological malignant disease. Med. J. Aust. 1977, 1, 292–294. [Google Scholar] [PubMed]
- Centkowski, P.; Brydak, L.; Machala, M.; Kalinka-Warzocha, E.; Blasinska-Morawiec, M.; Federowicz, I.; Walewski, J.; Wegrzyn, J.; Wolowiec, D.; Lech-Maranda, E.; et al. Immunogenicity of influenza vaccination in patients with non-Hodgkin lymphoma. J. Clin. Immunol. 2007, 27, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Brydak, L.B.; Machala, M.; Centkowski, P.; Warzocha, K.; Bilinski, P. Humoral response to hemagglutinin components of influenza vaccine in patients with non-Hodgkin malignant lymphoma. Vaccine 2006, 24, 6620–6623. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.A.; Moldovan, A.; Barnack, F.; Loh, L.; Eisenberg, R.A. Response to immunization in SLE patients treated with rituximab. In Proceedings of Annual Scientific Meeting, University of Pennsylvania, Philadelphia, PA, USA, 9–10 June 2006.
- Van der Kolk, L.E.; Baars, J.W.; Prins, M.H.; van Oers, M.H. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood 2002, 100, 2257–2259. [Google Scholar] [PubMed]
- Van Assen, S.; Holvast, A.; Benne, C.A.; Posthumus, M.D.; van Leeuwen, M.A.; Voskuyl, A.E.; Blom, M.; Risselada, A.P.; de Haan, A.; Westra, J.; et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010, 62, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Williams-Gould, J.; Watson, T.; Pai-Scherf, L.; Pastan, I. Pretreatment with rituximab does not inhibit the human immune response against the immunogenic protein LMB-1. Clin. Cancer Res. 2004, 10, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Ek, T.; Mellander, L.; Hahn-Zoric, M.; Abrahamsson, J. Intensive treatment for childhood acute lymphoblastic leukemia reduces immune responses to diphtheria, tetanus, and haemophilus influenzae type B. J. Pediatr. Hematol. Oncol. 2004, 26, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Cecinati, V.; Brescia, L.; Principi, N. Vaccinations in children with cancer. Vaccine 2010, 28, 3278–3284. [Google Scholar] [CrossRef] [PubMed]
- Zecca, M.; Nobili, B.; Ramenghi, U.; Perrotta, S.; Amendola, G.; Rosito, P.; Jankovic, M.; Pierani, P.; de Stefano, P.; Bonora, M.R.; et al. Rituximab for the treatment of refractory autoimmune hemolytic anemia in children. Blood 2003, 101, 3857–3861. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.; Ito, S.; Nozu, K.; Fujinaga, S.; Nakayama, M.; Sako, M.; Saito, M.; Yoneko, M.; Iijima, K. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr. Nephrol. 2009, 24, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Sellier-Leclerc, A.L.; Macher, M.A.; Loirat, C.; Guerin, V.; Watier, H.; Peuchmaur, M.; Baudouin, V.; Deschenes, G. Rituximab efficiency in children with steroid-dependent nephrotic syndrome. Pediatr. Nephrol. 2010, 25, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wiley, J.M.; Luddy, R.; Greenberg, J.; Feuerstein, M.A.; Bussel, J.B. Chronic immune thrombocytopenic purpura in children: Assessment of rituximab treatment. J. Pediatr. 2005, 146, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, G. Persistent B-cell depletion after rituximab for thrombocytopenic purpura. Eur. J. Pediatr. 2007, 166, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.; Beresford, M.; Maynes, C.; Pilkington, C.; Marks, S.; Glackin, Y.; Tullus, K. The indications, efficacy and adverse events of rituximab in a large cohort of patients with juvenile-onset SLE. Lupus 2014. [CrossRef]
- Dale, R.C.; Brilot, F.; Duffy, L.V.; Twilt, M.; Waldman, A.T.; Narula, S.; Muscal, E.; Deiva, K.; Andersen, E.; Eyre, M.R.; et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology 2014, 83, 142–150. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.; Grillo-Lopez, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [PubMed]
- Casulo, C.; Maragulia, J.; Zelenetz, A.D. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin. Lymphoma Myeloma Leuk. 2013, 13, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Piro, L.D.; White, C.A.; Grillo-Lopez, A.J.; Janakiraman, N.; Saven, A.; Beck, T.M.; Varns, C.; Shuey, S.; Czuczman, M.; Lynch, J.W.; et al. Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann. Oncol. 1999, 10, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Ghielmini, M.; Rufibach, K.; Salles, G.; Leoncini-Franscini, L.; Leger-Falandry, C.; Cogliatti, S.; Fey, M.; Martinelli, G.; Stahel, R.; Lohri, A.; et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: Clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: A study of the swiss group for clinical cancer research (SAKK). Ann. Oncol. 2005, 16, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Ghielmini, M.; Schmitz, S.F.; Cogliatti, S.B.; Pichert, G.; Hummerjohann, J.; Waltzer, U.; Fey, M.F.; Betticher, D.C.; Martinelli, G.; Peccatori, F.; et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 2004, 103, 4416–4423. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Hase, M.; Tokuman, N.; Yoshida, T. Immune reconstitution of B-cell lymphoma patients receiving CHOP-based chemotherapy containing rituximab. Hematol. Oncol. 2011, 29, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Buser, A.; Stern, M.; Arber, C.; Medinger, M.; Halter, J.; Rovo, A.; Favre, G.; Lohri, A.; Tichelli, A.; Gratwohl, A. Impaired B-cell reconstitution in lymphoma patients undergoing allogeneic HSCT: An effect of pretreatment with rituximab? Bone Marrow Transplant. 2008, 42, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Zhang, Y.; Wang, Z.; Esler, W.V.; Beggs, D.; Pruitt, B.; Hancock, P.; Townsend, M. Maintenance rituximab after autologous stem cell transplant for high-risk B-cell lymphoma induces prolonged and severe hypogammaglobulinemia. Bone Marrow Transplant. 2005, 35, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Esler, W.V.; Zhang, Y.; Zhang, J.; Periman, P.O.; Burris, C.; Townsend, M. B-cell depletion for 2 years after autologous stem cell transplant for NHL induces prolonged hypogammaglobulinemia beyond the rituximab maintenance period. Leuk. Lymphoma 2008, 49, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Imashuku, S.; Teramura, T.; Morimoto, A.; Naya, M.; Kuroda, H. Prolonged hypogammaglobulinemia following rituximab treatment for post transplant Epstein-Barr virus-associated lymphoproliferative disease. Bone Marrow Transplant. 2004, 33, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Zhao, Y.; Wang, S.H.; Li, H.H.; Huang, W.R.; Gao, C.J.; Yu, L. Change of serum immunoglobulin level in patients with diffuse large B cell lymphoma after rituximab combined with chemotherapy. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2011, 19, 676–679. [Google Scholar] [PubMed]
- Anolik, J.H.; Barnard, J.; Owen, T.; Zheng, B.; Kemshetti, S.; Looney, R.J.; Sanz, I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis rheum. 2007, 56, 3044–3056. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Ben-Bassat, I.; Shpilberg, O.; Polliack, A.; Raanani, P. The late adverse events of rituximab therapy—Rare but there! Leuk. Lymphoma 2009, 50, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Posfai, E.; Irsai, G.; Illes, A.; Mehes, G.; Marton, I.; Molnar, C.; Csipo, I.; Barath, S.; Gergely, L. Evaluation of significance of lymphocyte subpopulations and non-specific serologic markers in B-cell non-Hodgkin’s lymphoma patients. Pathol. Oncol. Res. 2014, 20, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.C.; Weitzman, S.; Weinstein, H.; Chang, M.; Cairo, M.; Hutchison, R.; Shiramizu, B.; Wiley, J.; Woods, D.; Barnich, M.; et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: A report from the children’s oncology group. Pediatr. Blood Cancer 2009, 52, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Schrappe, M.; Tiemann, M.; Ludwig, W.D.; Yakisan, E.; Zimmermann, M.; Mann, G.; Chott, A.; Ebell, W.; Klingebiel, T.; et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood 1999, 94, 3294–3306. [Google Scholar] [PubMed]
- Samochatova, E.V.; Maschan, A.A.; Shelikhova, L.N.; Myakova, N.V.; Belogurova, M.B.; Khlebnikova, O.P.; Shamardina, A.V.; Ryskal, O.V.; Roumiantseva, J.V.; Konovalov, D.M.; et al. Therapy of advanced-stage mature B-cell lymphoma and leukemia in children and adolescents with rituximab and reduced intensity induction chemotherapy (B-NHL 2004m protocol): The results of a multicenter study. J. Pediatr. Hematol. Oncol. 2014, 36, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, A.; Burkhardt, B.; Zimmermann, M.; Borkhardt, A.; Kontny, U.; Klingebiel, T.; Berthold, F.; Janka-Schaub, G.; Klein, C.; Kabickova, E.; et al. Phase ii window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and burkitt leukemia. J. Clin. Oncol. 2010, 28, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Lisfeld, J.; Burkhardt, B.; Meinhardt, A.; Zimmermann, M.; Kabıckova, E.; Bielack, S.; Kontny, U.; Gnekow, A.; Sauerbrey, A.; Reiter, A. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and burkitt leukemia: Dose-escalation does not increase the response rate. Br. J. Haematol. 2012, 112, Abstract 6. [Google Scholar]
- Goldman, S.; Smith, L.; Anderson, J.R.; Perkins, S.; Harrison, L.; Geyer, M.B.; Gross, T.G.; Weinstein, H.; Bergeron, S.; Shiramizu, B.; et al. Rituximab and FAB/LMB 96 chemotherapy in children with stage III/IV B-cell non-Hodgkin lymphoma: A children’s oncology group report. Leukemia 2013, 27, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Smith, L.; Galardy, P.; Perkins, S.L.; Frazer, J.K.; Sanger, W.; Anderson, J.R.; Gross, T.G.; Weinstein, H.; Harrison, L.; et al. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive burkitt lymphoma/leukaemia: A children’s oncology group report. Br. J. Haematol. 2014, 167, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Seidemann, K.; Mann, G.; Zimmermann, M.; Burkhardt, B.; Oschlies, I.; Ludwig, W.D.; Klingebiel, T.; Graf, N.; Gruhn, B.; et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: A report of the BFM group study NHL-BFM95. Blood 2005, 105, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Cairo, M.S.; Gerrard, M.; Sposto, R.; Auperin, A.; Pinkerton, C.R.; Michon, J.; Weston, C.; Perkins, S.L.; Raphael, M.; McCarthy, K.; et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 2007, 109, 2736–2743. [Google Scholar] [PubMed]
- Attias, D.; Weitzman, S. The efficacy of rituximab in high-grade pediatric B-cell lymphoma/leukemia: A review of available evidence. Curr. Opin. Pediatr. 2008, 20, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Bohlius, J.F.; Trelle, S.; Skoetz, N.; Reiser, M.; Kober, T.; Schwarzer, G.; Herold, M.; Dreyling, M.; Hallek, M.; et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2007, 99, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.I.; Kakisi, O.K.; Vardakas, K.; Falagas, M.E. Infectious complications of monoclonal antibodies used in cancer therapy: A systematic review of the evidence from randomized controlled trials. Cancer 2007, 109, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Kuriyama, K.; Tsuji, K.; Isoda, K.; Hibi, S.; Todo, S.; Sugimoto, T.; Imashuku, S. Use of rituximab to treat refractory diamond-blackfan anemia. Eur. J. Haematol. 2005, 74, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Forstpointner, R.; Dreyling, M.; Repp, R.; Hermann, S.; Hanel, A.; Metzner, B.; Pott, C.; Hartmann, F.; Rothmann, F.; Rohrberg, R.; et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with fcm alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the german low-grade lymphoma study group. Blood 2004, 104, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Lepage, E.; Briere, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; van den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pfreundschuh, M.; Trümper, L.; Österborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.-L.; et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Habermann, T.M.; Weller, E.A.; Morrison, V.A.; Gascoyne, R.D.; Cassileth, P.A.; Cohn, J.B.; Dakhil, S.R.; Woda, B.; Fisher, R.I.; Peterson, B.A.; et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J. Clin. Oncol. 2006, 24, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Hiddemann, W.; Kneba, M.; Dreyling, M.; Schmitz, N.; Lengfelder, E.; Schmits, R.; Reiser, M.; Metzner, B.; Harder, H.; Hegewisch-Becker, S.; et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005, 106, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Dreyling, M.; Hoster, E.; Wormann, B.; Duhrsen, U.; Metzner, B.; Eimermacher, H.; Neubauer, A.; Wandt, H.; Steinhauer, H.; et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J. Clin. Oncol. 2005, 23, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Eve, H.E.; Linch, D.; Qian, W.; Ross, M.; Seymour, J.F.; Smith, P.; Stevens, L.; Rule, S.A. Toxicity of fludarabine and cyclophosphamide with or without rituximab as initial therapy for patients with previously untreated mantle cell lymphoma: Results of a randomised phase II study. Leuk. Lymphoma 2009, 50, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Tuccori, M.; Focosi, D.; Maggi, F.; Cosottini, M.; Meini, B.; Lena, F.; Blandizzi, C.; del Tacca, M.; Petrini, M. Progressive multifocal leukoencephalopathy: A report of three cases in HIV-negative patients with non-Hodgkin’s lymphomas treated with rituximab. Ann. Hematol. 2010, 89, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Tuccori, M.; Focosi, D.; Blandizzi, C.; Pelosini, M.; Montagnani, S.; Maggi, F.; Pistello, M.; Antonioli, L.; Fornai, M.; Pepe, P.; et al. Inclusion of rituximab in treatment protocols for non-Hodgkin’s lymphomas and risk for progressive multifocal leukoencephalopathy. Oncologist 2010, 15, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Pelosini, M.; Focosi, D.; Rita, F.; Galimberti, S.; Caracciolo, F.; Benedetti, E.; Papineschi, F.; Petrini, M. Progressive multifocal leukoencephalopathy: Report of three cases in HIV-negative hematological patients and review of literature. Ann. Hematol. 2008, 87, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.L. Unusual viral infections (progressive multifocal leukoencephalopathy and cytomegalovirus disease) after high-dose chemotherapy with autologous blood stem cell rescue and peritransplantation rituximab. Blood 2002, 99, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Watanabe, T.; Maruyama, D.; Kim, S.W.; Kobayashi, Y.; Tobinai, K. Progressive multifocal leukoencephalopathy in a patient with B-cell lymphoma during rituximab-containing chemotherapy: Case report and review of the literature. Int. J. Hematol. 2008, 88, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Norris, L. Association between rituximab use and progressive multifocal leukencephalopathy among non-HIV, non-Hoddkin lymphoma Veteran’s Administration patients. J. Clin. Oncol. 2014, 32, Abstract e19540. [Google Scholar] [PubMed]
- Kaplan, L.D.; Lee, J.Y.; Ambinder, R.F.; Sparano, J.A.; Cesarman, E.; Chadburn, A.; Levine, A.M.; Scadden, D.T. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: Aids-malignancies consortium trial 010. Blood 2005, 106, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Gafter-Gvili, A.; Leibovici, L.; Dreyling, M.; Ghielmini, M.; hsu Schmitz, S.F.; Cohen, A.; Shpilberg, O. Rituximab maintenance for the treatment of patients with follicular lymphoma: Systematic review and meta-analysis of randomized trials. J. Natl. Cancer Inst. 2009, 101, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Gea-Banacloche, J.C. Rituximab-associated infections. Semin. Hematol. 2010, 47, 187–198. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worch, J.; Makarova, O.; Burkhardt, B. Immunreconstitution and Infectious Complications After Rituximab Treatment in Children and Adolescents: What Do We Know and What Can We Learn from Adults? Cancers 2015, 7, 305-328. https://doi.org/10.3390/cancers7010305
Worch J, Makarova O, Burkhardt B. Immunreconstitution and Infectious Complications After Rituximab Treatment in Children and Adolescents: What Do We Know and What Can We Learn from Adults? Cancers. 2015; 7(1):305-328. https://doi.org/10.3390/cancers7010305
Chicago/Turabian StyleWorch, Jennifer, Olga Makarova, and Birgit Burkhardt. 2015. "Immunreconstitution and Infectious Complications After Rituximab Treatment in Children and Adolescents: What Do We Know and What Can We Learn from Adults?" Cancers 7, no. 1: 305-328. https://doi.org/10.3390/cancers7010305